Abstract
Hypoparathyroidism is the inability of parathyroid hormone (PTH) to maintain calcium homeostasis. Patients with post-surgical hypoparathyroidism may have an increased risk of mortality; there is clinical and molecular evidence of the effects of this condition on the cardiovascular system. The aim of this study was to evaluate arterial stiffness by measuring the carotid-femoral pulse wave velocity (PWV) in post-surgical hypoparathyroidism patients. A cross-sectional study was conducted with 30 post-surgical hypoparathyroidism patients and 25 volunteers from the Endocrinology Outpatient Clinic of the Medical School. The SphygmoCor system was used to evaluate arterial stiffness by analyzing the PWV. The mean ages of the hypoparathyroidism (50.4 years) and control individuals (49.6 years) were similar. The mean PWVs were 8.7 and 7.5 m/s in the Hypoparathyroidism and Control groups, respectively (p-value = 0.084). Considering only normotensive patients, PWV was statistically higher in the Hypoparathyroidism Group (7.6 versus 6.5 m/s; p-value = 0.039). For this group, serum ionized calcium, phosphorus, and the calcium x phosphorus product levels were positively associated to PWV. Hypoparathyroidism increases arterial stiffness as assessed by PWV. Serum ionized calcium, phosphorus, and the calcium x phosphorus product are affected. A more effective investigative and therapeutic approach for patients with hypoparathyroidism can help control cardiovascular risk.
Similar content being viewed by others
Introduction
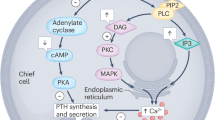
Hypoparathyroidism is an endocrine disease characterized by decreased levels of parathormone (PTH), hypocalcemia and high plasma levels of phosphorus. Insufficient hormone synthesis, accidental damage, or removal of the parathyroid glands during surgery are reported as the origin of hypoparathyroidism. In adults, surgical procedures of the neck, particularly thyroidectomy, are the most common cause of the disease; other causes include autoimmune diseases, genetic diseases, infiltration, radiation, and human immunodeficiency virus (HIV)1,2,3,4. The incidence rate of permanent post-surgical hypoparathyroidism is 0.4–33%.2
The effects of PTH on calcium and phosphorus levels have been described in respect to the cardiovascular system. PTH receptors in endothelial and myocardial cells and direct hypertrophic effects of PTH on myocardial cells have been reported5. Although the mechanism is uncertain, hypocalcemia is believed to reduce myocardial contractility; QT prolongation and arrythmias may occur due to hypocalcemia6. Moreover, persistent hyperphosphatemia has been associated to vascular calcification in patients with chronic kidney disease (CKD)7. As hyperphosphatemia is a characteristic of hypoparathyroidism, increased cardiovascular risk has been described in patients with this metabolic disorder8.
In turn, arterial stiffness (AS) is cited as an independent risk marker for cardiovascular disease (CVD)9,10. As it is a practical and reliable non-invasive method, pulse wave velocity (PWV) is considered the gold standard to evaluate AS11,12.
There are few studies that describe the effect of hypoparathyroidism on AS13,14. One study reported that PWV values were higher in patients with hypoparathyroidism compared to apparently healthy subjects (6.2 versus 5.9 m/s; p-value = 0.02).13 Other researchers enrolled 56 non-surgical hypoparathyroidism patients and found that PWV was higher in this group compared to patients with pseudohypoparathyroidism14. Although both studies demonstrated increased AS, only the first study found an association between ionized calcium (Ca2+), phosphorus (P) and the calcium x phosphorus product (Ca x P product) with PWV13.
The aim of the present research was to evaluate whether central hemodynamic parameters, particularly AS, are altered in post-surgical hypoparathyroidism patients and investigate any association between calcium metabolism parameters and AS.
Subjects and methods
Study population
This was a cross-sectional study performed in patients with permanent post-surgical hypoparathyroidism (≥ 12 months) compared to individuals without any parathyroid gland or systemic disease. The inclusion criteria were adult asymptomatic women (≥ 18 years), receiving adequate treatment with levothyroxine for permanent post-surgical hypoparathyroidism (≥ 12 months) and with serum calcium (Ca) ≥ 7 mg/dL. The exclusion criteria were patients with a glomerular filtration rate < 30 mL/min/1.73 m2 calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Eq.15, presence of diabetes, uncontrolled hypothyroidism (thyroid stimulating hormone - TSH > 10 mU/L) and other endocrine disorders. The study enrolled 30 women with hypoparathyroidism (Study Group) and 25 women without any history of surgical procedures of the neck or clinical evidence of thyroid dysfunction (Control Group). Both groups were age matched. All hypoparathyroidism patients had been treated with adequate doses of oral calcium and calcitriol over the previous three months. This study was approved by the Research Ethics Committee of the Medical School according to national and international guidelines (CAAE: 11934919.5.0000.5415: #3.314.696) and followed the ethical principles of the Declaration of Helsinki. All individuals read and signed an informed consent form.
Firstly, systolic (SBP) and diastolic (DBP) blood pressures (BPs) were measured at least three times for each individual with an automatic sphygmomanometer (OMROM-Automatic, Digital Blood Pressure Monitor, HEM-705CP), which uses a cuff-based oscillometric method to measure the brachial artery BP. The mean of three BP measurements was considered. AS was evaluated according to the PWV using an AtCor SphygmoCor-XCEL (Atcor Medical Pty Ltd, Sydney, NSW, Australia). This method has previously been validated for AS assessments16. The augmentation pressure and augmentation index (AI) adjusted for a 75 pulse/min heart rate (AIx75%) were also evaluated.
Anthropometric data were collected for patients and the body mass index (BMI) was calculated. Moreover, the PWV, electrocardiogram and laboratorial tests were performed. According to the VII Hypertension Brazilian Guidelines, a PWV ≥10 m per second (m/s) is indicative of AS17. Laboratory tests of the hypoparathyroidism group were recovered from the hospital computer database including fasting plasma glucose after eight hours of fasting (reference range: 74–100 mg/dL), serum creatinine (range: 0.57–1.11 mg/dL), TSH (range: 0.55–4 mIU/mL), free thyroxine (fT4 - range: 0.90–1.80 ng/mL), total Ca (range: 8.4–10.2 mg/dL), phosphorus (range: 2.5–4.5 mg/dL), magnesium (range: 1.8–2.4 mg/dL), 25-hydroxyvitamin D (25-OHD - range: 25–80 ng/mL), PTH (range: 15–65 pg/mL) and 24-hour urine calcium. The Ca x P product was calculated by multiplying the calcium and phosphorus levels. The exams of the Control Group were serum creatinine and fasting glucose.
Statistical analysis
Descriptive statistics together with the Kolmogorov-Smirnov test were used to analyze the trend of continuous variables. Student’s t-test was used to compare independent groups. Correlation analyses were achieved by Spearman’s and Pearson’s correlation tests. A multivariate regression analysis was performed to detect independent variables predicting hemodynamic parameters and PWV. The sample size was chosen considering the numbers of patients treated with hypoparathyroidism in the health service.
Statistical analyses were performed using the IBM Statistical Package for Social Sciences (SPSS) software, version 27 (IBM Corporation, NY, USA). P-values < 0.05 were considered significant.
Results
This study included 30 hypoparathyroidism patients and 25 control subjects. There was no significant difference in age between the two groups. However, the BMI and waist circumference were statistically greater in the Study Group. (Table 1). Hypertension was present in nine patients of each group, resulting in prevalences of 30% in the Study Group and 36% in the Control Group. BMI greater than 30 was found in 40% and 16% of the Study and Control Groups, respectively. However, obesity did not influence in the PWV values. Thus, no association was observed between BMI and PWV. Around 40% of both groups were menopausal women.
Indication for neck surgery was the presence of thyroid carcinoma in 66.6% of patients with the mean duration of hypoparathyroidism being nine years and six months in this group. Only four patients (13%) demonstrated mild increases in the TSH level. The hypoparathyroidism patients presented adequate Ca x P product (Table 2).
Both groups demonstrated normal PWVs and other hemodynamic variables. The PWV was higher, albeit non-significant, in the hypoparathyroidism group. However, an analysis of just the normotensive subjects showed that the PWV was statistically different between the two groups (7.64 ± 1.73 versus 6.50 ± 1.39 m/s for the Study and Control Groups, respectively; p-value = 0.039) (Table 3).
Table 4 shows data for the subgroups according to presence or not of hypertension (normotensive control, hypertensive control, normotensive hypoparathyroidism and hypertensive hypoparathyroidism). Central and peripheral hemodynamic parameters, including BP, pulse pressure and AIx75%, were higher in the Study Group compared to controls independently of whether hypertension was present. (Table 4).
Linear regression analysis of the Study Group showed a significant association between the biochemical test results (ionized calcium, phosphorus, Ca x P product) and the central and peripheral parameters (p-value < 0.05), including PWV. However, the PTH level was only associated to central systolic and diastolic BPs. (Table 5)
Discussion
Calcium metabolism disorders involving Ca, P and PTH are described as risk factors for vascular calcification, increased risk of CVD,18,19 and mortality20. The present study evaluated the AS by PWV in patients with post-surgical hypoparathyroidism. There are conflicting results and few studies that report the effect of hypoparathyroidism on AS. Clinical, BP and laboratorial parameters investigated in this study demonstrated that hypoparathyroidism is associated with higher BP and AS, thereby contributing to increased cardiovascular risk.
Two previous studies investigated PWV in patients with hypoparathyroidism and found higher velocities in hypoparathyroidism patients13,14. In the first study, Pamuk et al. compared hypoparathyroidism patients with volunteers in terms of PWV and BP13. The device used to assess PWV was a Mobil-o-graph, different from previous and the present study. Some of the subjects were not surgical hypoparathyroidism cases whereas the present research included only surgical hypoparathyroidism. The authors found a mean PWV of 6.2 m/s (range: 4.5–10.2), lower than that found in the current study which can be explained by the difference in the mean age (40 years), that is, lower than the mean age of the current study (mean 50.43 years) and the fact that hypertensive patients were not included13. In the second study, a sample of 56 individuals with non-surgical hypoparathyroidism was compared to 30 patients with pseudohypoparathyroidism in terms of BP and PWV. The method of PWV evaluation, a SphygmoCor-XCEL, which is considered the gold standard, was the same as in the present study14. Although the PWV was higher in the non-surgical hypoparathyroidism group, neither PWV nor BP were associated to the Ca x P product, or Ca and P levels14.
The current research demonstrated a higher prevalence of obesity in the Study Group, which could affect the PWV result. However, no correlation was observed between BMI and PWV. This finding agrees with a recent study that did not observe any association between BMI and PWV in women either. The authors concluded that obesity parameters have not influence on PWV in women21.
There was no difference in the percentage of menopausal women between groups in the present study. Furthermore, the effect of estrogen deprivation during menopause increases arterial stiffness in an age-dependent manner. As the groups were age-matched, the menopause had no influence on the results22.
According to multivariate linear regression analysis, ionized calcium, phosphorus, and the Ca x P product are independent predictors for PWV. Compared to the work by Pamuk et al., the present study agrees only with the phosphorus level, because phosphorus was found to be an independent predictor of increases in PWV;13 it was demonstrated that hyperphosphatemia is related to cardiovascular risk7. There is an increase of phosphorus uptake into smooth muscle cells mediated by the sodium-phosphate cotransporter following an increase in extracellular phosphorus. Phosphorus inside the cell stimulates the production of Core-binding factor alpha1 (Cbfa-1), a transcription factor that promotes osteogenic differentiation. The smooth muscle cells become calcified and gain osteoblast-like phenotypical characteristics, decreasing arterial elasticity which leads to AS. Consequently, the systolic BP and pulse pressure increase, and the diastolic blood pressure decreases7,23,24
Although the present study demonstrated that calcium, phosphorus and the Ca x P product were adequate in serum, these parameters were correlated to AS. These disorders have previously been described associated to vascular calcification and risk of death in patients with CKD.24,25,26,27 Recently, cross-sectional studies revealed that the phosphorus concentration has an important influence on calciprotein particles (CPP)28,29, which are mineral-protein products mainly composed of calcium phosphate and serum proteins, in particular fetuin-A30. CPPs are present in the blood and renal tubular fluid with phosphate toxicity being mediated by higher concentrations of CPP in the body. Serum CPP levels have been positively correlated with AS as determined using the PWV31. CPP levels have not been studied in hypoparathyroidism individuals. However, as described previously, calcium and phosphorus disorders are similar in CKD patients and so this allows the use of this model to explain the effects of calcification in hypoparathyroidism patients.
Meena et al. studied the intima-media thickness of the carotid artery, aorta, and renal artery of 30 sporadic idiopathic hypoparathyroidism patients compared to healthy controls. They detected a higher intima-media thickness in the hypoparathyroidism group compared to the control group. On the other hand, laboratorial parameters such as calcium, phosphorus, the Ca x P product, 25-OHD and PTH were not correlated to intima-media thickness.32 Another study by Agarwal et al. detected different stages of calcification of the coronary artery in three patients in the hypoparathyroidism group whereas no calcification was found in the 40 volunteers of the control group by multidetector computer tomography (CT). Although the difference was not statistically significant, the hypoparathyroidism group demonstrated a higher calcium score of the coronary artery which was negatively correlated with serum calcium levels18. Such studies confirm the effect of hypoparathyroidism on arterial stiffness AS. However, the authors did not find a positive correlation between phosphorus concentration and vascular function.
Two previous studies described an association between hypoparathyroidism and mortality8,20, while hyperphosphatemia and Ca x P product were found to be risk factors for fatal outcomes19. These results support the results in the present study and may confirm the effect of surgical hypoparathyroidism on AS and cardiovascular risk13,14. Additionally, the positive correlation between phosphorus level and the Ca x P product and PWV suggests that AS may be due to an increment in vascular calcification.
A systematic review demonstrated associations between serum calcium, stroke, and arterial calcification in individuals with normal calcium levels.33 A study with 565 Korean adults showed that serum calcium levels were independently and positively associated with AS and ten-year CVD risk by the Framingham risk score.34 Thus, it was concluded that early detection of higher serum calcium levels may be important for the assessment of AS and the future risk of a cardiovascular event.
Although hypoparathyroidism patients present with undetectable or low levels of PTH, the positive association described in this research between PTH and central BP parameters is compatible with other studies.34,35 In the SPRINT trial, higher PTH levels in CKD patients increased the risk of cardiovascular events, especially in the BP intensive control group35. PTH receptors have been found in cardiomyocytes and vascular wall and so, PTH acts direct or indirectly on the heart and arterial system36. Contrasting with these results, PTH and 25-OHD levels were not associated with progression of carotid AS37.
This study has some limitations. As the study has a cross-sectional design, patient outcomes are not reported. Furthermore, laboratory tests of the calcium metabolism were not performed in the Control Group. However, we consider that control subjects had normal levels since they were asymptomatic and did not have any history of radiation or surgical procedures of the neck. In addition, lipid tests were not collected in the Control Group. However, we consider that this did not interfere in the results as Pamuk et al. demonstrated that lipid parameters were similar in both hypoparathyroidism and control individuals13.
An important characteristic of the present study was that only women made up both groups, and there was no difference between the groups in terms of cardiovascular risk factors and age. Furthermore, possible causes of AS in hypoparathyroidism patients were discussed. Additionally, the method used to assess AS was the SphygmoCor-XCEL, considered the gold standard to evaluate PWV.
Conclusion
The present study demonstrated an increase of AS in hypoparathyroidism patients, which is a reliable indicator for CVD risk. In addition, a positive correlation between hyperphosphatemia and PWV was detected. Therefore, patients with hypoparathyroidism should be closely monitored for the phosphorus-calcium balance and development of CVD. The relationship can no longer be neglected as a possible cause of CVD. Other studies are needed in patients with this endocrine disease. The current study can guide further comprehensive studies.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Abbreviations
- AS:
-
Arterial stiffness
- PWV:
-
Pulse wave velocity
- BP:
-
Blood pressure
- PTH:
-
Parathormone
- HIV:
-
Human immunodeficiency virus
- CKD:
-
Chronic kidney disease
- CPP:
-
Calciprotein particles
- Ca x P product:
-
Calcium x phosphorus product
- 25-OHD:
-
25-hydroxyvitamin D
- CT:
-
Computer tomography
- CVD:
-
Cardiovascular disease
- TSH:
-
Thyroid stimulating hormone
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- AIx75%:
-
Augmentation index adjusted for a 75 pulse/min heart rate
References
Bilezikian, J. P. Hypoparathyroidism. J. Clin. Endocrinol. Metab. 105(6), 1722–1736. https://doi.org/10.1210/clinem/dgaa113 (2020).
Zhou, B., Cheng, F., Zhu, X., Zhu, L. & Li, Z. Effect of intraoperative active exploration of parathyroid glands to reduce the incidence of postoperative hypoparathyroidism, and risk factors of hypoparathyroidism after total thyroidectomy: A single-center study. Front. Surg. 10, 1203595. https://doi.org/10.3389/fsurg.2023.1203595 (2023).
Cianferotti, L., Marcucci, G. & Brandi, M. L. Causes and pathophysiology of hypoparathyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 32(6), 909–925. https://doi.org/10.1016/j.beem.2018.07.001 (2018).
Giusti, F. & Brandi, M. L. Clinical presentation of hypoparathyroidism. Front. Horm. Res. 51, 139–146. https://doi.org/10.1159/000491044 (2019).
Hakami, Y. & Khan, A. Hypoparathyroidism. Front. Horm. Res. 51, 109–126. https://doi.org/10.1159/000491042 (2019).
Brown, S. J. et al. The parathyroid gland and heart disease. Methodist Debakey Cardiovasc. J. 13(2), 49–54. https://doi.org/10.14797/mdcj-13-2-49 (2017).
Shang, D. et al. Hyperphosphatemia and hs-CRP initiate the coronary artery calcification in peritoneal dialysis patients. Biomed. Res. Int. 2017, 2520510. https://doi.org/10.1155/2017/2520510 (2017).
Vadiveloo, T., Donnan, P. T., Leese, C. J., Abraham, K. J. & Leese, G. P. Increased mortality and morbidity in patients with chronic hypoparathyroidism: A population-based study. Clin. Endocrinol. (Oxf). 90(2), 285–292. https://doi.org/10.1111/cen.13895 (2019).
Boutouyrie, P., Chowienczyk, P., Humphrey, J. D. & Mitchell, G. F. Arterial stiffness and cardiovascular risk in hypertension. Circ. Res. 128(7), 864–886. https://doi.org/10.1161/CIRCRESAHA.121.318061 (2021).
Vlachopoulos, C. et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 241(2), 507–532. https://doi.org/10.1016/j.atherosclerosis.2015.05.007 (2015).
Ben-Shlomo, Y. et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 63(7), 636–646. https://doi.org/10.1016/j.jacc.2013.09.063 (2014).
Baldo, M. P. et al. Carotid-femoral pulse wave velocity in a healthy adult sample: The ELSA-Brasil study. Int. J. Cardiol. 251, 90–95. https://doi.org/10.1016/j.ijcard.2017.10.075 (2018).
Pamuk, N. et al. Central and peripheral blood pressures and arterial stiffness increase in hypoparathyroidism. Arch. Endocrinol. Metab. 64(4), 374–382. https://doi.org/10.20945/2359-3997000000234 (2020).
Underbjerg, L., Sikjaer, T. & Rejnmark, L. Cardiovascular findings in patients with nonsurgical hypoparathyroidism and pseudohypoparathyroidism: A cohort study. Clin. Endocrinol. (Oxf). 90(4), 592–600. https://doi.org/10.1111/cen.13927 (2019).
Calculation of glomerular filtration rate for Health Care Professionals—National Kidney Foundation. (2011). Available in: http://www.kidney.org/professionals/KDOQI/gfr_calculator.cfm.
Milan, A. et al. Current assessment of pulse wave velocity: Comprehensive review of validation studies. J. Hypertens. 37(8), 1547–1557. https://doi.org/10.1097/HJH.0000000000002081 (2019).
7th Brazilian Guideline of Arterial Hypertension. Arq Bras Cardiol 107 (3 Supl. 3) https://doi.org/10.5935/abc.20160140 (2016).
Agarwal, P. et al. To assess vascular calcification in the patients of hypoparathyroidism using multidetector computed tomography scan. Indian J. Endocrinol. Metab. 19(6), 785–790. https://doi.org/10.4103/2230-8210.167545 (2015).
Underbjerg, L., Sikjaer, T. & Rejnmark, L. Long-term complications in patients with hypoparathyroidism evaluated by biochemical findings: A case-control study. J. Bone Miner. Res. 33(5), 822–831. https://doi.org/10.1002/jbmr.3368 (2018).
Almquist, M., Ivarsson, K., Nordenström, E. & Bergenfelz, A. Mortality in patients with permanent hypoparathyroidism after total thyroidectomy. Br. J. Surg. 105(10), 1313–1318. https://doi.org/10.1002/bjs.10843 (2018).
Eikås, J. G. et al. Arterial stiffness in overweight and obesity: Association with sex, age, and blood pressure. High Blood Press. Cardiovasc. Prev. 30(5), 435–443. https://doi.org/10.1007/s40292-023-00593-2 (2023).
Stefanska, A., Bergmann, K. & Sypniewska, G. Metabolic syndrome and menopause: Pathophysiology, clinical and diagnostic significance. Adv. Clin. Chem. 72, 1–75. https://doi.org/10.1016/bs.acc.2015.07.001 (2015).
Underbjerg, L., Sikjaer, T., Mosekilde, L. & Rejnmark, L. The epidemiology of nonsurgical hypoparathyroidism in Denmark: A nationwide case finding study. J. Bone Miner. Res. 30(9), 1738–1744. https://doi.org/10.1002/jbmr.2501 (2015).
Reynolds, J. L. et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J. Am. Soc. Nephrol. 15(11), 2857–2867. https://doi.org/10.1097/01.ASN.0000141960.01035.28 (2004).
Reiss, A. B. et al. CKD, arterial calcification, atherosclerosis and bone health: Inter-relationships and controversies. Atherosclerosis 278, 49–59. https://doi.org/10.1016/j.atherosclerosis.2018.08.046 (2018).
Sigrist, M. K. et al. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2(6), 1241–1248. https://doi.org/10.2215/CJN.02190507 (2007).
London, G. M. et al. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol. Dial. Transplant. 18(9), 1731–1740. https://doi.org/10.1093/ndt/gfg414 (2003).
Kuro-O, M. Calcium phosphate microcrystallopathy as a paradigm of chronic kidney disease progression. Curr. Opin. Nephrol. Hypertens. 32(4), 344–351. https://doi.org/10.1097/MNH.0000000000000890 (2023).
Miura, Y. et al. Identification and quantification of plasma calciprotein particles with distinct physical properties in patients with chronic kidney disease. Sci. Rep. 8(1), 1256. https://doi.org/10.1038/s41598-018-19677-4 (2018).
Jahnen-Dechent, W. et al. Mud in the blood the role of protein-mineral complexes and extracellular vesicles in biomineralisation and calcification. J. Struct. Biol. 212(1), 107577. https://doi.org/10.1016/j.jsb.2020.107577 (2020).
Smith, E. R. et al. Phosphor ylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with predialysis CKD. Nephrol. Dial. Transplant. 27(5), 1957–1966. https://doi.org/10.1093/ndt/gfr609 (2012).
Meena, D., Prakash, M., Gupta, Y., Bhadada, S. K. & Khandelwal, N. Carotid, aorta and renal arteries intima-media thickness in patients with sporadic idiopathic hypoparathyroidism. Indian J. Endocrinol. Metab. 19(2), 262–266. https://doi.org/10.4103/2230-8210.149320 (2015).
Reid, I. R., Gamble, G. D. & Bolland, M. J. Circulating calcium concentrations, vascular disease and mortality: A systematic review. J. Intern. Med. 279(6), 524–540. https://doi.org/10.1111/joim.12464 (2016).
Park, B. & Lee, Y. J. Borderline high serum calcium levels are associated with arterial stiffness and 10-year cardiovascular disease risk determined by Framingham risk score. J. Clin. Hypertens. (Greenwich). 21(5), 668–673. https://doi.org/10.1111/jch.13532 (2019).
Ginsberg, C. et al. PTH, FGF23, and intensive blood pressure lowering in chronic kidney disease participants in SPRINT. Clin. J. Am. Soc. Nephrol. 13(12), 1816–1824. https://doi.org/10.2215/CJN.05390518 (2018).
Bosworth, C. et al. Parathyroid hormone and arterial dysfunction in the multi-ethnic study of atherosclerosis. Clin. Endocrinol. (Oxf). 79(3), 429–436. https://doi.org/10.1111/cen.12163 (2013).
Gepner, A. D. et al. 25-hydroxyvitamin D and parathyroid hormone levels do not predict changes in carotid arterial stiffness: The multi-ethnic study of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 34(5), 1102–1109. https://doi.org/10.1161/ATVBAHA.113.302605 (2014).
Acknowledgements
The authors would like to thank the reviewer for correcting both spelling and grammar of the English text. The authors thank the participants and investigators of this research.
Funding
Prof Jose F. Vilela-Martin is a researcher at The National Council for Scientific and Technological Development (CNPq - grant number #313715/2021-1) and of Sao Paulo Research Foundation (FAPESP − 2016/08203-6).
Author information
Authors and Affiliations
Contributions
The study was conceived by LNC-M and JFV-M. LNC-M and JFV-M provided the research platform. RD, JRRU, VSL, LABF, KAO and AOS performed the literature search, contributed to the methodology and data acquisition. LNC-M and JFV-M wrote the manuscript. JCY-T and MAVS critically revised the study. The final version of the manuscript was reviewed and agreed to be submitted by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All data are from publicly available databases that have received ethical approval from their respective institutional review boards and informed consent from all participants. No administrative permissions were required to access the data. This study was approved by the Research Ethics Committee of the Medical School according to national and international guidelines (CAAE: 11934919.5.0000.5415: #3.314.696).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cosenso-Martin, L.N., Souza, R.D.M., Uyemura, J.R.R. et al. Increased arterial stiffness in normotensive individuals with hypoparathyroidism. Sci Rep 15, 8817 (2025). https://doi.org/10.1038/s41598-025-92708-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92708-z