Abstract
Vaginal atrophy is a prevalent symptom in menopausal women, affecting over 50% of older women and patients with loss of ovarian function. The role of factors other than estrogen, such as the vaginal microbiota (VM), in the development of vaginal atrophy has not been fully explored. Therefore, we selected 8-week-old C57 mice with bilateral ovariectomy for experimentation. After four weeks of treatment, we observed that the vaginal epithelium of ovariectomized mice showed signs of atrophy. There were also significant differences in the structure and metabolites of VM. Vaginal transplantation of microbiota from ovary-intact mice significantly alleviated the vaginal atrophy of ovariectomized mice and altered the structure and metabolism of VM. These findings indicate that ovarian activity significantly affects the structure and metabolism of VM. VM of ovary-intact mice may promote vaginal health by upregulating the estrogen receptor alpha gene (ESR1, one-way ANOVA, F4, 25 = 17.76, P < 0.0001) in vaginal epithelial cells in ovariectomized mice, which in turn promotes cell proliferation (the number of vaginal epithelial cell layers, one-way ANOVA, F4, 25 = 28.04, P < 0.0001). Further studies are needed to investigate the interactions between VM and vaginal health. This finding can help develop new therapeutic strategies and interventions for patients suffering from vaginal atrophy.
Similar content being viewed by others
Introduction
Genitourinary syndrome of menopause (GSM) is a term used to describe a series of symptoms and signs related to the reproductive and urinary tracts of women after menopause due to decreased estrogen levels. This term encompasses not only symptoms associated with vaginal atrophy but also those pertaining to the urinary system1. Over 50% of women experience genitourinary symptoms during the menopausal transition2. These symptoms include genital dryness, burning, irritation, sexual discomfort, pain, and lack of lubrication. Additionally, women may experience urinary urgency, difficulty urination, and recurrent urinary tract infections3. This is recognized as a determinant of impaired quality of life in postmenopausal women4. Decreased estrogen level after menopause is a major contributor to GSM5. However, not all postmenopausal women suffer from GSM, suggesting that there may be other factors that affect the development of GSM. For example, smoking, alcohol abuse, and lack of exercise increase the incidence of GSM, which may be related to decreased circulation and impaired receptor function6. A deeper insight into these factors can help better understand the mechanisms leading to GSM and provide more effective preventive and therapeutic approaches.
Previous studies have demonstrated a correlation between VM and GSM, particularly in the postmenopausal period7. Reduction in estrogen levels following menopause decreases glycogen accumulation in the vaginal epithelium, thereby limiting the availability of carbohydrates for lactobacillus fermentation8. Maintaining vaginal health and function heavily relies on the balance of VM9. In women with GSM, a low hormone level can lead to pathophysiologic changes in the vagina, affecting VM and mucosal immunity. Disruption of VM and dysregulation of the mucosal immune response promotes GSM10. VM diversity and Lactobacillus abundance were significantly increased in patients with GSM receiving hormone therapy. This suggests that hormone therapy may alleviate the symptoms of GSM11.
In addition to hormone therapy, several new studies have focused on the role of vaginal microbiota transplantation (VMT) in the treatment of vaginal systemic disorders. A study explored the potential of VMT for treating recurrent bacterial vaginosis (BV). Five patients with recurrent BV were treated with VMT. VM were obtained from a healthy donor. The results showed that four patients achieved long-term remission and their symptoms significantly improved. These findings suggest that VMT may be a potential treatment for recurrent BV12. In addition, microbiota transplantation has shown great promise in the treatment of gynecologic malignancies. Studies have shown that microbiota can modulate the host’s genetic composition, metabolism, and disease susceptibility. The diversity of microbiota enables the host to resist tumorigenesis through immunomodulation13. Therefore, researchers suggested that regulating the composition and function of microbiota can enhance the efficacy of anti-cancer drugs14.
These studies demonstrated that VM is deeply involved in vaginal health. VM is not only affected by the vaginal environment but also maintains it. Probiotics are effective in alleviating the symptoms of GSM. But instead of using species commonly found in the human vagina, probiotic preparations can be chosen as species already present in the gut microbiota. Thus, they may not fully restore the diversity and function of the normal VM15. Therefore, it is worth exploring the role of VMT in the development of GSM. Dominant strains of VM, such as Lactobacillus, produce surface-active molecules with anti-biofilm, antioxidant, antiviral, pathogen-inhibitory, and immunomodulatory effects. These molecules are involved in the interaction between the human host and VM16. This therapeutic approach may lead to a more comprehensive and personalized restoration of VM and alleviate GSM symptoms and quality of sexual life.
GSM symptoms are caused by decreased estrogen production, and the ovaries are the main organs producing estrogen in women. Previous studies have shown that the vaginal epithelium of mice is significantly atrophied and the number of cell layers is reduced four weeks after bilateral ovariectomy17,18. This study aimed to investigate the effect of vaginal atrophy in bilateral ovariectomized mice by VMT and changes in intravaginal metabolites. Our study also explored the relationship between VM and vaginal health. By screening vaginal metabolites, new treatment modalities can be identified for GSM patients who are unwilling or unable to use hormone therapy.
Results
VMT significantly alleviated vaginal atrophy in ovariectomized mice
Bilateral ovariectomy is a common method to induce menopause in mice. Researchers typically perform this procedure to decrease estrogen levels, mimicking patients who have undergone ovariectomy for various reasons. After four weeks, we observed that vaginal orifices of mice were closed in the surgical group, while mice in the control group did not show this phenomenon. We measured the body weights of mice in each group once a week during the next eight weeks of treatment (Fig. 1A). The results showed that mice in the bilateral ovariectomy group (OVX group, n = 6) and bilateral ovariectomy with normal saline lavage group (OVX + NST group, n = 6) consistently gained weight and were heavier than mice in other groups (P < 0.0001). The bilateral ovaries of both groups of mice were removed and their estrogen levels were reduced, leading to fat accumulation. As shown in Fig. 1B, the body weights of mice in the control group (CON group, n = 6) and bilateral ovariectomy with estriol-treated group (OVX + EST group, n = 6) remained at the basal level of 20 g (Fig. 1B). Estriol treatment compensated for the loss of estrogen and maintained the body weight of mice at normal levels. The body weights of mice in the bilateral ovariectomy with VMT (OVX + VMT group, n = 6) continuously increased, but weight gain in this group was significantly less than that in the OVX group (Fig. 1B). As shown in Fig. 1C, at the end of the treatment period, mouse vaginal dissection revealed that the vaginas of mice in the OVX group (0.020 ± 0.001 g) were significantly atrophied compared to the CON group (0.078 ± 0.013 g) (Fig. 1C–E). Although there was no significant difference in the length of the isolated vaginas, we observed a significant improvement in vaginal atrophy and a significant increase in the weight of the isolated vaginas in the OVX + EST group (0.042 ± 0.004 g) (Fig. 1E) and the OVX + VMT group (0.040 ± 0.009 g) (Fig. 1D, E). We measured the serum levels of estriol and estradiol in all groups to investigate the effect of different treatments on estrogen levels in mice. Figure 1G showed that bilateral ovariectomy decreased estradiol levels in mice (Fig. 1F). None of the treatment groups increased estradiol levels in mice compared to the OVX group (Fig. 1G).
Differences in vaginal morphology among experimental groups. (A) Mice were used to measure the effects of time, treatment period, and post-treatment retrieval time. (B) Body weight changes in each group of mice during treatment. (C) Images of isolated vagina in each group (D) Vaginal length (one-way ANOVA, F4,25 = 1.544, P = 0.2202). (E) Vaginal weight (one-way ANOVA, F4, 25 = 49.26, P < 0.0001). (F) Serum levels of estradiol (one-way ANOVA, F4, 25 = 30.23, P < 0.0001). (G) Serum levels of estriol (one-way ANOVA, F4,25 = 1.156, P = 0.3536). Data are shown as mean ± SD, n = 6 for each group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ANOVA: analysis of variance. CON: control group (n = 6); OVX + EST: bilateral ovariectomy with estriol-treated group (n = 6); OVX + VMT: bilateral ovariectomy with VMT (n = 6); OVX + NST: bilateral ovariectomy with normal saline lavage group (n = 6); OVX: bilateral ovariectomy group (n = 6).
VMT enhanced vaginal cell proliferation and suppressed the expression of inflammatory factors in ovariectomized mice
Previous studies have demonstrated that ovariectomy leads to thinning of the vaginal mucosa, reduces the number of vaginal epithelial cells, and decreases vaginal blood vessel density in mice. Therefore, we investigated the histological changes in vaginal epithelial cells. As shown in Fig. 2A–C, the thickness of vaginal epithelium (20 ± 2.0 μm) significantly decreased in the OVX group. Similarly, the number of cell layers decreased in this group (2.8 ± 0.26) (Fig. 2A). Treatment with estriol and VMT both improved vaginal atrophies, increasing vaginal epithelial thickness and the number of epithelial cell layers (Fig. 2B, C). However, saline irrigation alone did not improve vaginal atrophy. We investigated the effect of VMT on the proliferative capacity of vaginal epithelial cells. In Fig. 2D, immunohistochemical analysis of proliferating cell nuclear antigen (PCNA) and ESR1 revealed that both estriol (0.18 ± 0.015) and VMT (0.21 ± 0.013) upregulated PCNA expression in vaginal epithelial cells (Fig. 2D). Figure 2H shows that VMT significantly increased the H score of ERSI immunohistochemistry, which in turn promoted the proliferation of vaginal epithelial cells (Fig. 2H, I). Previous studies have shown that estriol promotes the proliferation of vaginal epithelial cells, thereby improving the symptoms of vaginal atrophy. However, despite promoting cell proliferation, estriol did not alter the expression of vaginal epithelial ER (0.11 ± 0.0092) in this study. In contrast, VMT upregulated the expression of ER (0.17 ± 0.01) and promoted vaginal epithelial cell proliferation (Fig. 2E). VMT may use estrogen more efficiently to promote cell proliferation by upregulating vaginal epithelial ER expression. This suggests that VMT may alleviate vaginal atrophy through other mechanisms.
Next, we investigated the effects of VMT on vaginal inflammation. We measured the levels of IL-1β and TNF-α in vaginal lavage fluid. The results showed that the levels of IL-1β (62.3 ± 2.5ng/l) and TNF-α (412.5 ± 43.7ng/l) were increased in the vaginal lavage fluid of the OVX group compared with the CON group (34.2 ± 3.7ng/l, 186.3 ± 31.7ng/l). Notably, both estriol (54.2 ± 4.2ng/l, 230.5 ± 42.9ng/l) and VMT (53.7 ± 4.1ng/l, 327.0 ± 18.1ng/l) reduced the levels of inflammatory factors in vaginal lavage fluid (Fig. 2F, G). These results suggest that VMT may ameliorate vaginal inflammation by modulating the production of inflammatory factors. However, further studies are needed to investigate the specific mechanisms.
VMT enhanced vaginal cell proliferation and suppressed the expression of inflammatory factors in ovariectomized mice. (A) Representative images of HE staining and immunohistochemical staining for ER and PCNA in the vagina of mice (magnification 200×). (B) Average vaginal epithelial thickness in each group (one-way ANOVA, F4, 25 = 135.8, P < 0.0001). (C) The number of vaginal epithelial cell layers in each group (one-way ANOVA, F4, 25 = 28.04, P < 0.0001). (D) Average intensity of PCNA in the immunostaining of vaginal epithelial cells in each group (one-way ANOVA, F4, 25 = 22.71, P < 0.0001). (E) Average intensity of ER in the immunostaining of vaginal epithelial cells (one-way ANOVA, F4, 25 = 17.76, P < 0.0001). (F) Serum levels of IL-1β (one-way ANOVA, F4, 25 = 61.47, P = 0.2202). (G) Serum levels of TNF-α (one-way ANOVA, F4, 25 = 47.03, P = 0.2202). (H) ERS1 IHC H-score (one-way ANOVA, F4, 25 = 73.94, P < 0.0001). (I) PCNA IHC H-score (one-way ANOVA, F4, 25 = 180.6, P < 0.0001). Data are shown as mean ± SD, n = 6 for each group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ANOVA: analysis of variance.
The diversity of VM differed among the CON, OVX + EST, OVX + VMT, and OVX groups
VM diversity prevents the proliferation of other pathogenic bacteria. This in turn forms a healthy VM and builds defenses against invading pathogens. We measured the VM of mice in the five groups (CON, OVX + EST, OVX + VMT, OVX + NST, and OVX) using 16 S rDNA sequencing. We aimed to explore the differences in microbiota between the groups. As shown in Fig. 3A, the number of ASVs in VM were as follows: CON (430), OVX + EST (533), OVX + VMT (345), OVX + NST (786), and OVX (1,746) (Fig. 3A). The CON and OVX + VMT groups had a similar number of ASVs and a greater proportion of shared ASVs. The α-diversity indices of VM were compared between groups. The Chao1 index is a diversity analysis based on the distribution of the abundance of individual species in a sample, and can be a good predictor of the number of undiscovered species, which is important for estimating the number of species. The results indicated that the OVX group had a higher number of VM species compared to other groups (Fig. 3B). The Simpson and Shannon indices combine species richness and species evenness and reflect the degree of microbiota diversity. Larger Simpson values indicate lower community diversity and larger Shannon values indicate higher community diversity. The diversity of microbiota was not significantly different between groups according to the Simpson index (Fig. 3D). The Shannon index (Fig. 3C), which considers both abundance and homogeneity, showed only weak differences in the diversity of microbiota. We also conducted a β-diversity analysis of VM. The PCoA analysis based on weighted Unifrac distance revealed that the VM of the OVX + VMT group was more similar to that of the CON group (Fig. 3E), while the VM of the OVX group differed more from that of the CON group. Bilateral ovariectomy significantly altered the VM of mice. VMT regulated the composition of the VM. The analysis of beta diversity based on unweighted Unifrac distance revealed that the OVX + EST group had the lowest diversity of VM (Fig. 3F).
Analysis of the diversity of VM. (A) Venn diagram showing ASV distribution in the CON (n = 6), OVX + EST (n = 6), OVX + VMT (n = 6), OVX + NST (n = 6), and OVX (n = 6) groups. (B) Chao 1 index for each group (n = 6 per group) (one-way ANOVA, F4, 25 = 12.27, P < 0.0001). (C) Shannon diversity index for each group (n = 6 per group) (one-way ANOVA, F4, 25 = 5.589, P = 0.0023). (D) Simpson diversity index for each group (n = 6 per group) (one-way ANOVA, F4, 25 = 1.821, P = 0.1563). (E) PCoA analysis based on weighted Unifrac. (F) Analysis of beta diversity based on unweighted Unifrac distance (n = 6 per group) (one-way ANOVA, F4, 25 = 19.95, P < 0.0001). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ANOVA: analysis of variance. Only comparison with P value less than 0.05 in (B–D, F).
Different compositions and functions of VM in the CON, OVX + EST, OVX + VMT, and OVX groups
As shown in Fig. 4A, we conducted a cluster analysis and generated a clustering tree to measure the hierarchical relationships between various samples. We utilized an unweighted pair-group method with arithmetic mean (UPGMA) clustering analysis based on a weighted Unifrac distance matrix. We then combined the clustering outcomes with the histograms of the community structure of each sample at the gate level to demonstrate the clustering results (Fig. 4A). The results of the clustering tree showed that the VM composition was closer in the CON and OVX + VMT groups, while the other three groups appeared to be relatively independent. The analysis of the community structure components revealed that there were significant differences at the phyla level among the samples. These observations were objective and solely based on the data analyzed. Differences and similarities in microbiota across groups may lead to different metabolic features that can affect vaginal health. Therefore, we counted the top 5 species in terms of abundance at the phylogenetic level (Fig. 4B-F). The results showed that mice in the CON (0.62 ± 0.14) and OVX + VMT (0.70 ± 0.19) groups had a higher abundance of Gammaproteobacteria. Mice in the OVX + NST (0.63 ± 0.09) group had a higher abundance of Bacilli, while mice in the OVX (0.23 ± 0.33, 0.25 ± 0.15, and 0.32 ± 0.17, respectively) and OVX + EST (0.16 ± 0.07, 0.33 ± 0.11, and 0.20 ± 0.03, respectively) groups had a higher abundance of Gammaproteobacteria, Bacilli, and Clostridia. We performed a STAMP difference analysis at the genus level to analyze species abundance and identify differential species for each group. Figure 4G demonstrates the variability of species in each group of microbiotas (Fig. 4G). We used PICRUSt2 software to analyze the 16 S sequencing data and unravel the functional gene composition of the samples. In addition, differences in function between different samples or subgroups were analyzed. The bar cumulative plot of the relative abundance of COG functional entries indicated no significant difference between groups in terms of the COG functional entries with high relative abundance (Fig. 4H). However, analysis of the relative abundance of the top 24 COG functional entries indicated that the CON and OVX + VMT groups exhibited a high abundance of unknown functional COGs (Fig. 4I), whereas the OVX group had a high abundance of Cell cycle control, cell division, chromosome partitioning (Fig. 4J). Differences in GOG function show differences in VM metabolism in different groups of mice, and metabolic differences also affect vaginal health.
Compositional analysis and functional prediction of VM in different groups of mice. (A) UPGMA cluster analysis based on Unifrac weighted distance matrix. (B) Gammaproteobacteria (one-way ANOVA, F4, 25 = 12.35, P < 0.001). (C) Bacilli (one-way ANOVA, F4, 25 = 7.774, P = 0.0003). (D) Clostridia (one-way ANOVA, F4, 25 = 11.56, P < 0.0001). (E) Actinobacteria (one-way ANOVA, F4, 25 = 13.88, P < 0.001). (F) Bacteroidia (one-way ANOVA, F4, 25 = 3.028, P = 0.0364). (G) Differences in VM between groups at the genus level STAMP. (H) Relative abundance bar chart displaying COG function for each sample. (I) COG function differences be-tween groups: function unknown (one-way ANOVA, F4, 25 = 8.954, P < 0.0001). (J) COG function differences between groups: cell cycle control, cell division, chromosome partitioning (one-way ANOVA, F4, 25 = 11.05, P < 0.0001). (B–F) The five most abundant species in each group of VM at the phylum level. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ANOVA: analysis of variance. Only differences with P value less than 0.05 are shown.
VM metabolism differed between the CON, OVX + EST, OVX + VMT, OVX + NST, and OVX groups
Figure 5A illustrates the structural identification of metabolites in biological samples, with 1707 metabolites identified above level 2. Lipid and lipid-like molecules and organic acids and their derivatives accounted for 27.8% and 22.7% of the total metabolites, respectively (Fig. 5A). The volcano plots based on univariate analysis of variance revealed significant differences in metabolites, including unidentified metabolites, between the groups. As shown in Fig. 5B, compared to the CON group, the OVX group exhibited a greater number of upregulated metabolites than downregulated ones. The most significantly downregulated metabolites in the OVX group were organic oxygen compounds (Fig. 5B). The OVX + VMT group had more upregulated metabolites than the other groups, with organic acids and their derivatives being the most significantly upregulated metabolites (Fig. 5C). This suggests that organic acids and their derivatives may be involved in maintaining vaginal health. The OPLS-DA score plots indicated that the intra-group differences of samples in each group were smaller than the inter-group differences, suggesting the reliability of data. Additionally, significant differences in metabolites were observed between groups (Fig. 5D). The samples and differential metabolites were clustered using a Hierarchical cluster (Hierarchical cluster). Figure 5E revealed that the OVX + EST and OVX + VMT groups had more similar metabolites and were more different from other groups (Fig. 5E). The heatmap displays correlations between metabolites, with red and blue colors indicating positive and negative correlations, respectively. Metabolites with expression correlation may share a common involvement in a biological process, i.e., functional correlation. Positive correlations may suggest that the metabolites originate from the same synthetic pathway, while negative correlations may indicate that they are catabolized for the synthesis of other metabolites, i.e., synthetic transformation relationships (Fig. 5F). Differential metabolites in the OVX and CON groups were mostly synthetic pathways.
Differences in VM metabolism between groups. (A) Proportions of each metabolite. (B, C) The graph displays the multiplicity of differential expression (fold change) on the horizontal axis, represented as log2 value, and the p-value on the vertical axis, represented as log10 value for -log10. A fold change (FC) greater than 1 and a p-value less than 0.05 (in the upper right corner) indicate upregulation, while an FC less than 1.00 and a p-value less than 0.05 (in the upper left corner) indicate downregulation. Metabolites with non-significant differences are shown in gray. (D) Orthogonal partial least squares discriminant analysis (OPLS-DA) was used to compare the OVX and CON groups. (E) A clustering tree was generated for each group of samples. (F) Metabolite correlation heatmap showing significant differences between the OVX and CON groups. Red and blue colors were used to indicate positive and negative correlations, respectively. The shade of the color corresponds to the absolute magnitude of the correlation coefficient, with darker colors indicating stronger correlations.
Differential metabolites are predominantly implicated in amino acid biosynthesis and pyrimidine metabolism
We screened for differential metabolites in each group to thoroughly investigate metabolic differences of VM between different groups and explore the role of VM metabolites in alleviating vaginal atrophy in ovariectomized mice. We identified two metabolites with increased abundance, namely N-acetyl-d-galactosamine and lamivudine (Fig. 6A, B), as well as seven metabolites with decreased abundance, namely urocanic acid, octadecanoic acid, 1-palmitoyl-2-linoleoyl-rac glycerol, arachidoyl ethanolamide, hydrocortisone 21-acetate, bufalin, and 1,2-dioleoyl-sn-glycero-3-glycol (glycero-3-phosphate) (Fig. 6C-I). Increases in lamivudine levels enhance resistance to bacterial viral infections, and arachidoyl ethanolamide is an indicator of many diseases. Metabolic pathway enrichment analyses were conducted to determine the degree of metabolite enrichment for each pathway. Figure 6J shows several metabolic and signaling pathways that were significantly affected, including ABC transporters, central carbon metabolism in cancer, protein digestion and absorption, pyrimidine metabolism, aminoacyl-tRNA biosynthesis, bile secretion, mineral absorption, biosynthesis of amino acids, alcoholism, and cocaine addiction (Fig. 6J). Figures A-I show the metabolic differential enrichment pathways for amoebiasis, histidine metabolism, fatty acid biosynthesis, and biosynthesis of unsaturated fatty acids. We conducted a MetPA enrichment analysis to identify key pathways with a high correlation with metabolite differences. The top five identified pathways were phenylalanine, tyrosine, and tryptophan biosynthesis, pyrimidine metabolism, and aminoacyl-tRNA (Fig. 6K). Amino acids are extensively involved in key pathways that maintain cell growth, metabolism, and immunity. These pathways may be associated with differences in VM metabolism, providing important clues for further research.
Differences in the abundance of VM metabolites between groups and functional analysis. (A) N-acetyl-d-galactosamine; (B) lamivudine; (C) urocanic acid; (D) octadecanoic acid; (E) 1-palmitoyl-2-linoleoyl-rac glycerol; (F) arachidoyl ethanolamide; (G) hydrocortisone 21-acetate; (H) bufalin; (I) 1,2-dioleoyl-sn-glycero-3-glycol; (J) Metabolic pathway enrichment is represented by bubbles in the graph. Each bubble corresponds to a metabolic pathway, with the horizontal coordinate indicating the p-value of the enrichment analysis. The size of each bubble indicates the number of different metabolites enriched in the pathway, with larger bubbles indicating a greater number of metabolites. The color of each bubble represents the corresponding rich factor of each pathway, with redder colors indicating higher values. (K) The figure displays the results of the MetPA enrichment analysis. Each circle represents a metabolic pathway, with the horizontal coordinate indicating the degree of pathway impact. The size of each circle corresponds to the pathway impact, with larger circles indicating greater impact values. The vertical coordinate represents the negative logarithm of the P-value obtained from the pathway enrichment analysis. The color of the circle changes from blue to red as the negative logarithm of the P-value decreases, indicating a higher level of significance for the pathway change. The color change of the point from blue to red indicates a positive correlation with the negative logarithm of the P value of the pathway change. A redder color indicates a more significant enrichment of the pathway. The figure shows the top 5 pathways with smaller P values and their respective names.
Discussion
Vaginal atrophy is caused by decreased estrogen levels leading to dryness, thinning, and loss of elasticity of the vulvar skin, which in turn leads to vulvar atrophy. This greatly damages women physically and mentally. High levels of estrogen promote the growth of subepithelial capillaries, thickening the epithelium and increasing vaginal secretions.
This study showed that bilateral ovariectomy in mice leads to vaginal atrophy, evidenced by decreased vaginal weight and epithelial layers. In addition to vaginal atrophy, the abundance and various VM were also affected. Transplanting the VM of mice with intact ovaries significantly alleviated vaginal atrophy in ovariectomized mice. This observation was accompanied by changes in the abundance and species of VM, which differed from those caused by estrogen. Therefore, it can be concluded that the abundance and species of VM in mice are affected by estrogen secretion from the ovaries. In humans, estrogen modulates the environment of the VM by altering vaginal epithelial cell metabolism, such as amylase levels19. VM of mice with VMT alleviated vaginal atrophy in ovariectomized mice, which may be attributed to the species of VM and their metabolites. Studies have shown that gut microbiota can alter beta-glucuronidase activity, potentially disrupting or dysregulating circulating estrogen levels20.
GSM affects approximately 50% of women after menopause21. Its pathogenesis is primarily related to ovarian hypoplasia, which causes a gradual decline in estrogen levels22. This decline changes the genital and urinary systems. Annually, many patients undergo ovariectomy for ovarian cancer or other benign diseases. Therefore, these women may suffer from GSM23. To explore the role of VM and its metabolites in vaginal health, we established a bilateral ovariectomized mouse model that simulates patients who have undergone ovariectomy for various diseases. Bilateral ovariectomized mouse models are frequently utilized to investigate diseases and physiological processes associated with ovarian hypoplasia, including osteoporosis24, cardiovascular disease25, and metabolic syndrome26. We aimed to investigate the pathogenesis of these diseases and evaluate potential treatments. These models are also used to study vaginal dryness, atrophy, inflammation, and other vaginal disorders associated with estrogen deficiency27. Numerous studies have investigated the association between VM and vaginal atrophy. They have used VMT to treat vaginal microecological imbalances28. However, there is a lack of research on the role of VM in improving vaginal atrophy. Therefore, it is crucial to explore the role of VMT in treating vaginal atrophy.
This study utilized a bilateral ovariectomized mouse model to simulate patients who underwent ovariectomy for various reasons. We aimed to explore the relationship between VM and reproductive health. Previous studies have shown that after bilateral ovariectomy, the mouse vagina undergoes atrophy, presenting with reduced vaginal weight, decreased number of epithelial cell layers29, and alterations in VM. The results indicated that the bilateral ovariectomy mouse model is appropriate for investigating the correlation between vaginal health and VM after ovariectomy. This study compared the microbiota structure and metabolites of VM between the two groups: the CON group and OVX group. The results showed significant changes in the composition and abundance of the VM and their metabolites after bilateral ovariectomy. This suggests that the ovaries can affect VM and metabolites. Consistently, Nuzhat et al. reported dynamic interactions in the diversity of estrogenic VM30.
After eight weeks of treatment, it was observed that both estriol and VMT increased the weight and number of epithelial cell layers in the vagina. In contrast, saline irrigation alone did not induce these changes. These findings suggest that both estriol and VMT can effectively alleviate vaginal atrophy31. Estriol did not affect serum estriol levels in mice after ovariectomy; however, it maintained mouse body weight at control levels, suggesting that estriol treatment may lead to systemic effects32. However, we did not detect elevated estrogen levels in the estriol-treated group, which is consistent with the findings of Nur Amanina et al.33. Immunohistochemical staining of vaginal tissues revealed that PCNA expression was upregulated in the epithelial cells of the vagina in the OVX + EST and OVX + VMT groups. This finding suggests that both estriol and VMT increased the proliferative capacity of vaginal epithelial cells. Additionally, high expression of ER was observed in vaginal epithelial cells in the OVX + VMT group. Therefore, we hypothesized that VMT can enhance the proliferative capacity of vaginal epithelial cells by upregulating ER. The expression of vaginal inflammatory factors was reduced by both estriol and VMT. Previous studies have demonstrated that VMT inhibits NF-κB, thereby reducing the expression of IL-1β and TNF-α34. Therefore, we hypothesized that VMT could promote the expression of ER in vaginal epithelial cells through this mechanism.
Clustering analysis of ASVs based on 16 S DNA sequencing showed that the CON and OVX + VMT groups had similar numbers of ASVs and a greater proportion of shared ASVs. This suggests significant differences in the abundance and structure of VM between the CON and OVX groups30. The Chao1 index has been demonstrated to be a reliable metric for evaluating the richness of microbiota. It was observed that the VM of OVX mice exhibited higher species richness, which may be attributable to increased colonization by rare VM. Shannon’s index, which considers both community richness and evenness, provides a more accurate reflection of the diversity of VM. The higher species diversity of VM observed in the OVX group may be attributed to the increased VM richness characteristic of this group. Simpson’s index is a more appropriate metric for evaluating the homogeneity of VM, and the results demonstrated that there was no significant difference in microbiota homogeneity among the groups. However, the structure of VM in ovariectomized mice was significantly altered by estriol, VMT, and saline irrigation. Beta diversity analysis based on unweighted Unifrac distance showed that the VM structure of the OVX + EST group was significantly different from other groups. The structure of VM in the OVX + VMT group was more similar to that in the CON group. Mice in the CON and OVX + VMT groups had a higher abundance of Gammaproteobacteria. These results suggest that the uterus significantly affects the composition and structure of the VM. VMT can significantly alter the microbiota’s structure. Healthy VM, together with ovaries, maintain vaginal health35. Three possible mechanisms of action are involved in VMT therapy, including increased nutrient competition, production of beneficial metabolites, and reduction of inflammatory factors.
Volcano plots based on univariate analysis of variances showed significant differences between the vaginal lavage metabolites between groups. We identified several differential metabolites, including N-acetyl-d-galactosamine, lamivudine, urocanic acid, octadecanoic acid, 1-palmitoyl-2-linoleoyl-rac glycerol, arachidoyl ethanolamide, hydrocortisone 21-acetate, bufalin, and 1,2-dioleoyl-sn-glycero-3-glycol. These metabolite changes may alleviate vaginal atrophy by altering cell proliferation, metabolism, and oxidative stress and increasing angiogenesis20,36. Metabolic pathway enrichment analysis revealed several metabolic and signaling pathways, including ABC transporters, central carbon metabolism in cancer, protein digestion and absorption, pyrimidine metabolism, aminoacyl-tRNA biosynthesis, bile secretion, mineral absorption, biosynthesis of amino acids, alcoholism, and cocaine addiction. Both metabolic pathway enrichment analyses and MetPA enrichment analyses revealed differences in pyrimidine metabolism and aminoacyl-tRNA in the metabolism of vaginal microorganisms.
In summary, this study utilized bilateral ovariectomized mice to investigate the association of vaginal atrophy with VM. The results showed that mice developed vaginal atrophy. Significant changes were observed in the composition and metabolites of the VM after bilateral ovariectomy. VMT from mice with intact ovaries into ovariectomized mice improved vaginal atrophy and changed the abundance and species of VM. ER expression in vaginal epithelial cells was upregulated after VMT. Thus, we hypothesized that estrogen secreted by ovaries can affect VM in mice and VMT can improve vaginal atrophy and downregulate inflammatory factors. We identified several differential metabolites, providing new insights into non-hormonal treatment of vaginal atrophy. This is certainly good news for those suffering from estrogen-dependent diseases. Exploring non-hormonal treatments for GSM is especially important when their ovaries are removed due to natural menopause or diseases. Non-hormonal therapies typically use moisturizers based on water or vegetable oils. Although they show promising clinical results in treating GSM-related symptoms, these compounds do not reverse vaginal atrophy37. Our study can well bridge this gap and holds great promise.
However, there are some limitations to the current study. 1.The microbiota in VMT was obtained from the VM of C57 mice that had not undergone ovariectomy. There may have been a partial loss of microbiota during VMT and it was not possible to restore the distribution of the VM in the mouse vagina. This may have a bearing on the recovery VM. 2.The VM composition of mice in the OVX + VMT group exhibited a greater similarity to that of the CON group. The remission rate of vaginal epithelial atrophy in mice in the OVX + VMT group was almost equivalent to that of the OVX + EST group (100%), however, the degree of improvement was less significant than that observed in the OVX + EST group. Consequently, there was a necessity to conduct further exploration into the long-term effects of VMT frequency and duration vaginal atrophy. 3.As VM is regulated by estrogen, it is necessary to investigate the association between VM and other estrogen-related diseases. Examples include pelvic organ disease and metabolic syndrome interactions with VM. 4.16 S rDNA sequencing can only explore species composition and species diversity, but not gene composition and function and related metabolic pathways. In the future, the specific mechanisms of VM and vaginal health can be explored through macro genomic sequencing. 5.Considering microbiome-host interactions, gut microbiota can modulate sympathetic nerve activity through the gut-brain axis. We believe there is a strong need to explore the VM and the interactions between gut microbiota and systemic health.
To further explore the impact of VMT and its metabolites on vaginal health. We are working on screening differential metabolites in order to screen key metabolites that can be used to treat vaginal atrophy. We hope to be able to combine differential metabolites and vaginal delivery mechanisms to construct a drug for the treatment of vaginal atrophy. In order to provide a new treatment modality for patients who are unable or unwilling to use estrogen therapy. Given the differences in VM in different human populations, we should seek regional collaborations in the future, hopefully with the support of the medical industry, in order to benefit patients with vaginal atrophy more quickly and better.
The activity of VM is affected by ovaries. VM and metabolites of ovary-intact mice can promote vaginal epithelial proliferation and maintain vaginal health by upregulating ER and PCNA of vaginal epithelial cells in ovariectomized mice. This provides a basis for studying the relationship between VM and vaginal health. It also provides a direction for exploring non-hormonal treatment of GSM.
Materials and methods
Materials
Estrogen replacement therapy is effective for the treatment of GSM. There are many side effects associated with the use of estradiol therapy. Estriol (8 mg/day) does not induce endometrial proliferation and can improve vaginal maturation without significant side effects. Thus, we decided to use estriol as the control treatment32,38. Ovestinon® 0.1% (Estriol) (hereinafter referred to as estriol cream) is a common and commercially available pharmaceutical product for treating genital tract atrophy. It contains 15 mg of estriol per 15 g of ointment. VM in VMT was derived from CON group. Control and VMT mice were randomly matched and fixed for transplantation.
Establishment of menopausal model
C57 mice are widely used in animal models of menopause due to easy breeding and modeling. The vaginal epithelium of mice showed significant atrophy after bilateral removal of ovaries. Female SPF-grade C57BL/6J mice (eight-week-old) were purchased from Hangzhou Ziyuan Laboratory Animal Science and Technology Co. Ltd. and housed in the Animal Experiment Center of Anhui Medical University. Mice were housed under specific pathogen-free conditions and maintained under standard laboratory conditions with 12 h:12 h light-dark cycles, and fed regular chow. Food and water were freely available. After one week of acclimatization, 30 mice were randomly divided into five groups as follows: (a) control group (CON group, n = 6); (b) bilateral ovariectomy with estriol-treated group (OVX + EST group, n = 6); (c) bilateral ovariectomy with VMT group (OVX + VMT group, n = 6); (d) bilateral ovariectomy with normal saline lavage group (OVX + NST group, n = 6); and (e) bilateral ovariectomy group (OVX group, n = 6). Except for the CON group, all groups underwent bilateral ovariectomy to simulate patients undergoing ovariectomy. After injecting sodium pentobarbital to anesthetize the mice, they were placed in the supine position. A 2 cm incision was made in the midline of the abdomen to explore the ovaries and surrounding fat pads. The ovaries were ligated, while in the control group, only bilateral fat pads were removed without ligating the ovaries. Finally, the muscles and skin were sutured. The study’s overall design and protocol were approved by the Laboratory Animal Ethics Committee of Anhui Medical University (LLSC20241413). The animal experiments conducted in this study were performed in accordance with the ARRIVE guidelines, and all methods were performed in accordance with the relevant guidelines and regulations.
Animal treatment
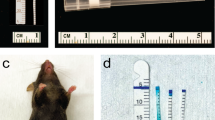
Four weeks after resection of bilateral ovaries, we observed closure of the vaginal orifices in mice. For each group, the following treatment was administered (Fig. 7):
-
CON: Only as the VMT donor in OVX + VMT group;
-
OVX + VMT: 100 µl of fresh VM lavage solution was given;
-
OVX + NST: 100 µl of normal saline was given;
-
OVX + EST: Based on the drug insert’s recommended dose for human administration, we conducted dosing conversions. Estriol cream was administered 0.33 mg per mouse vaginally (20 g mouse body weight/60 kg human body weight);
-
OVX: No treatment was administered;
-
Mice in the OVX + EST group were administered estriol cream on a daily basis. A 100 µl bolus of fresh VM lavage solution was administered vaginally every 3 days in the OVX + VMT group, while normal saline was used for the OVX + NST group (Equivalent to 0.33 mg Ovestinon® 0.1% by volume administered vaginally in OVX + EST). All treatments were administered at 8 p.m. for a duration of eight weeks.
Sample collection
Collection of VM lavage solution: To collect VM lavage solution, first we ensured that the researchers are wearing protective gear. With one hand, they gently held the mouse and used a sterile cotton swab to stimulate the outlet of the reproductive tract. We encouraged the mouse to urinate, and after urination, we used the cotton swab to absorb the urine. Next, we disinfected the mouse’s vulva with iodophor, avoiding its contact with the vagina. A 0.5 ml disposable sterile dropper was used to suck up 200 µl of sterile saline, which was then inserted into the mouse’s vagina for about 5 mm. The saline was blown repeatedly for 50 times to ensure adequate washing of the vagina. An equal amount of the saline lavage was then injected into the freezing tube and stored39,40.
Mice were injected intraperitoneally with sodium pentobarbital (30–90 mg/kg) and blood was collected from the heart for plasma collection. After obtaining vaginal tissues, mice were euthanized by cervical dislocation and observed for loss of respiration and heartbeat to ensure that they met the requirements for euthanasia. The weight and length of the isolated vagina were measured using an electronic balance and ruler. The upper third of the vagina was fixed in formalin, dehydrated, and paraffin-embedded for histological analysis.
Histological and immunohistochemical analysis
The vaginal wall was treated with formalin, dehydrated, and embedded. A cross-section of the specimen (5 μm) was cut and HE staining was conducted. One vaginal cross-section was selected for each sample to measure vaginal thickness and count the number of vaginal epithelial layers. Raw images were acquired using a slide scanner (Pannoramic MIDI II, 3D histech). Another researcher measured the vaginal thickness and vaginal epithelial layers of each sample using CaseViewer 2.4 without knowing the specimen number. Vaginal thickness and the number of vaginal epithelial layers were measured ten times for each sample and the final result was expressed as the average of ten measurements. Immunohistochemical techniques were utilized to measure the expression of ER and proliferating cell nuclear antigen (PCNA) in the vaginal epithelium and endometrium of mice. Another researcher measured the mean intensity of ER positivity and PCNA positivity in the whole epithelial layer of the vagina using Image J software without knowing the specimen number. And QuPath was used to analyse H-score for ERS1 and PCNA in the entire epithelial layer of the vagina. Ten fields of view were randomly selected for each specimen, and the final result was expressed as the average of ten measurements. ESR1 antibody (1:800, proteintech, catalog:21244-1-AP) and PCNA antibody (1:800, proteintech, catalog:24036-1-AP) were purchased from Wuhan Sanying Biotechnology Co. Immunohistochemistry rabbit two-part method kit (PV-6001) was purchased from Zhongshan Jinqiao.
The concentration of estrogen and inflammatory factors in serum and vaginal lavage fluid were measured by ELISA
IL-1β and TNF-α are common inflammatory factors in chronic inflammation and are involved in various inflammatory responses in the body. Previous studies have suggested that changes in estrogen levels may affect IL-1β and TNF-α levels in VM lavage fluid in mice. VMT may affect inflammatory factor levels in vaginal lavage fluid, further affecting the development of GSM. ELISA kits (Elabscience, Wuhan, China) were used to measure the levels of estriol and estradiol in mouse serum and the levels of inflammatory factors IL-1β and TNF-α in mouse serum and VM lavage fluid. Each sample was diluted five-fold and the instructions of the product manual were followed for measurement. We added the diluted samples to wells. Thereafter, an appropriate amount of enzyme labeling reagent was added. The mixture was then incubated for a specified duration. Next, the well plate was washed and the substrate solution was added, followed by another incubation period. Finally, the termination solution was added to complete the process. Absorbance was measured at 450 nm using a microplate reader (Thermo Fisher Varioskan LUX Multifunctional Microplate Reader). The concentration of each sample was calculated based on the standard curve provided by the kit.
Analysis of 16 S RNA in vaginal lavage fluid
The OMEGA Soil DNA Kit (D5625-01) (Omega BioTek, Norcross, GA, USA) was used to extract genomic DNA from samples and assess the purity and concentration of DNA. According to the selection of sequencing region, the selected V3-V4 variable region was amplified by PCR using specific primers with Barcode and high-fidelity DNA polymerase. The specific steps of PCR amplification were as follows: pre-denaturation at 94 °C for 5 min; denaturation at 94 °C for 30 s; annealing at 55 °C for 30 s; extension at 72 °C for 1.5 min; and extension at 72 °C for 10 min. The middle three steps were set for 30 cycles. We performed an agarose gel electrophoresis check before sequencing. The extracted DNA samples were electrophoresed on an agarose gel to observe whether the bands of DNA were clear and intact, and whether there was any degradation or contamination. The target fragments were cut and recovered by the Quant-iT PicoGreen dsDNA detection kit. Based on the quantitative results of electrophoresis, the PCR amplification recovered products were detected and quantified using a microplate reader (BioTek, FLx800) fluorescence quantitative system. The corresponding proportions were mixed according to the sequencing requirements of each sample. The library was constructed using the TruSeq Nano DNA LT Library Prep Kit from Illumina. The constructed library was inspected by Agilent Bioanalyzer 2100 and Promega QuantiFluor. After qualifying the library, it was sequenced.
Data analysis: Raw sequencing data were obtained in FASTQ format. Paired-end reads were then preprocessed using Cutadapt 4.9 software to detect and cut off the adapter. After trimming, paired-end reads were filtered for low-quality sequences, denoised, merged, and detected. The chimera reads were cut off using DADA2 1.16 with the default parameters of QIIME2. The software provided the representative reads and the ASV abundance table.
Annotation: The representative reads of each ASV were selected using the QIIME2 2.0 package. All representative reads were annotated and blasted against Silva database Version 138 (16s/18s rDNA) using classify-sklearn with the default parameters.
Diversity analysis: We calculated the α and β diversity indices using Qiime2 software. α diversity is used to analyze the diversity of microbiota communities in each sample. The richness and diversity of microbiota communities in the sample can be reflected through the diversity analysis of each sample (α diversity, Supplementary Table 1). Diversity analysis includes the use of species accumulation box chart, species diversity curve, and a series of statistical analysis indexes to measure the differences in species richness and diversity of microbiota communities in each sample. β diversity is a comparative analysis of microbiota community composition in different samples (Supplementary Table 2).
Difference analysis: LEfSe (Linear discriminant analysis effect size, linear discriminant analysis, and influence factor) can be used to identify species characteristics that can best explain the differences between groups in two or more groups of samples, and determine the influence degree of these characteristics on the differences between groups. Using the inter-group difference test method, species differences between two groups/multiple groups of samples were measured, and the information of species with significant differences between the two groups was obtained.
The STAMP difference analysis was used to compare the species abundance between two groups (Wilcox test) or multiple groups (Kruskal Wallis test). Species with significant differences were obtained.
Functional analysis: PICRUSt2 2.3.0 (Phylogenetic Investigation of Communities by Recommendation of Unobserved States) is a software that predicts functional abundance only based on the sequence of the marker gene (16 S/18S). It provides the gene function spectrum of their common ancestors based on the tree of OTU from the Greengene database and the gene information from OTU. It provides the gene function spectrum of other undetected species in the Greengenes database, builds the gene function prediction spectrum of the whole pedigree of archaea and bacteria domain, and finally “maps” the composition of the flora obtained by sequencing into the database to predict the metabolic function of flora.
High-resolution off-target metabolomics of VM
LC-MS/MS analysis
The analysis was performed using a UHPLC (1290 Infinity LC, Agilent Technologies) coupled to a quadrupole time-of-flight (AB Sciex TripleTOF 6600, Shanghai Applied Protein Technology Co., Ltd.).
For HILIC separation, samples were analyzed using a 2.1 mm × 100 mm ACQUIY UPLC BEH Amide 1.7 μm column (waters, Ireland). In both ESI-positive and ESI-negative modes, the mobile phase contained A = 25 mM ammonium acetate and 25 mM ammonium hydroxide in water and B = acetonitrile. The gradient was 95% B for 0.5 min and linearly reduced to 65% in 6.5 min. Then, the gradient was reduced to 40% in 1 min and kept for 1 min. Then, the gradient increased to 95% in 0.1 min, with a 3 min re-equilibration period.
The ESI source conditions were as follows: Ion Source Gas1 as 60, Ion Source Gas2 as 60, curtain gas as 30, source temperature: 600℃, IonSpray Voltage Floating: ± 5500 V. In MS-only acquisition, the instrument was set to acquire over the m/z range 60-1000 Da, and the accumulation time for TOF MS scan was set at 0.20 s/spectra. In auto MS/MS acquisition, the instrument was set to acquire over the m/z range 25-1000 Da, and the accumulation time for product ion scan was set at 0.05 s/spectra. The product ion scan was acquired using information-dependent acquisition with high sensitivity mode. The parameters were as follows: the collision energy was fixed at 35 V with ± 15 eV; declustering potential was 60 V (+) and − 60 V (−); isotopes within 4 Da were excluded, and 10 candidate ions were used to monitor per cycle.
The analysis was performed using a UHPLC (Vanquish UHPLC, Thermo) coupled to an Orbitrap Exploris™ 480 (Shanghai Applied Protein Technology Co., Ltd.).
For HILIC separation, samples were analyzed using a 2.1 mm × 100 mm ACQUIY UPLC BEH Amide 1.7 μm column (Waters, Ireland). In both ESI-positive and ESI-negative modes, the mobile phase contained A = 25 mM ammonium acetate and 25 mM ammonium hydroxide in water and B = acetonitrile. The gradient was 95% B for 0.5 min and was linearly reduced to 65% in 6.5 min. Then, the gradient was reduced to 40% in 1 min and kept for 1 min. Thereafter, it increased to 95% in 0.1 min and was kept at 95% for 2.9 min.
The ESI source conditions were set as follows: Ion Source Gas1 as 50, Ion Source Gas2 as 2, source temperature: 350℃, IonSpray Voltage Floating: +3500 V/-2800 V. In MS-only acquisition, the instrument was set to acquire over the m/z range 70-1200 Da, the resolution was set at 60,000, and the accumulation time was set at 100 ms. In auto MS/MS acquisition, the instrument was set to acquire over the m/z range 70-1200 Da, the resolution was set at 60,000, and the accumulation time was set at 100 ms, excluding time within 4 s.
Raw MS data were converted to MzXML files using ProteoWizard MSConvert before importing them into freely available XCMS software. The following parameters were used for peak picking: centWave m/z = 10 ppm, peak width = c (10, 60), and prefilter = c (10, 100). For peak grouping, bw = 5, mzwid = 0.025, and minfrac = 0.5 were used. CAMERA (Collection of Algorithms of MEtabolite pRofile Annotation) was used for annotation of isotopes and adducts. In the extracted ion features, only variables with more than 50% of the nonzero measurement values in at least one group were kept. The compounds of metabolites were identified by comparing the accuracy m/z value (< 10 ppm), and MS/MS spectra with an in-house database were established with available authentic standards.
After sum-normalization, the processed data were analyzed using R package (ropls). Multivariate data analysis, including Pareto-scaled principal component analysis and orthogonal partial least-squares discriminant analysis (OPLS-DA), were conducted. The 7-fold cross-validation and response permutation test were used to evaluate the robustness of the model. The variable importance in the projection (VIP) value of each variable in the OPLS-DA model was calculated to indicate its contribution to the classification. Student’s t-test was applied to determine the significance of differences between two groups of independent samples. VIP > 1 and p value < 0.05 were used to screen significantly changed metabolites. Pearson’s correlation analysis was performed to measure the correlation between two variables.
Statistical analysis
The data are presented as the mean ± standard deviation. Independent sample t-test was used for statistical comparisons between two groups, while one-way ANOVA was used for statistical comparisons between multiple groups (All data have been checked for normality). Parametric tests can be used when normality and homoscedasticity are satisfied. Finally, Bonferroni correction was used for comparisons between groups. Some cartoon components come from www.figdraw.com for model drawing. GraphPad Prism 9.3.0 software (GraphPad Software, Inc.) was used for detailed statistical processing and plotting. A P-value < 0.05 was considered to be statistically significant.
Data availability
The materials described in the manuscript, including all relevant raw data, will be made available to any researcher wishing to use them for non-commercial purposes, provided that participant confidentiality is not breached. Please contact the corresponding author (wenyanautumn@sina.com) for further information.
References
Portman, D. J., Gass, M. L. & Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: New terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 21 (10),1063–1068. https://doi.org/10.1097/GME.0000000000000329 (2014).
Crandall, C. J., Mehta, J. M. & Manson, J. E. Management of menopausal symptoms: A review. JAMA 329 (5), 405–420. https://doi.org/10.1001/jama.2022.24140 (2023).
Jin, J. Vaginal and urinary symptoms of menopause. JAMA 317 (13), 1388. https://doi.org/10.1001/jama.2017.0833 (2017).
Nappi, R. E. et al. The burden of vulvovaginal atrophy on women’s daily living: implications on quality of life from a face-to-face real-life survey. Menopause 26 (5), 485–491. https://doi.org/10.1097/GME.0000000000001260 (2019).
Faubion, S. S., Sood, R. & Kapoor, E. Genitourinary syndrome of menopause: Management strategies for the clinician. Mayo Clin. Proc. 92 (12):1842–1849. https://doi.org/10.1016/j.mayocp.2017.08.019 (2017).
Gandhi, J. et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am. J. Obstet. Gynecol. 215 (6), 704–711. https://doi.org/10.1016/j.ajog.2016.07.045 (2016).
Shardell, M., Gravitt, P. E., Burke, A. E., Ravel, J. & Brotman, R. M. Association of vaginal microbiota with signs and symptoms of the genitourinary syndrome of menopause across reproductive stages. J. Gerontol. Biol. Sci. Med. Sci. 76 (9), 1542–1550. https://doi.org/10.1093/gerona/glab120 (2021).
Lebeer, S. et al. A citizen-science-enabled catalogue of the vaginal Microbiome and associated factors. Nat. Microbiol. 8 (11), 2183–2195. https://doi.org/10.1038/s41564-023-01500-0 (2023). Epub 2023 Oct 26.
Zhu, B., Tao, Z., Edupuganti, L., Serrano, M. G. & Buck, G. A. Roles of the microbiota of the female reproductive tract in gynecological and reproductive health. Microbiol. Mol. Biol. Rev. 86 (4), e0018121. https://doi.org/10.1128/mmbr.00181-21 (2022). Epub 2022 Oct 12.
Qi, W. et al. The effect of pathophysiological changes in the vaginal milieu on the signs and symptoms of genitourinary syndrome of menopause (GSM). Menopause 28 (1), 102–108. https://doi.org/10.1097/GME.0000000000001644 (2020).
Geng, L. et al. Effect of menopausal hormone therapy on the vaginal microbiota and genitourinary syndrome of menopause in Chinese menopausal women. Front. Microbiol. 11, 590877. https://doi.org/10.3389/fmicb.2020.590877 (2020).
Lev-Sagie, A. et al. Vaginal Microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 25 (10), 1500–1504. https://doi.org/10.1038/s41591-019-0600-6 (2019). Epub 2019 Oct 7.
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342 (6161), 971–976. https://doi.org/10.1126/science.1240537 (2013).
Fessler, J., Matson, V. & Gajewski, T. F. Exploring the emerging role of the Microbiome in cancer immunotherapy. J. Immunother Cancer. 7 (1), 108. https://doi.org/10.1186/s40425-019-0574-4 (2019).
France, M., Alizadeh, M., Brown, S., Ma, B. & Ravel, J. Towards a deeper Understanding of the vaginal microbiota. Nat. Microbiol. 7 (3), 367–378. https://doi.org/10.1038/s41564-022-01083-2 (2022).
Chee, W. J. Y., Chew, S. Y. & Than, L. T. L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell. Fact. 19 (1), 203. https://doi.org/10.1186/s12934-020-01464-4 (2020).
Quispe Calla, N. E. et al. Ovariectomized mice and postmenopausal women exhibit analogous loss of genital epithelial integrity. Tissue Barriers. 9 (2), 1865760. https://doi.org/10.1080/21688370.2020.1865760 (2021).
Huang, J. et al. Fecal microbiota transplantation mitigates vaginal atrophy in ovariectomized mice. Aging (Albany NY). 13 (5), 7589–7607. https://doi.org/10.18632/aging.202627 (2021). Epub 2021 Feb 26.
Nunn, K. L. et al. Amylases in the human vagina. mSphere 5 (6), e00943–e00920. https://doi.org/10.1128/mSphere.00943-20 (2020).
Baker, J. M., Al-Nakkash, L. & Herbst-Kralovetz, M. M. Estrogen-gut Microbiome axis: physiological and clinical implications. Maturitas 103, 45–53. https://doi.org/10.1016/j.maturitas.2017.06.025 (2017).
Jang, Y. C., Leung, C. Y. & Huang, H. L. Comparison of severity of genitourinary syndrome of menopause symptoms after carbon dioxide laser vs vaginal Estrogen therapy: A systematic review and Meta-analysis. JAMA Netw. Open. 5 (9), e2232563. https://doi.org/10.1001/jamanetworkopen.2022.32563 (2022).
Mitchell, C. M. et al. Association of vaginal estradiol tablet with serum Estrogen levels in women who are postmenopausal: secondary analysis of a randomized clinical trial. JAMA Netw. Open. 5 (11), e2241743. https://doi.org/10.1001/jamanetworkopen.2022.41743 (2022).
Michelsen, T. M., Pripp, A. H., Tonstad, S., Tropé, C. G. & Dørum, A. Metabolic syndrome after risk-reducing salpingo-ovariectomy in women at high risk for hereditary breast ovarian cancer: a controlled observational study. Eur. J. Cancer. 45 (1), 82–89. https://doi.org/10.1016/j.ejca.2008.09.028 (2009).
Xie, X. et al. Metformin alleviates bone loss in ovariectomized mice through Inhibition of autophagy of osteoclast precursors mediated by E2F1. Cell. Commun. Signal. 20 (1), 165. https://doi.org/10.1186/s12964-022-00966-5 (2022).
Dutta, S. R., Singh, P. & Malik, K. U. Ovariectomy via 12/15-lipoxygenase augments angiotensin II-Induced hypertension and its pathogenesis in female mice. Hypertension 80 (6), 1245–1257. https://doi.org/10.1161/HYPERTENSIONAHA.122.20836 (2023).
Stokar, J. et al. Hepatic Adropin is regulated by Estrogen and contributes to adverse metabolic phenotypes in ovariectomized mice. Mol. Metab. 60, 101482. https://doi.org/10.1016/j.molmet.2022.101482 (2022).
Han, N. R. et al. Improvement effects of a mixed extract of flowers of Pueraria Thomsonii Benth. And peels of Citrus Unshiu Markovich on postmenopausal symptoms of ovariectomized mice. Biomed. Pharmacother. 103, 524–530. https://doi.org/10.1016/j.biopha (2018).
Li, Y., Zhu, W., Jiang, Y., Lessing, D. J. & Chu, W. Synthetic bacterial consortia transplantation for the treatment of Gardnerella vaginalis-induced bacterial vaginosis in mice. Microbiome 11 (1), 54. https://doi.org/10.1186/s40168-023-01497-y (2023).
McCracken, J. M. et al. Cellular and extracellular vaginal changes following murine ovarian removal. Physiol. Rep. 11 (15), e15762. https://doi.org/10.14814/phy2.15762 (2023).
Rahman, N., Mian, M. F., Nazli, A. & Kaushic, C. Human vaginal microbiota colonization is regulated by female sex hormones in a mouse model. Front. Cell. Infect. Microbiol. 13, 1307451. https://doi.org/10.3389/fcimb.2023.1307451 (2023).
Nicol, T., Vernon-Roberts, B. & Quantock, D. C. The effect of various anti-oestrogenic compounds on the reticulo-endothelial system and reproductive tract in the ovariectomized mouse. J. Endocrinol. 34 (3), 377–386. https://doi.org/10.1677/joe.0.0340377 (1966).
Kolokythas, A., Betschart, C., Wunder, D., Janka, H. & Stute, P. Impact of vaginal estriol on serum hormone levels: a systematic review. Climacteric 27 (2), 137–153 (2024). Epub 2024 Jan 2.
Tan, N. A. S., Giribabu, N., Karim, K., Nyamathulla, S. & Salleh, N. Intravaginal treatment with Marantodes pumilum (Kacip Fatimah) ameliorates vaginal atrophy in rats with post-menopausal condition. J. Ethnopharmacol. 236, 9–20. https://doi.org/10.1016/j.jep.2019.02.027 (2019).
Chen, T. et al. Dysbiosis of the rat vagina is efficiently rescued by vaginal microbiota transplantation or probiotic combination. Int. J. Antimicrob. Agents. 57 (3), 106277. https://doi.org/10.1016/j.ijantimicag.2021.106277 (2021).
Jašarević, E. et al. The composition of human vaginal microbiota transferred at birth affects offspring health in a mouse model. Nat. Commun. 12 (1), 6289. https://doi.org/10.1038/s41467-021-26634-9 (2021).
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157 (1), 121–141. https://doi.org/10.1016/j.cell.2014.03.011 (2014).
Chen, J. et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J. Sex. Med. 10 (6), 1575–1584. https://doi.org/10.1111/jsm.12125 (2013).
Tzingounis, V. A., Aksu, M. F. & Greenblatt, R. B. Estriol in the management of the menopause. JAMA 239 (16), 1638–1641 (1978).
Wei, G. et al. A probiotic nanozyme hydrogel regulates vaginal microenvironment for Candida vaginitis therapy. Sci. Adv. 9 (20), eadg0949. https://doi.org/10.1126/sciadv.adg0949 (2023).
Lebeau, A. et al. HPV infection alters vaginal Microbiome through down-regulating host mucosal innate peptides used by lactobacilli as amino acid sources. Nat. Commun. 13 (1), 1076. https://doi.org/10.1038/s41467-022-28724-8 (2022).
Acknowledgements
We are grateful to the Research and Experimental Center of the Second Affiliated Hospital of Anhui Medical University for their valuable assistance in our experiment. We also thank Zhongke New Life for providing technical support for our experiment. We would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided. We thank ImageJ (https://imagej.net) and QuPath (https://qupath.github.io) for their assistance in analyzing the immunohistochemistry statistical data.
Funding
This study was funded by the Young Scientists Fund of the National Natural Science Foundation of China (No.81100412) and the Anhui Provincial Natural Science Foundation (2008085MH283).
Author information
Authors and Affiliations
Contributions
W. W. and Q. Z. conceptualized and designed this project. Z. X., Q. Z., J. Z., Y. L. and L. W. conducted the experiments, analyzed and statistically analyzed, and generated charts. Z. X.and Q. Z. wrote the first draft of the manuscript. J. Z., Y. L. and L. W. made revisions to the manuscript. All authors studied and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The animal study protocol was approved by the Laboratory Animal Ethics Committee of Anhui Medical University (LLSC20241413).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, Z., Zhu, Q., Zou, J. et al. Vaginal microbiota transplantation alleviates vaginal atrophy in ovariectomized mice. Sci Rep 15, 8390 (2025). https://doi.org/10.1038/s41598-025-92881-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92881-1