Abstract
Glioblastoma (GBM) is the most aggressive form of diffuse glioma, characterized by high lethality. Temozolomide (TMZ)-based chemotherapy is a standard treatment for GBM, but development of chemoresistance poses a significant therapeutic challenge. Despite advances in understanding GBM biology, the mechanisms driving TMZ resistance remain unclear. Identifying vital molecular players involved in this resistance is crucial for developing new therapies. Our results indicated that periostin (POSTN) was significantly upregulated in GBM cell lines and patient samples, correlating with poorer clinical outcomes. POSTN overexpression enhanced GBM cell proliferation, migration, invasion, and chemoresistance, while lentiviral suppression of POSTN significantly reduced these behaviors. In vivo, bioluminescence imaging further confirmed the enhanced tumor growth associated with POSTN overexpression. Bioinformatics analysis was performed to explore the underlying molecular mechanism. The results revealed a strong correlation between POSTN and epithelial-mesenchymal transition (EMT) process and the tumor necrosis factor α (TNFα)-NF-κB signaling pathway. Moreover, exogenous POSTN silencing reduced IκB-kinase α (IKKα) phosphorylation, thereby decreasing NF-κB expression by limiting IκBα degradation. Collectively, our study demonstrated that POSTN-induced activation of NF-κB signaling and EMT processes promoted the malignancy and chemoresistance of GBM, suggesting that POSTN may serve as a reliable prognostic biomarker and potential therapeutic target for GBM.
Similar content being viewed by others
Introduction
Diffuse gliomas are the most prevalent type of primary brain malignancies, accounting for approximately 50% of all brain tumors1. Glioblastoma (GBM), classified as WHO grade IV glioma, is the most clinically aggressive neoplasm2. Despite advances in neurosurgery, radiotherapy, and chemotherapy, the prognosis for GBM patients remains bleak, with a median survival time of approximately 15 months. Over the past three decades, there has been minimal improvement in these outcomes, primarily due to the tumor’s substantial resistance to conventional therapies and its high recurrence rate3.
Temozolomide (TMZ), the standard chemotherapy for gliomas, exerts its anti-tumor effects by damaging DNA and inducing cell death. The effectiveness of TMZ is significantly impacted by O6-methylguanine-DNA methyltransferase (MGMT), which repairs TMZ-induced DNA alterations, thus inhibiting apoptosis4. Evidence suggests that increased MGMT activity is crucial in driving resistance to TMZ in GBM, as it removes TMZ-induced alkylation from nucleotides5. A significant proportion of GBM patients lack MGMT due to promoter hypermethylation, making them initially more susceptible to TMZ, but resistance often develops over time6. Overcoming TMZ resistance remains a critical challenge with emerging research highlighting other contributing factors like alternative DNA repair systems, signaling pathways, autophagy, and epigenetic changes7,8,9,10. Understanding these mechanisms and developing novel molecular markers is essential for improving GBM treatment.
Periostin (POSTN), encoded by the POSTN gene located on chromosome 13q13, is an extracellular matrix protein crucial for cellular adhesion, migration, and invasion, thereby establishing it as a significant component within the tumor microenvironment11. Belonging to the fasciclin family, POSTN is essential for maintaining tissue architecture and plays a significant role in the epithelial-mesenchymal transition (EMT) in tumors12. Recent studies have shown significant variation in POSTN expression across different cancer types: it is markedly downregulated in bladder cancer and some gastric cancers, yet significantly upregulated in gliomas and breast cancer, often accompanied by higher tumor grades and unfavorable outcomes13,14,15. Moreover, POSTN has been reported to be involved in gliomas angiogenesis, contributing to tumor growth and spread16. However, the specific functions and molecular mechanism of POSTN in GBM still require further investigation.
EMT has been proven to enhance malignant tumor properties such as stemness, therapeutic resistance, and cell invasiveness17. Emerging research indicates that key EMT markers are significantly increased in cancers such as lung cancer, glioma, breast cancer, and melanoma, and this increase correlates with poor prognosis18,19,20,21. Additionally, nuclear factor κB (NF-κB), a key transcription factor regulating immune and inflammatory responses, has been shown to facilitate cancer progression and may be associated with EMT process22. Similarly, POSTN is believed to drive EMT in several types of tumors, leading to increased malignancy and metastatic potential15. Moreover, accumulating evidence suggests that POSTN also enhances tumor cell proliferation and migration by activating NF-κB-related signaling pathways23,24,25. While previous studies have explored POSTN’s role in EMT or NF-κB signaling in other tumor types, its contributions in GBM remain largely unexplored. Understanding the precise roles and underlying mechanisms of POSTN within these pathways is essential for developing new therapeutic approaches to improve outcomes for GBM patients.
This study identified POSTN as a key gene linked to temozolomide resistance in GBM using RNA-sequencing data analysis. Increased POSTN levels were associated with poor outcomes in GBM patients. Overexpression of POSTN significantly enhanced malignant behaviors, including TMZ resistance, while its suppression greatly reduced these effects. Pathway enrichment analysis suggested that POSTN expression was involved in the EMT process and NF-κB signaling. POSTN overexpression may promote EMT-like phenotypes and chemoresistance in GBM cells. Mechanistic studies revealed that POSTN facilitated the phosphorylation of the inhibitor of nuclear factor kappa-B kinase subunit α (IKKα), thereby enhancing NF-κB signaling. Overall, our study highlighted that POSTN could be a potential prognostic marker and therapeutic target for GBM.
Results
Underlying chemoresistance genes could identify different prognostic subgroups for GBM
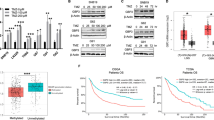
Gene expression profiles between TMZ-resistant and TMZ-sensitive cell lines were compared to investigate potential core genes associated with TMZ resistance in GBM. Differential expression analysis using the limma package identified 1,010 and 2,357 significantly upregulated genes in drug-resistant cell lines from the GSE199689 and GSE100736 databases, respectively (Fig. 1A and B). Additionally, to identify candidate genes, an intersection analysis of the upregulated genes was performed, revealing 220 genes consistently overexpressed across both TMZ-resistant datasets. (Fig. 1C). Further clustering analysis was conducted on the TCGA dataset, using the ConsensusClusterPlus package, identified two distinct clusters two distinct clusters (Fig. 1D, E). A gene set comprising 220 chemoresistance-related genes was subsequently constructed, and resistance scores were analyzed using the ssGSEA algorithm. The results indicated that the poor prognosis group exhibited significantly higher resistance scores (Fig. 1F). Similar results were observed in the CGGA database (Fig. 1G, H). Moreover, results obtained through the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis indicated a positive correlation between the candidate genes and the structural constituents of the extracellular matrix (Fig. 1). Taken together, the identified chemoresistance risk genes accurately classified patients into distinct prognostic categories. Notably, higher resistance scores were associated with poorer prognoses, indicating the significant TMZ resistance heterogeneity in GBM.
Underlying chemoresistance genes could identify different prognostic subgroups for GBM. A and B, Differentially expressed genes (DEGs) in TMZ-resistant versus TMZ-sensitive cell lines, identified via limma analysis. Expression profiles were obtained from the GSE199689 (A) and GSE100736 (B) datasets. DEGs were defined as Log2FC > 2.0 and adjusted P < 0.05. C, Venn diagram depicting intersection of upregulated genes from both datasets, revealing 220 shared candidate genes. D, E and G, Clustering analysis using The Cancer Genome Atlas (TCGA) database (E) and the Chinese Glioma Genome Atlas (CGGA) database (G), categorizing the candidate genes into two distinct clusters. Cluster optimization was achieved using the ConsensusClusterPlus package through distance analysis, resulting in two distinct clusters (D). F and H, Kaplan-Meier analysis and the distribution of resistance scores for the two clusters extracted from the TCGA (F) and CGGA (H) databases (TCGA: P = 0.00061, CGGA: P = 0.012, log-rank test; ****P < 0.001, independent sample t-test). I, Bubble plots illustrating the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of the 220 identified candidate genes, showing a positive correlation with extracellular matrix structural constituents26,27.
Elevated POSTN expression was strongly associated with poor outcomes in GBM patients
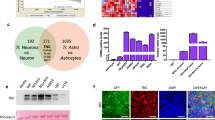
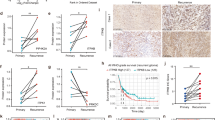
To further identify underlying chemoresistance genes associated with different prognoses in GBM, differential expression analyses were performed on patient datasets from TCGA (Fig. 1F) and CGGA (Fig. 1H). In the TCGA database, 20 genes were identified as significantly upregulated in different prognosis groups (Fig. 2A), while in the CGGA database, 5 genes were significantly upregulated (Fig. 2B). A subsequent Venn diagram analysis identified two candidate genes, periostin (POSTN) and Chitinase-3-like protein 1 (CHI3L1), associated with prognostic differences. POSTN was notably elevated, indicating its potential role in GBM prognosis (Fig. 2C). The expression of POSTN across pathological subtypes was further examined in the CGGA and TCGA datasets to better understand its clinical relevance, showing a progressive upregulation in alignment with glioma pathology (Fig. 2D, G). Similarly, when grouped by subtype (classical, mesenchymal, proneural), significant differences in POSTN mRNA levels were observed in both databases (Fig. 2E, H). Moreover, patients with lower POSTN expression exhibited prolonged survival compared to those with higher expression levels in IDH wild-type (IDH-wt) GBM (Fig. 2F, I). To further investigate the potential of POSTN as a biomarker for GBM, we performed expression analysis of POSTN in different tissues. By comparing POSTN expression across different grades of glioma, we aimed to assess its relationship with glioma progression. Immunohistochemistry (IHC) analysis of POSTN expression in glioma tissues of varying grades demonstrated that POSTN primarily localized to the cytoplasmic membranes of glioma cells and was also observed in the extracellular matrix, with significant enrichment in WHO grade IV gliomas (Fig. 2J). Additionally, Western blot analysis was conducted to compare POSTN expression levels between GBM tumor tissues and matched peritumoral infiltration tissues. The findings demonstrated that POSTN was markedly increased in GBM compared to the peritumoral infiltration tissues, with qRT-PCR analysis corroborating these results (Fig. 2K), suggesting its potential as a biomarker. Furthermore, POSTN expression was analyzed in three GBM cell lines (U87, LN229, and U251), as well as in normal human astrocytes (NHAs). Consistent with earlier findings, significant increases in POSTN expression were observed in the GBM cell lines, as revealed by both Western blot and qRT-PCR assays (Fig. 2L). Altogether, POSTN exhibited high expression levels in GBM cell lines and patient samples, with increased expression closely associated with poor patient outcomes.
Periostin (POSTN) was markedly upregulated in GBM samples and cell lines, and increased expression of POSTN was strongly linked to unfavorable outcomes in GBM patients. A and B, Volcano plots depicting DEGs identified in patients with different prognoses using the datasets from TCGA (A) and CGGA (B). C, Venn diagram showing intersection analysis of upregulated genes from TCGA and CGGA datasets to identify common candidate genes. D and G, POSTN mRNA expression in gliomas categorized by WHO grade using the CGGA database (D) and TCGA database (G) (***P < 0.001, **P < 0.01, *P < 0.05, one-way ANOVA followed by Tukey’s Honest Significant Difference (HSD) test). E and H, Analysis of POSTN mRNA expression levels across different glioma subtypes (classical, mesenchymal, proneural) using data from the CGGA database (E) and TCGA database (H) (***P < 0.001, one-way ANOVA followed by Tukey’s HSD post-test). F and I, Kaplan-Meier survival analysis of POSTN expression in IDH-wt GBM samples across all subtypes from the CGGA (F) and TCGA datasets (I) (CGGA: P = 0.012, TCGA: P = 0.0002, log-rank test). J, IHC images showing POSTN distribution in gliomas of different WHO grades, including oligodendrogliomas (grade II), astrocytomas (grade III), and glioblastomas (grade IV), respectively. K, Western blot assays of POSTN expression in four glioma tissues compared to matched peritumoral infiltration tissues, using GAPDH as the reference control. qRT-PCR assays of POSTN mRNA levels in four gliomas tumor tissues versus matched peritumoral infiltration tissues (****P < 0.0001, **P < 0.01, Student’s t-test). Upper: Western blot analysis. Lower: qRT-PCR analysis. L, Western blot assays of POSTN expression in U87, LN229, and U251 GBM cells relative to normal human astrocytes (NHA), using GAPDH as the reference control. qRT-PCR assays for detecting POSTN mRNA levels in U87, LN229, and U251 GBM cell lines versus NHA (****P < 0.0001, Student’s t-test). Upper: Western blot analysis. Lower: qRT-PCR analysis.
POSTN suppression reduced the malignancy of GBM cells
To further examine the functional role of POSTN, U87 cells with relatively high endogenous POSTN expression were used for knockdown experiments and transfected with shPOSTN and shNT lentiviruses. Immunofluorescence imaging was used to assess the transduction efficiency and confirmed successful POSTN knockdown with consistent efficiency across all panels, without altering cell morphology (Fig. 3A). Western blot (Fig. 3B) and qRT-PCR assays (Fig. 3C) confirmed that POSTN expression was significantly reduced in U87 cells transfected with shPOSTN#1 and shPOSTN#2 compared to those transfected with shNT. Additionally, the impact of POSTN suppression on tumor proliferation was further explored using the EDU staining assays. Cells transfected with shPOSTN#1 and shPOSTN#2 demonstrated a marked decrease in EDU-positive cell counts versus shNT-transfected cells, indicating that suppression of POSTN could reduce cell proliferation in GBM cells (Fig. 3D). This finding was supported by proliferation assay, which demonstrated that POSTN knockdown significantly reduced U87 cell growth in vitro (Fig. 3E). Moreover, wound healing, Matrigel invasion, and colony formation assays were conducted to investigate the functional significance of POSTN silencing in GBM malignancy. The findings revealed a notable decline in the migratory capacity of U87 cell lines with suppressed POSTN expression, relative to the control cells (Fig. 3F). Additionally, these POSTN-knockdown U87 cell lines exhibited a markedly diminished invasion capacity relative to the control cells following a 24-hour incubation period (Fig. 3G). Furthermore, POSTN silencing brought about a substantial reduction in cell proliferation and self-renewal capacity (Fig. 3H). Collectively, POSTN knockdown suppressed key malignant biological processes, such as proliferation, migration, invasion and self-renewal.
POSTN knockdown suppressed the malignant characteristics of glioblastoma cell lines. A, Immunofluorescence images illustrating U87 cell transduction efficiency with lentiviral shPOSTN#1, shPOSTN#2, and control (shNT). B, Western blot analysis of POSTN expression in U87 cells transfected with shPOSTN lentivirus or control, using GAPDH as the reference control. C, qRT-PCR assays of POSTN mRNA levels in U87 cells transfected with shPOSTN lentivirus or control (****P < 0.0001, one-way ANOVA followed by Dunnett’s post-test). D, EDU staining assay for measuring the proliferation of U87 cells transfected with shPOSTN lentivirus or control. Microscopic counts of EDU-positive cells demonstrated proliferation capacity (***P < 0.001, one-way ANOVA followed by Dunnett’s post-test). E, In vitro proliferation assay evaluating the proliferation of U87 cells transfected with shPOSTN lentivirus or control (***P < 0.001, one-way ANOVA followed by Dunnett’s post-test). F, Wound healing assays of U87 cells transfected with shPOSTN lentivirus or control at baseline (top) and day 3 (bottom). The cell migration distance was significantly reduced in the shPOSTN treatment group compared to the control group (*P < 0.05, one-way ANOVA followed by Dunnett’s post-test). G, Cell invasion assays of U87 cells transfected with shPOSTN lentivirus or control (*P < 0.05, one-way ANOVA followed by Dunnett’s post-test). H, Colony formation assays of U87 cells transfected with shPOSTN lentivirus or control (**P < 0.01, one-way ANOVA followed by Dunnett’s post-test).
POSTN overexpression induced malignancy and drug resistance in GBM cells
The endogenous POSTN expression was relatively low in LN229 cells; exogenous POSTN was introduced into these cells via lentivirus transduction for overexpression studies. Immunofluorescence imaging confirmed successful POSTN overexpression with consistent transduction efficiency across all panels, without altering cell morphology (Fig. 4A). Western blot (Fig. 4B) and qRT-PCR (Fig. 4C) analyses revealed markedly elevated POSTN expression in LN229 cells transduced with POSTN overexpression lentivirus compared to controls. The EDU staining assay confirmed more EDU-positive clones in POSTN-transduced cells compared to the control, reflecting enhanced cell proliferation due to POSTN overexpression (Fig. 4D). Similarly, in vitro proliferation assays corroborated these findings, showing that POSTN overexpression significantly enhanced GBM cell proliferation (Fig. 4E). Furthermore, wound healing, Matrigel invasion, and colony formation assays revealed that POSTN overexpression significantly enhanced the migration, invasion, and self-renewal capacities of GBM cells (Fig. 4F-H). Moreover, cytotoxicity assays indicated that LN229 cells overexpressing POSTN showed resistance to temozolomide, requiring higher drug concentrations to achieve similar levels of cell proliferation inhibition as seen in the control group (Fig. 4I). In vivo bioluminescence imaging further demonstrated an elevated proliferation rate in mice implanted with POSTN-overexpressing LN229 cells, highlighting the critical role of POSTN in driving tumor progression in GBM (Fig. 4J). Altogether, these data demonstrated that POSTN overexpression promoted tumor malignancy and drug resistance of GBM cells.
POSTN overexpression may contribute to enhanced malignancy and resistance to temozolomide, thereby corroborating its involvement in cancer processes. A, Immunofluorescence illustrating the efficiency of POSTN overexpression lentiviral transduction in the control (EV group) and POSTN group. B, Western blot analysis of POSTN protein expression in LN229 cells with or without POSTN overexpression lentivirus transduction, using GAPDH as the internal control. C, qRT-PCR analysis of POSTN mRNA levels in LN229 cells following POSTN lentiviral transduction (****P < 0.0001, Student’s t-test). D, EDU staining of LN229 cells with or without POSTN overexpression lentiviral transduction. Microscopic counts of EDU-positive cell clones represent proliferation ability (**P < 0.01, Student’s t-test). E, In vitro proliferation assay of LN229 cells with lentiviral POSTN or control transduction (**P < 0.01, one-way ANOVA followed by Dunnett’s post-test). F, Wound healing assays on LN229 cells transduced with POSTN overexpression lentivirus or control at day 0 (top) and day 3 (bottom). The migration distance was significantly increased in the POSTN-overexpressing group compared to the control group (*P < 0.05, Student’s t-test). G, Invasion assays of LN229 cells transfected with POSTN lentivirus or control (**P < 0.01, Student’s t-test). H, Colony formation assays of LN229 cells transfected with or without POSTN overexpression lentivirus (**P < 0.01, Student’s t-test). I, Cytotoxicity assays of LN229 cells transfected with POSTN lentivirus or control (**P < 0.01, one-way ANOVA followed by Dunnett’s post-test). J, In vivo bioluminescence imaging of POSTN-overexpressing LN229 cells at week 1 (top) and week 4 (bottom). The bar graph quantified the mean fluorescence intensity ratio at 4 weeks relative to 1 week (**P < 0.01, one-way ANOVA followed by Dunnett’s post-test).
POSTN enhanced the malignancy and chemoresistance of GBM through EMT dynamics and NF-κB signaling
POSTN, acting as a potential oncogene, escalates with glioma grades and exacerbates GBM malignancies and drug resistance. Bioinformatics analyses were conducted to elucidate its underlying mechanisms. Initial GSEA revealed significant enrichment of the TNFα/NF-κB pathway and epithelial-mesenchymal transition (EMT) phenotype in groups exhibiting high POSTN expression within both TCGA and CGGA datasets (Fig. 5A–D), affirming POSTN’s role in crucial cellular transformations during glioma progression. To corroborate these bioinformatics findings, Western blot and qRT-PCR assays were conducted. The results confirmed that POSTN overexpression significantly upregulated mesenchymal markers while downregulated epithelial biomarkers. EMT typically involves a loss of epithelial characteristics, cytoskeletal reorganization, decreased cellular adhesion, and the acquisition of mesenchymal traits, which enhance motility and invasiveness. Moreover, certain EMT-related transcription factors are implicated in broader cellular processes cell growth and apoptosis, contributing extensively to tumor progression beyond conventional EMT stages. Our findings highlighted that POSTN promoted invasion, migration, and drug resistance in GBM through EMT-like phenotype induction (Fig. 5E, F).
For further validation of the mechanism underlying POSTN-dependent regulation of NF-κB signaling, U87 cells were transduced with shPOSTN and control shNT lentiviruses. Western blot analysis revealed that POSTN knockdown significantly reduced IKKα phosphorylation, leading to decreased degradation of IκBα proteins and subsequent attenuation of p65 protein activation. However, these effects were moderately reversed by TNFα treatment (Fig. 6A). These results suggested that POSTN-dependent NF-κB activation could proceed through TNFα-driven stimulation. Additionally, the EDU staining assay showed that POSTN suppression substantially decreased U87 cell proliferation, with TNFα intervention moderately countering this effect (Fig. 6B). Cytotoxicity assays revealed that the resistance to temozolomide in U87 cells with POSTN knockdown was partially reversed upon the addition of TNFα (Fig. 6C). Moreover, Wound healing, Matrigel invasion, and colony formation assays demonstrated that migration, invasion, and self-renewal were notably inhibited in U87 cells with reduced POSTN levels. However, these characteristics could be partially enhanced with the administration of TNFα (Fig. 6D–F). Overall, POSTN knockdown effectively inhibited the NF-κB signaling pathway, subsequently attenuating malignant biological behaviors and chemoresistance.
POSTN showed positive associations with EMT-like phenotype invasion and migration, as well as TNFα/ NF-κB signaling. A-D, Bubble plots were performed to elucidate pathways significantly enriched in the TCGA (A) and CGGA (C) databases, showing a positive association of POSTN with EMT and TNFα/NF-κB signaling. GSEA analysis indicated a substantial link between EMT and POSTN expression in the TCGA (B) and CGGA (D) databases using normalized enrichment scores (NES) and p-values. E, Western blot analysis was undertaken to determine EMT downstream target protein levels in control and POSTN knockdown samples, using GAPDH as the reference control. F, qRT-PCR was performed to analyze mRNA levels of EMT downstream targets in U87 cells transduced with lentiviral POSTN and controls. Relative mRNA levels were normalized to GAPDH (****P < 0.0001, one-way ANOVA followed by Dunnett’s post-test).
POSTN regulated NF-κB signaling activation by modulating IkB-kinase α (IKKα) phosphorylation. A, Western blot analysis of NF-κB-regulated downstream proteins in U87 cells transfected with control (shNT) or shPOSTN#1 lentivirus and subsequently administered TNFα or maintained untreated, using GAPDH as the internal control. B, EDU staining assay on U87 cells transfected with shPOSTN#1 or control lentivirus and subsequently administered TNFα or maintained untreated. Proliferation levels were indicated by microscopic counts of EDU-positive cells (**P < 0.01, ***P < 0.001, Student’s t-test). C, Cytotoxicity assays on U87 cells transfected with shNT or shPOSTN#2 lentivirus and subsequently administered TNFα or maintained untreated (**P < 0.01, ***P < 0.001, one-way ANOVA followed by Dunnett’s post-test). D, Wound healing assays on U87 cells transfected with shNT or shPOSTN#1 lentivirus and subsequently administered TNFα or maintained untreated at day 0 (top) and day 3 (bottom) (*P < 0.05, **P < 0.01, one-way ANOVA followed by Dunnett’s post-test). E, Cell invasion assay on U87 cells transfected with shPOSTN#1 or control lentivirus and subsequently administered TNFα or maintained untreated (*P < 0.05, **P < 0.01, one-way ANOVA followed by Dunnett’s post-test). F, Colony formation assays on U87 cells transfected with shPOSTN#1 or control lentivirus and subsequently administered TNFα or maintained untreated (*P < 0.05, **P < 0.01, one-way ANOVA followed by Dunnett’s post-test).
Discussion
For over a decade, TMZ has been the primary treatment for GBM owing to its anti-tumor effect induced by DNA damage. However, combining TMZ with radiotherapy often fails to offer substantial additional benefits beyond those observed with radiotherapy alone, as GBM patients typically develop resistance during extended therapy, with about 90% relapsing28. This highlights GBM as the most aggressive diffuse glioma, posing significant challenges in neuro-oncology29. Consequently, elucidating TMZ resistance mechanisms could potentially enhance the efficacy of TMZ therapy in patients with GBM and help identify novel therapeutic targets. This study found significant upregulation of POSTN in GBM cell lines and patient samples, correlating with poorer clinical outcomes. Furthermore, POSTN was shown to promote the EMT process and NF-κB signaling, enhancing glioma cell proliferation, migration, invasion, and chemoresistance. These findings indicated that POSTN could be pivotal in tumor progression and chemoresistance.
MGMT, a crucial enzyme for removing TMZ-induced DNA damage, when overexpressed, promotes TMZ resistance30. While promoter methylation of MGMT enhances GBM sensitivity to TMZ by reducing DNA repair ability, certain patients with methylated MGMT promoters still have high MGMT expression and exhibit significant resistance to TMZ31,32, indicating that other regulatory mechanisms may also influence TMZ therapy. Consequently, there’s an urgent need for reliable predictors of TMZ treatment responses. Given this challenging issue, we analyzed bioinformatic data from the CGGA and TCGA databases, and performed differential expression analysis on gene expression profiles from drug-resistant and non-resistant cell lines, identifying POSTN as a critical gene. Previous studies have shown that POSTN, located on chromosome 13q13.3, encodes periostin, a protein involved in cell adhesion and migration, and is closely associated with tumor progression33. Zhou et al. found that POSTN could be secreted by glioblastoma stem cells, promoting tumor progression and growth through the recruitment of M2-type tumor-associated macrophages34. Additionally, research by Valdor et al. and Huizer et al. showed that POSTN could also be secreted by the tumor perivascular microenvironment, specifically by pericytes, contributing to GBM angiogenesis and progression35,36. Moreover, evidence had shown that POSTN was a potential biomarker for gliomas37,38. Importantly, Molina et al. suggested that POSTN could serve as a GBM biomarker, as GBM-conditioned pericytes exhibit pro-tumor properties and secrete a pro-tumor secretome, including POSTN. Our findings from CGGA and TCGA data, supported by immunohistochemistry, qRT-PCR, and Western blot analyses, indicated that POSTN was consistently upregulated with the pathological grade of glioma. Our study further showed that its overexpression was significantly associated with shorter survival in patients39,40, supporting its potential as a prognostic biomarker for GBM.
Accumulating evidence indicates that POSTN interacts with cell surface receptors, particularly integrins, and activates signaling pathways that promote cancer cell survival, migration, invasion, and EMT41,42,43. Nakazawa et al.44 discovered that POSTN overexpression was closely linked to EMT-dependent chemoresistance in triple-negative breast cancer. Similarly, Li et al.45 highlighted the potential role of POSTN in EMT, tumor relapse, and immune therapy response in hepatocellular carcinoma. While POSTN has been recognized for its pivotal role in multiple cancers, the mechanism by which POSTN acts as pro-tumoral in GBM, leading to its malignancy and chemoresistance, remains poorly understood. In this study, we systematically revealed the role of POSTN in GBM, particularly its key involvement in cell proliferation, migration, invasion, and TMZ resistance. Using data from the TCGA and CGGA datasets along with integrated analytical techniques, we demonstrated that POSTN-driven GBM malignancy involved EMT, leading to changes in adhesion molecules and promoting the mesenchymal phenotype associated with migration and invasion. Moreover, we provided evidence for the first time that POSTN overexpression was closely associated with TMZ resistance in GBM cells, suggesting that POSTN might be a key regulator in the development of drug resistance in GBM.
NF-κB is a key transcription factor modulating gene expression. In the canonical pathway, receptors like TLR and TNFR activate IKKβ, leading to IκB protein degradation and NF-κB release22. Evidence suggests that aberrant NF-κB pathway activation is closely related to chemotherapy resistance across multiple malignancies, such as ovarian, colorectal, and breast cancer14,24,25. Moreover, POSTN-induced NF-κB signaling has been implicated in the emergence of treatment resistance across these cancers. Lin et al. found that in ovarian cancer, POSTN enhanced the recruitment and polarization of M2 macrophages and cancer-associated fibroblasts through integrin-dependent TGF-β2 and NF-κB signaling, fostering a microenvironment that promoted tumor drug resistance25. Similarly, research by Wei et al. on colorectal cancer revealed that deficiency of POSTN decreased the infiltration of PD-1 + tumor-associated macrophages via integrin-NF-κB signaling24, thereby enhancing anti-PD-1 therapy effectiveness. In GBM, as in other cancers, NF-κB signaling is crucial for driving tumor progression and therapeutic chemoresistance46. Nonetheless, the mechanisms driving NF-κB hyperactivity in GBM, particularly the involvement of POSTN in this pathway, remain to be elucidated. This study, for the first time, investigated POSTN’s role in modulating the NF-κB signaling in GBM cells, providing new insights into its contribution to this aggressive brain cancer. Exogenous POSTN silencing was found to diminish IKKα phosphorylation and IκB degradation, thereby reducing NF-κB pathway activation. The alterations were moderately reversed with TNFα intervention, suggesting that POSTN may promote malignant properties and chemoresistance, through NF-κB-mediated mechanisms.
This study thoroughly explored POSTN’s roles in GBM chemoresistance; however, further molecular investigations are needed to better define the underlying mechanisms. Despite the insights gained, the precise mechanisms of POSTN’s interaction with the NF-κB pathway and its effect on downstream molecular markers. While we have established that POSTN is involved in IKKα phosphorylation, the fact that POSTN encodes an extracellular matrix protein and cannot directly phosphorylate IKKα suggests the existence of an unknown intermediary mechanism within the POSTN-NF-κB axis. While EMT and NF-κB may be interconnected, this study did not further validate their potential interaction. Additionally, altering a single candidate biomarker could lead to unpredictable outcomes due to the inherent heterogeneity of GBM. Future studies should include additional cell lines and ensure cell culture purity. Therefore, comprehensive strategies for assessing GBM patients are crucial before clinical management.
Overall, our study revealed that POSTN expression was significantly elevated in GBM cell lines and patient samples, with higher levels strongly associated with poorer clinical outcomes. Moreover, POSTN-induced activation of NF-κB pathway and EMT process was found to promote the malignancy and chemoresistance of GBM, suggesting that POSTN could be a reliable prognostic indicator and a promising therapeutic target in GBM management.
Materials and methods
Ethical approval and participant consent
The Scientific Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China approved use of all the patient samples in this study (No. 2022NSFC-035) and obtained written informed consent from all patients, all experimental protocols used in the study were in accordance with the guidelines of the Declaration of Helsinki. Four glioma samples and matched peritumoral infiltration tissues were collected from patients who underwent surgery between 2022 and 2023 at our institution and preserved in paraffin blocks following established methods.
Analysis of differential gene expression
Gene expression and clinical data for GBM were sourced from the GSE199689, GSE100736, TCGA and CGGA datasets. The data were preprocessed in R (version 4.2) for background correction, gene symbol conversion, and normalization. The limma toolkit identified differentially expressed genes (DEGs). DEGs were determined by log2(Fold change) and adjusted P value, with log2FC > 2 and adjusted P < 0.05 indicating upregulation.
Gene ontology (GO) analysis, gene set enrichment analysis (GSEA), and gene set variation analysis (GSVA)
The transcriptional profiles from the databases were imported into R for preprocessing, including background correction, gene ID conversion, and normalization. GBM samples were then categorized into two cohorts: high expression and POSTIN low expression. Differential gene expression (DEG) analysis was conducted using the limma package. DEGs with a log2FC > 2 and an adjusted P value < 0.05 were annotated via Gene Ontology (GO) using the DAVID tool. The GSVA toolkit and GSEA software were employed for further confirmation, and findings were visualized with clusterProfiler. For additional analysis, GSEA software was used to identify significantly enriched KEGG pathways in the POSTIN high group, following standard procedures with 1000 permutations.
Cell culture and in vitro growth assay
Primary GBM cell lines (U87, LN229, U251) and the NHA cell line were obtained from ATCC. Cells were cultured in DMEM-F12 medium with 10% FBS and 1% penicillin-streptomycin, maintained at 37 °C with 5% CO2. The medium was refreshed every three days. Cell proliferation assays were performed following standard protocols. Briefly, cells were counted using a trypan blue cell counter, seeded into 96-well plates at 2 × 103 cells/100 µL per well, and left to incubate for 8 h at 37 °C with 5% CO2. Proliferation was measured using alamarBlue.
EdU staining for proliferation assay
Cell proliferation was assessed by EdU (5-ethynyl-2’-deoxyuridine) staining according to the manufacturer’s instructions provided with the EdU staining kit (Servicebio, Catalog No. G5879). Cells were seeded at an appropriate density, treated with specific conditions (e.g., POSTN overexpression or knockout), and incubated with EdU (10 µM) in the culture medium for 2 h at 37 °C. After incubation, cells were washed twice with PBS, fixed with 4% paraformaldehyde (PFA) at room temperature for 15 min, and permeabilized with 0.5% Triton X-100 for 20 min. EdU incorporation was visualized using the EdU staining kit, and nuclei were counterstained with Hoechst 33,342. Positive signal cells were photographed using an EVOSTM FL Auto 2 imaging system (Thermo Fisher Scientific) and quantitatively counted using ImageJ.
Immunohistochemistry (IHC)
For immunohistochemical analysis, tissue blocks were fixed in 4% paraformaldehyde (PFA) at 4 °C for 48 h, embedded in paraffin, and sectioned to 4 μm thickness. Sections were blocked with 5% bovine serum albumin (BSA, EZ2811C238; BioFroxx) for 1 h at room temperature, then incubated overnight at 4 °C with anti-POSTN primary antibodies (sc-398631; Santa Cruz Biotechnology; 1:100). The subsequent day, sections received biotinylated secondary antibody treatment (KIT-9730; MXB Biotechnologies; 1:2000) for 1 h at room temperature, washed with PBS, and incubated with streptavidin-peroxidase (KIT-9730; MXB Biotechnologies) for 50 min. After another PBS wash, sections were stained with 3,3-diaminobenzidine (30 mg in 100 ml Tris buffer with 0.03% H2O2) for 5 min, rinsed with water, and counterstained with hematoxylin for 3 min. POSTN expression was evaluated by analyzing 10 randomly selected visual fields per section under a light microscope, using ImageJ software (v.1.8.0; NIH).
Lentivirus production and transduction
Lentiviruses for POSTN overexpression and knockdown constructs were engineered and synthesized by GeneChem (Shanghai, China). Following the manufacturer’s protocol, these lentiviruses were introduced into LN229 and U87 cell lines. After 48 h, cells were diluted, seeded in 6-well plates at 5 × 104 cells per well, and treated with 1 µg/ml puromycin for 72 h. The cells were then cultured for 4 weeks at 37 °C with 5% CO2 in 0.1 µg/ml puromycin to establish stable POSTN and shPOSTN clones. The shPOSTN lentiviral particles targeted POSTN with two specific sequences (shPOSTN_#1: CGAGCCTTGTATGTATGTTAT and shPOSTN_#2: CACTTGTAAGAACTGGTATAA), with non-targeting shRNA as the control.
Quantitative RT-PCR (qRT-PCR)
qRT-PCR assays followed established procedures. Total RNA was extracted with RNeasy mini kits and quantified using a Nanodrop 2000. cDNA was synthesized, and qRT-PCR was performed. GAPDH was used as the internal control. Relative mRNA expression was calculated via the 2-ΔΔCt method. Primer sequences are detailed in Table 1.
Western blot
Western blot analysis followed standard protocols. Primary antibodies included: anti-POSTIN (Santa Cruz Biotechnology), anti-N-cadherin, anti-E-cadherin, anti-vimentin, anti-p-IKKα Ser176/180, and anti-NF-κB (Cell Signaling Technology); anti-IKKα and anti-IκBα (ZENBIO); and anti-Snail 1 and anti-Occludin (Wanlei Bio). Secondary antibodies, anti-rabbit IgG and anti-mouse IgG, were from Cell Signaling Technology and used as controls. GAPDH, from Proteintech, served as the internal control. Finally, band gray values were quantified by Image J.
Colony formation assays
Colony formation assays were performed to evaluate the tumorigenic potential of U87 cell lines. U87 cells, stably transduced with shNT, shPOSTIN, or POSTIN overexpression lentivirus, were seeded at varying densities in 6-well plates. After 2 weeks of cultivation, colonies were fixed with methanol and stained with methylene blue. The colonies were then imaged using an inverted microscope (IX53, OLYMPUS, Tokyo, Japan) and enumerated using ImageJ software.
Wound healing assays
Wound healing assays were conducted to evaluate the migratory capacity of U87 cells. Cells transfected with shNT, shPOSTIN, or POSTIN overexpression lentivirus were seeded into 6-well plates. Once the cells reached confluence, a wound was created using a sterile pipette tip. The cells were then washed and cultured in FBS-free medium. Wound closure was monitored by capturing images with an inverted microscope (IX53, OLYMPUS, Tokyo, Japan), and the migration distance of cells over time was quantified using ImageJ software. The initial wound width at day 0 served as the baseline, and its decrease at day 3 was measured to evaluate cell migration capacity.
Matrigel invasion assays
Matrigel invasion assays were performed to evaluate the invasiveness of GBM cells. Pre-transduced U87 or LN229 cells (1 × 105) were seeded into the upper chambers of ThinCert inserts (8 μm pore size, Greiner Bio-One) pre-coated with Matrigel (Corning, NY, USA). The lower chambers were filled with 500 µL of culture medium containing 10% FBS, serving as a chemoattractant. After 24 h of incubation at 37 °C in a humidified atmosphere, the inserts were carefully removed. Non-invaded cells on the upper surface of the membrane were gently wiped off with a cotton swab. Invaded cells on the lower surface of the membrane were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet for 15 min, and subsequently washed with PBS. Images of the invaded cells were captured using an inverted phase-contrast microscope (Olympus). Cell invasion was quantified by analyzing the images with ImageJ software, where the number of invaded cells was determined in three randomly selected fields per membrane.
Cell count kit‑8 (CCK8) cytotoxicity assays
The cytotoxicity of Temozolomide (TMZ) on GBM cells was evaluated using the CCK-8 assay. Cells were seeded at 2 × 103 per well in 96-well plates with 100 µL of complete medium and incubated overnight. The medium was exchanged for fresh medium containing different TMZ concentrations (0, 100, 200, 400, 800, 1600 µM). After 48 h at 37 °C in 5% CO2, 10 µL of CCK-8 reagent was added to each well and incubated for 2 h. Absorbance at 450 nm was measured with a microplate reader, and cell viability was calculated relative to untreated controls.
In vivo bioluminescence imaging
6-week-old BALB/c nude mice were selected from Shanghai experimental animal center of Chinese Academy of Sciences. To establish To establish an orthotopic transplantation tumor model, 5 × 105 LN229 cells were suspended in 5 µL of L15 medium and injected into the right striatum of mice. A week later, D-Luciferin potassium (15 mg mL–1) was injected intravenously into each mouse (10 mg·kg–1), and luciferase fluorescence intensity was measured using an in vivo imaging system (Caliper, Princeton, NJ, USA) to confirm successful glioma model construction. The mice were then divided into two groups (n = 5) based on fluorescence intensity. Bioluminescence images were taken at key time points (week 1 and week 4) using the IVIS imaging system, and tumor burden was quantified and analyzed for growth with Living Image software. Finally, the nude mice were sacrificed by euthanized by isofluorane anesthesia. The Animal Experimental Ethics Committee of Xi’an Jiaotong University approved all animal experiments. Furthermore, all animal experiments were performed in accordance with relevant guidelines and regulations and reporting in the manuscript follows the recommendations in the ARRIVE guidelines 2.0.
Statistical analysis
Data are presented as mean ± SD. The figure legends detail the number of independent replicates. Two-tailed t-tests assessed pairwise comparisons, while one-way ANOVA with Tukey’s HSD or Dunnett’s post-test was used for multigroup analyses. Kaplan-Meier survival curves were evaluated via the log-rank test. Statistical analyses were conducted with GraphPad Prism 9 or SPSS 24.0 software. Significance was defined as a two-sided P value below 0.05, unless specified otherwise.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the united States in 2016–2020. Neuro Oncol. 25, iv1–iv99. https://doi.org/10.1093/neuonc/noad149 (2023).
Weller, M. et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 18, 170–186. https://doi.org/10.1038/s41571-020-00447-z (2021).
Schaff, L. R. & Mellinghoff, I. K. Glioblastoma and other primary brain malignancies in adults: a review. Jama 329, 574–587. https://doi.org/10.1001/jama.2023.0023 (2023).
Ma, R., Taphoorn, M. J. B. & Plaha, P. Advances in the management of glioblastoma. J. Neurol. Neurosurg. Psychiatry. 92, 1103–1111. https://doi.org/10.1136/jnnp-2020-325334 (2021).
Shaw, R., Basu, M., Karmakar, S. & Ghosh, M. K. MGMT in TMZ-based glioma therapy: multifaceted insights and clinical trial perspectives. Biochim. Biophys. Acta Mol. Cell. Res. 1871, 119673. https://doi.org/10.1016/j.bbamcr.2024.119673 (2024).
Smerdi, D., Moutafi, M., Kotsantis, I., Stavrinou, L. C. & Psyrri, A. Overcoming resistance to Temozolomide in glioblastoma: a scoping review of preclinical and clinical data. Life (Basel) 2024, 14. https://doi.org/10.3390/life14060673 (2024).
Lang, F., Liu, Y., Chou, F. J. & Yang, C. Genotoxic therapy and resistance mechanism in gliomas. Pharmacol. Ther. 228, 107922. https://doi.org/10.1016/j.pharmthera.2021.107922 (2021).
Elshazly, A. M. & Gewirtz, D. A. Is autophagy inhibition in combination with temozolomide a therapeutically viable strategy? Cells 2023, 12. https://doi.org/10.3390/cells12040535 (2023).
Li, S., Xie, X., Peng, F., Du, J. & Peng, C. Regulation of Temozolomide resistance via LncRNAs: clinical and biological properties of LncRNAs in gliomas (Review). Int. J. Oncol. 61 https://doi.org/10.3892/ijo.2022.5391 (2022).
Tomar, M. S., Kumar, A., Srivastava, C. & Shrivastava, A. Elucidating the mechanisms of Temozolomide resistance in gliomas and the strategies to overcome the resistance. Biochim. Biophys. Acta Rev. Cancer 1876, 188616. https://doi.org/10.1016/j.bbcan.2021.188616 (2021).
Gonzalez-Gonzalez, L. & Alonso, J. Periostin: a matricellular protein with multiple functions in cancer development and progression. Front. Oncol. 8, 225. https://doi.org/10.3389/fonc.2018.00225 (2018).
Brabletz, S., Schuhwerk, H., Brabletz, T. & Stemmler, M. P. Dynamic EMT: a multi-tool for tumor progression. EMBO J. 40, e108647. https://doi.org/10.15252/embj.2021108647 (2021).
Zhao, Z., Zhang, Y., Guo, E., Zhang, Y. & Wang, Y. Periostin secreted from podoplanin-positive cancer-associated fibroblasts promotes metastasis of gastric cancer by regulating cancer stem cells via AKT and YAP signaling pathway. Mol. Carcinog. 62, 685–699. https://doi.org/10.1002/mc.23517 (2023).
Wu, T. et al. Matrix stiffening facilitates the collective invasion of breast cancer through the periostin-integrin mechanotransduction pathway. Matrix Biol. 121, 22–40. https://doi.org/10.1016/j.matbio.2023.05.006 (2023).
Dorafshan, S. et al. Periostin: biology and function in cancer. Cancer Cell. Int. 22, 315. https://doi.org/10.1186/s12935-022-02714-8 (2022).
Bernardes, S. S. et al. Glioma pericytes promote angiogenesis by producing periostin. Cell. Mol. Neurobiol. 42, 557–564. https://doi.org/10.1007/s10571-020-00975-3 (2022).
Huang, Y., Hong, W. & Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 15, 129. https://doi.org/10.1186/s13045-022-01347-8 (2022).
Noronha, C. et al. Cadherin expression and EMT: A focus on gliomas. Biomedicines 9, 74. https://doi.org/10.3390/biomedicines9101328 (2021).
Shirley, C. A., Chhabra, G., Amiri, D., Chang, H. & Ahmad, N. Immune escape and metastasis mechanisms in melanoma: breaking down the dichotomy. Front. Immunol. 15, 1336023. https://doi.org/10.3389/fimmu.2024.1336023 (2024).
Xie, S., Wu, Z., Qi, Y., Wu, B. & Zhu, X. The metastasizing mechanisms of lung cancer: recent advances and therapeutic challenges. Biomed. Pharmacother. 138, 111450. https://doi.org/10.1016/j.biopha.2021.111450 (2021).
Hashemi, M. et al. EMT mechanism in breast cancer metastasis and drug resistance: revisiting molecular interactions and biological functions. Biomed. Pharmacother. 155, 113774. https://doi.org/10.1016/j.biopha.2022.113774 (2022).
Yu, H., Lin, L., Zhang, Z., Zhang, H. & Hu, H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal. Transduct. Target. Ther. 5, 209. https://doi.org/10.1038/s41392-020-00312-6 (2020).
Wang, Z. et al. Periostin: an emerging activator of multiple signaling pathways. J. Cell. Commun. Signal. 16, 515–530. https://doi.org/10.1007/s12079-022-00674-2 (2022).
Wei, T. et al. Periostin deficiency reduces PD-1(+) tumor-associated macrophage infiltration and enhances anti-PD-1 efficacy in colorectal cancer. Cell. Rep. 42, 112090. https://doi.org/10.1016/j.celrep.2023.112090 (2023).
Lin, S. C. et al. Periostin promotes ovarian cancer metastasis by enhancing M2 macrophages and cancer-associated fibroblasts via integrin-mediated NF-κB and TGF-β2 signaling. J. Biomed. Sci. 29, 109. https://doi.org/10.1186/s12929-022-00888-x (2022).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53, D672–d677. https://doi.org/10.1093/nar/gkae909 (2025).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Tesileanu, C. M. S. et al. Temozolomide and radiotherapy versus radiotherapy alone in patients with glioblastoma, IDH-wildtype: post hoc analysis of the EORTC randomized phase III CATNON trial. Clin. Cancer Res. 28, 2527–2535. https://doi.org/10.1158/1078-0432.CCR-21-4283 (2022).
Tan, A. C. et al. Management of glioblastoma: state of the Art and future directions. CA Cancer J. Clin. 70, 299–312. https://doi.org/10.3322/caac.21613 (2020).
Butler, M. et al. MGMT status as a clinical biomarker in glioblastoma. Trends Cancer 6, 380–391. https://doi.org/10.1016/j.trecan.2020.02.010 (2020).
Egaña, L. et al. Methylation of MGMT promoter does not predict response to temozolomide in patients with glioblastoma in Donostia hospital. Sci. Rep. 10, 18445. https://doi.org/10.1038/s41598-020-75477-9 (2020).
Oldrini, B. et al. MGMT genomic rearrangements contribute to chemotherapy resistance in gliomas. Nat. Commun. 11, 3883. https://doi.org/10.1038/s41467-020-17717-0 (2020).
Ruan, K., Bao, S. & Ouyang, G. The multifaceted role of periostin in tumorigenesis. Cell. Mol. Life Sci. 66, 2219–2230. https://doi.org/10.1007/s00018-009-0013-7 (2009).
Zhou, W. et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell. Biol. 17, 170–182. https://doi.org/10.1038/ncb3090 (2015).
Huizer, K. et al. Periostin is expressed by pericytes and is crucial for angiogenesis in glioma. J. Neuropathol. Exp. Neurol. 79, 863–872. https://doi.org/10.1093/jnen/nlaa067 (2020).
Valdor, R. et al. Glioblastoma ablates pericytes antitumor immune function through aberrant up-regulation of chaperone-mediated autophagy. Proc. Natl. Acad. Sci. U S A 116, 20655–20665. https://doi.org/10.1073/pnas.1903542116 (2019).
Faried, A., Hermanto, Y., Tjahjono, F. P., Valentino, A. & Arifin, M. Z. Identification of Periostin as a potential biomarker in gliomas by database mining. World Neurosurg. 135, e137–e163. https://doi.org/10.1016/j.wneu.2019.11.077 (2020).
Molina, M. L. et al. Chaperone-Mediated autophagy ablation in pericytes reveals new glioblastoma prognostic markers and efficient treatment against tumor progression. Front. Cell. Dev. Biol. 10, 797945. https://doi.org/10.3389/fcell.2022.797945 (2022).
Neuzillet, C. et al. Periostin- and podoplanin-positive cancer-associated fibroblast subtypes cooperate to shape the inflamed tumor microenvironment in aggressive pancreatic adenocarcinoma. J. Pathol. 258, 408–425. https://doi.org/10.1002/path.6011 (2022).
Null, J. L. et al. Periostin + Stromal cells guide lymphovascular invasion by Cancer cells. Cancer Res. 83, 2105–2122. https://doi.org/10.1158/0008-5472.CAN-22-2412 (2023).
Sonongbua, J. et al. Periostin induces epithelial–to–mesenchymal transition via the integrin α5β1/TWIST–2 axis in cholangiocarcinoma. Oncol. Rep. 43, 1147–1158. https://doi.org/10.3892/or.2020.7485 (2020).
Chen, L. et al. Periostin mediates epithelial-mesenchymal transition through the MAPK/ERK pathway in hepatoblastoma. Cancer Biol. Med. 16, 89–100. https://doi.org/10.20892/j.issn.2095-3941.2018.0077 (2019).
Jia, Y. Y., Yu, Y. & Li, H. J. POSTN promotes proliferation and epithelial-mesenchymal transition in renal cell carcinoma through ILK/AKT/mTOR pathway. J. Cancer 12, 4183–4195. https://doi.org/10.7150/jca.51253 (2021).
Nakazawa, Y. et al. Periostin Blockade overcomes chemoresistance via restricting the expansion of mesenchymal tumor subpopulations in breast cancer. Sci. Rep. 8, 4013. https://doi.org/10.1038/s41598-018-22340-7 (2018).
Li, Z. et al. Presence of onco-fetal neighborhoods in hepatocellular carcinoma is associated with relapse and response to immunotherapy. Nat. Cancer 5, 167–186. https://doi.org/10.1038/s43018-023-00672-2 (2024).
Shi, P., Xu, J. & Cui, H. The recent research progress of NF-κB signaling on the proliferation, migration, invasion, immune escape and drug resistance of glioblastoma. Int. J. Mol. Sci. 24, 896. https://doi.org/10.3390/ijms241210337 (2023).
Funding
This study was funded by the National Natural Science Foundation of China (No. 82102014), the Shaanxi Provincial Key Industrial Innovation Chain Project (No. 2024SF-ZDCYL-02-10), the Clinical Research Program of the First Affiliated Hospital of Xi’an Jiaotong University of China (No. XJTU1AF-CRF-2018-006).
Author information
Authors and Affiliations
Contributions
Y.S., Y.X.L. and W.W. designed the experiments; B.C.Z., and W.W. conducted most of the experiments; Y.H.P. and J.W. collected the clinical tissue samples. Y.S., B.C.Z. and W.W. analyzed the data. Y.S. and Y.X.L. wrote the manuscript, and M.Z. and N.C. revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shang, Y., Liang, Y., Zhang, B. et al. Periostin-mediated activation of NF-κB signaling promotes tumor progression and chemoresistance in glioblastoma. Sci Rep 15, 13955 (2025). https://doi.org/10.1038/s41598-025-92969-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92969-8