Abstract
This study aimed to examine the relationships of osteoporosis with Osteoporosis indices in elderly patients, and investigate the associations of novel inflammatory markers with variations of Osteoporosis indices. Senior citizens were recruited from the National Health and Nutrition Examination Survey (NHANES). Dual-energy X-ray absorptiometry was used to detect bone mineral density tests. Osteoporosis indices and diagnosis of osteoporosis were evaluated using multivariate weighted logistic regression models. Novel inflammatory markers were calculated based on lymphocyte, neutrophil, monocyte, platelet, and albumin counts. The relationships between the Osteoporosis indices and novel inflammatory markers were evaluated with multivariate weighted logistic regression models. Totally 837 elderly patients were enrolled, including 494 men and 343 women, and their weighted average age was 68.28 ± 7.60 years. Our results indicated that the osteoporosis indices were positively correlated with the presence of osteoporosis and that these three indices measured the severity of osteoporosis. After multivariate weighted logistic regression model analysis of the novel inflammatory markers and osteoporosis index, AIRI, SIRI, and SII were significantly correlated with the osteoporosis indices. There may be a close relationship between inflammation and senile osteoporosis. The novel inflammatory markers are convenient and objective for predicting low BMD or osteoporosis risk in older patients. Among these markers, elderly patients with high levels of AISI, SIRI, and SII should focus on the risk of osteoporosis. However, this study has some limitations. It is essential to expand the sample size to a wider population to investigate the relationship between inflammation and osteoporosis.
Similar content being viewed by others
Introduction
Osteoporosis, characterized by diminished bone mass and compromised bone microarchitecture, represents the widespread skeletal disorder that significantly elevates the risk of fractures, particularly in the geriatric population. The global burden of osteoporosis is significant, impacting approximately 200 million individuals worldwide1,2,3. It compromises patients’ life quality and imposes a substantial economic strain on healthcare systems4,5. Among the various manifestations of osteoporosis, vertebral fractures are significantly prevalent6,7,8.
Osteoporosis is diagnosed through dual-energy X-ray absorptiometry (DXA)9. This technique contributes to measuring bone mineral content (BMC), bone mineral density (BMD), bone area, and other relevant parameters10,11. To assess the presence of osteoporosis in the spine, DXA can also be used to measure the spinal BMD, spinal BMC, and spinal area12.
The novel immunoinflammatory markers, including Aggregate Index of Systemic Inflammation (AISI), Systemic Inflammatory Response Index (SIRI), Neutrophil-to-Lymphocyte Ratio (NLR), System Immune Inflammation Index (SII), Platelet-to-Lymphocyte Ratio (PLR), Monocyte-to-Lymphocyte Ratio (MLR), and Neutrophil Percentage Adjusted Ratio (NPAR) serve as the new indices based on lymphocyte, neutrophil cell, monocyte, platelet, and albumin counts. A growing body of evidence shows that these novel inflammatory markers can well indicate the body’s inflammatory and immune state and predict different diseases13,14,15,16. Recently, inflammation has aroused wide attention owing to its impact on osteoporosis pathogenesis. Systemic inflammatory and immune statuses are closely related to osteoporosis, probably because immune cells, directly and indirectly, affect bone cell physiological processes17,18,19,20,21,22. Studies have also indicated that chronic inflammation causes bone loss by inducing osteoclast activation and inhibiting osteoblasts23.
According to the established theoretical framework, this study aimed to explore the association of novel inflammatory indices (AISI, SIRI, SII, NLR, PLR, MLR, and NPAR) with osteoporosis. By evaluating the relationships between total spinal osteoporosis indices (including TS-BMD, TS-BMC, and TS-area) and osteoporosis status, these markers were used to quantify the osteoporosis status. This approach contributes to elucidating the connection between novel inflammatory markers and osteoporosis. Therefore, this study offers insights into the interplay between inflammation and osteoporosis, paving the way for developing strategies aiming at the prevention of spinal osteoporosis.
Materials and methods
Study subjects
Subject data were collected according to the National Health and Nutrition Examination Survey (NHANES) to evaluate health and nutritional status among the general US population through the cross-sectional study. The NHANES is conducted by the Centers for Disease Control and Prevention (USA) and is updated every two years. The NHANES keeps on going, providing precious health-related information for the adult and pediatric US populations. It includes representative U.S. population samples with the stratified, multi-stage probability design and is conducted every two years. NHANES interview covers questions regarding demographics, socioeconomics, health, and diet-associated aspects. Examinations include physiological, dental, and medical measurements as well as laboratory tests performed in the hands of trained medical staff. Data acquisition was conducted by structured interviews with individuals at home, by sample tests in the laboratory, and by health screening at mobile examination centers24. The NHANES 1999–2018 data were obtained in this study. All the participants were recruited. Subjects below were excluded: (i) participants with no complete data on osteoporosis indices (TS-BMD, TS-BMC, TS-Area), (ii) participants who did not respond to the survey (ever told had osteoporosis/brittle bones), (iii) participants with missing indices of interest for this study (lymphocyte number, monocyte number, neutrophils number, platelet count, and albumin), (iv) participants who were not the elderly patients (≤ 60 and ≤ 55 years for men and women, respectively), and (v) participants with missing data on variables associated with this study. Subjects enrolled in the present work offered informed consent. Our study was approved by the ethics review board of the National Center for Health Statistics24.
Osteoporosis status definition
Osteoporosis status was defined according to a self-reported health questionnaire. In any case, the answer of “yes” to the question “Ever told had osteoporosis/brittle bones” was included in the osteoporosis group (OP group); otherwise, it was included in the non-osteoporosis group (NOP group). Detailed information on the self-reported reproductive health questionnaire can be obtained from the National Health and Social Services website25.
Novel inflammatory markers
Recent advancements in the field of inflammatory marker research have introduced several novel indices to better characterize inflammatory processes, including AISI, SIRI, SII, PLR, MLR, NLR, and NPAR, according to previous studies21,22,26,27. Figure 1 shows the distribution of the difference in novel inflammatory markers for OP compared with NOP groups. All indices were determined based on the complete blood count experimental and biochemical results. Complete blood count tests and biochemical methodologies can be obtained from the NHANES website28, where monocyte, neutrophil, lymphocyte, and platelet counts are measured in 109 cells/L. A regression analysis of the novel inflammatory markers and the osteoporosis index was conducted.
Distribution of novel inflammatory markers among osteoporosis participants included in the final analysis. SIRI, systemic inflammation response index; AISI, The Aggregate Index of Systemic Inflammation; SII, systemic immune-inflammation index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; NPAR, neutrophil-to-albumin ratio. 0 means the NOP group, and 1 means the OP group.
Osteoporosis indices
TS-BMD refers to total spine bone mineral density; TS-BMC indicates total spine bone mineral content; and TS-Area represents total spine bone area. In Table 1, TS-BMD, TS-BMC, and TS-Area had significant differences among people with or without osteoporosis (P < 0.001). Therefore, in this study, TS-BMD, TS-BMC, and TS-Area were employed to mark the osteoporosis index for quantifying osteoporosis.
Covariates
Considering that additional factors may influence osteoporosis indices, covariates were recruited for analyses. Covariates were selected from the NHANES database according to prior literature29,30. Age, gender, height, weight, body mass index (BMI), annual household income, education level, smoke status, alcohol consumption, diabetes, high-density lipoprotein (HDL), alanine transaminase (ALT), aspartate transaminase (AST), blood calcium, cholesterol total, triglycerides, albumin, protein total, white blood cell, red blood cell, platelet count, and hemoglobin were identified as candidate covariates. More details are presented in Table 1.
Statistical analysis
Analysis was conducted on subjects with sufficient data. Therefore, those who had missing covariates were eliminated from the analyses. Basic characterstics were expressed as weighted proportion (categorical variables, analyzed by weighted chi-square tests) and weighted mean and standard error (SE) (continuous variables, analyzed by weighted t-tests). Weights utilized in the analysis were selected concerning the NHANES database31. As a result, the mobile examination center (MEC) exam weight (WTMEC2YR) was used in the analysis since certain variables in this study were obtained from MEC. Associations between osteoporosis indices and osteoporosis status (osteoporosis vs. Non-osteoporosis) were evaluated with multivariable weighted logistic regression. The same multivariable weighted linear regression method was used after classifying the independent variables according to the order of 4 values (Q1-Q4). This method improves the authenticity of the osteoporosis index to quantify osteoporosis. Associations of the novel inflammatory markers with osteoporosis indices were analyzed with multivariable weighted linear regression models. We also carried out the same analysis method after classifying 4 values according to the value of the independent variable, aiming to determine the interval with the most significant correlation. All examinations were performed in Stata/SE 18.0 or R software 4.0.3. P < 0.05 (two-sided) represented statistical significance.
Results
Basic characteristics
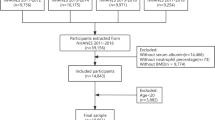
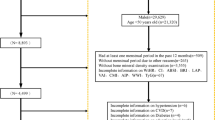
Figure 2 presents the patient screening process. Data for 101,1316 people registered in the NHANES database were collected from 1999 to 2018. The information of 101,1316 participants was obtained based on NHANES (1999–2000: N = 9965; 2001–2002: N = 11039; 2003–2004: N = 10122; 2005–2006: N = 10348; 2007–2008: N = 10149; 2009–2010: N = 10537; 2011–2012: N = 9756; 2013–2014: N = 10175; 2015–2016: N = 9971; 2017–2018: N = 9254). Participants with missing information on the osteoporosis indices (TS-BMD, TS-BMC, TS- area; N = 80495) were eliminated. Participants who did not respond to the survey were excluded (Ever told had osteoporosis or brittle bone; N = 7699). Participants with missing indices of interest (Lymphocyte number, Monocyte number, Neutrophils number, Platelet count, Albumin; N = 523) were excluded. Participants who were older than the age limit required for the study were excluded (≤ 60 and ≤ 55 years for men and women, respectively; N = 8611). Participants with missing data on variables in this study were excluded (N = 3149). Following the eventual selection, a total of 837 diabetics satisfying the eligibility criteria were recruited, including 9.8% (82/837) who had osteoporosis (Fig. 2; Table 2).
Table 2 displays basic characteristics. The weighted average age for osteoporosis participants enrolled in this study was 68.28 ± 7.60 years, the average weight was 79.21 ± 16.59 kg, the average height was 168.02 ± 9.49 cm, and the average BMI was 28.01 ± 5.22 kg/m2. The mean TS-BMD, TS-BMC, and TS-area were 1.02 ± 0.18 g/cm2, 65.68 ± 17.61 g, 63.44 ± 8.93 cm2, respectively. This study analyzed the novel inflammatory markers in OP compared with NOP groups using a violin plot (Fig. 3).
Differences in the novel inflammatory markers in osteoporosis participants enrolled for eventual analysis. SIRI, systemic inflammation response index = Monocyte number × Segmented neutrophils number / Lymphocyte number. AISI, The Aggregate Index of Systemic Inflammation = Platelet count × Monocyte number × Segmented neutrophils number / Lymphocyte number. SII, systemic immune-inflammation index = Platelet count × Segmented neutrophils number/Lymphocyte number. NLR, neutrophil-to-lymphocyte ratio = Segmented neutrophils number / Lymphocyte number. PLR, platelet-to-lymphocyte ratio = Platelet count / Lymphocyte number. MLR, monocyte-to-lymphocyte ratio = Monocyte number/Lymphocyte number. NPAR, neutrophil-to-albumin ratio = Segmented neutrophils number / Albumin. Bold fonts stand for P < 0.05.
Associations of osteoporosis indices (TS-BMD, TS-BMC, TS-Area) with osteoporosis
All cases were classified as OP and NOP groups based on whether they developed osteoporosis or not, and the data were again correlated. We observed a significant correlation between osteoporosis indices (TS-BMD, TS-BMC, TS-Area) and whether they had osteoporosis or not, as listed in Table 1 and shown in Fig. 4. Therefore, this study was chosen to further explore whether the osteoporosis indices indicated osteoporosis. Table 3 displays the associations of osteoporosis indices with osteoporosis. After not adjusting for any covariate (Model 1), TS-BMD, TS-BMC, and TS-Area showed a significant association (P < 0.05) with outcome (osteoporosis or not). This relationship was equally significant between Q1 to Q4. After adjusting for age, gender, and BMI (Model 2), TS-BMD, and TS-BMC remained significantly correlated with outcome, while TS-Area did not suggest a correlation with outcome. Subsequently, after we categorized the dependent variables again between Q1 to Q4, TS-BMD showed a close relationship to the outcome in groups Q3 and Q4, TS-BMC exhibited a significant correlation with the outcome in groups Q2 and Q3, and TS-Area was significantly related to the outcome in Q2. After adjusting for all covariates (Model 3), TS-BMD was significantly associated with the outcome in groups Q2, Q3, and Q4, TS-BMC showed a significant relationship to the outcome in group Q2, while TS-Area was significantly associated with the outcome in groups Q2 and Q4. The results from the above analyses and the analyses after grouping the dependent variables suggested a significant correlation between the osteoporosis indices (TS-BMD, TS-BMC, TS-Area) and outcome. Therefore, in the following analyses, the osteoporosis index was used to quantify and replace the outcome (osteoporosis or not).
Association between osteoporosis indices (TS-BMD, TS-BMC, TS-Area) and novel inflammatory markers
The associations of the osteoporosis indices with inflammatory markers are listed in Table 1. In the analysis of the inflammatory marker AISI and outcome, AISI had a close relationship to the Q3 group of TS-BMD (Model1 P = 0.004, Modle 2 P < 0.001, Model 3 P = 0.001), and the Q3 group of TS-BMC (Modle 2 P = 0.010, Model 3 P = 0.025). SIRI was significantly associated with the Q3 group of TS-BMD (Modle 2 P = 0.002, Model 3 P = 0.003), Q4 group of TS-BMD (Model1 P = 0.044, Modle 2 P = 0.002, Model 3 P = 0.002), the Q3 group of TS-BMC (Modle 2 P = 0.039, Model 3 P = 0.042), Q3 group of TS-Area (Modle 1 P = 0.014), and Q4 group of TS-Area (Modle 1 P < 0.001). SIRI was significantly correlated with the Q3 group of TS-BMD (Model 1 P = 0.006, Modle 2 P = 0.003, Model 3 P = 0.005), and Q4 group of TS-BMD (Modle 1 P = 0.021, Model 2 P = 0.042). NLR was significantly associated with the Q2 group of TS-BMD (Model 1 P = 0.037, Modle 2 P = 0.025, Model 3 P = 0.030), Q3 group of TS-BMD (Modle 2 P = 0.009, Model 3 P = 0.014), Q4 group of TS-BMD (Modle 2 P = 0.003, Model 3 P = 0.002), Q3 group of TS-Area (Modle 1 P = 0.016), and Q4 group of TS-Area (Modle 1 P = 0.001). MLR was significantly related to the Q2 group of TS-BMD (Model 1 P = 0.013, Modle 2 P = 0.004, Model 3 P = 0.003), Q3 group of TS-BMD (Modle 2 P = 0.022, Model 3 P = 0.007), Q4 group of TS-BMC (Modle 1 P < 0.001), Q3 group of TS-Area (Modle 1 P = 0.003), and Q4 group of TS-Area (Modle 1 P < 0.001, Modle 2 P = 0.017, Model 3 P = 0.034). PLR was significantly associated with the Q4 group of TS-Area (Modle 2 P = 0.046, Model 3 P = 0.045). NPAR was significantly correlated with the Q3 group of TS-BMD (Modle 2 P = 0.004, Model 3 P = 0.004), Q4 group of TS-BMD (Modle 2 P = 0.005, Model 3 P = 0.008), and Q3 group of TS-BMC (Modle 2 P = 0.033, Model 3 P = 0.028).
Discussion
The interplay between the inflammatory system and osteoporosis has been a subject of considerable interest, with recent research underscoring that systemic inflammation may be involved in osteoporosis pathogenesis27,32,33,34,35. Inflammatory markers have emerged as a significant predictor of various outcomes in patients with osteoporosis. Various novel inflammatory markers have been vital prognostic indices of osteoporosis patients.
An increasing number of evidence underscores the intimate link between chronic inflammation and bone remodeling, a connection that may be impacted by age-related oxidative stress and immune system activation22. As individuals age, the immune system tends to remain in a persistent subclinical inflammatory state, which can affect T and B lymphocytes to varying extents36,37. This disruption can upset the balance between inflammatory factors and protective immunity elements which are crucial for bone metabolism.
Moreover, the research performed by Monaco et al. has revealed a significant positive correlation between total lymphocyte count and femoral bone mineral density (BMD) in healthy postmenopausal women38. Importantly, this assessment requires only peripheral blood samples, causing minimal participant discomfort. Thus, these novel inflammatory indices have drawn considerable attention from researchers39.
Interleukin-1 (IL-1), Interleukin-9 (IL-9), Tumor Necrosis Factor-alpha (TNF-α), and other inflammatory mediators have been found to promote osteoclast differentiation or inhibit the osteogenic differentiation of bone marrow mesenchymal stem cells (MSCs). These effects occur by activating the RANKL/RANK/OPG pathway and the Wnt signaling pathway40,41.
Compared with previous studies, this study has the corresponding advantages. At first, we studied the relationships between osteoporosis index and novel inflammatory markers among old spine osteoporosis patients in the USA and provided new evidence. Secondly, seven novel inflammatory markers (AISI, SIRI, SII, NLR, PLR, MLR, NPAR) were evaluated to reflect more fully the relationship between osteoporosis and inflammation. These seven different markers provide a comprehensive overview of our inflammatory system and a more comprehensive assessment of the relationship between the two. Subgroup analyses were performed. The possible association of the osteoporosis index with the inflammatory markers can be clarified through subgroup analysis.
Firstly, in the initial research phase, significant differences were observed in the osteoporosis indices (TS-BMD, TS-BMC, TS-Area) between the two groups (Table 2; Fig. 1, P < 0.001). By multivariable weighted logistic regression, the osteoporosis index was significantly related to whether the patient had osteoporosis (Table 3, P < 0.001). By stratifying the osteoporosis index (Table 3, Q1-Q4), the difference still existed. This suggests that the osteoporosis index can be used to quantify whether osteoporosis exists.
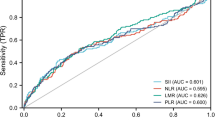
For the following study, a multivariable weighted linear regression of the novel inflammatory markers (AISI, SIRI, SII, NLR, PLR, MLR, NPAR) and the osteoporosis indices was performed (TS-BMD, TS-BMC, TS-Area)(Table 4). We found significant differences in SII and TS-BMD between Models 1 and 2 (Table 4, P = 0.033, = 0.046), whereas no significant differences were observed after adding covariates (Model 3). NLR and Ts-Area showed significant differences among different covariates (P < 0.001, P = 0.008, P = 0.003). The remaining novel inflammatory markers (AISI, SIRI, NLR, PLR, MLR, NPAR) did not differ significantly in the process of increasing or decreasing covariates. After classifying the novel inflammatory markers in the order of 4 values (Q1-Q4), weighted multifactor linear regression analysis was conducted with the osteoporosis indices (TS-BMD, TS-BMC, TS-Area) again. Group Q3 of AISI (P = 0.004, P < 0.001, P = 0.001), group Q4 of SIRI (P = 0.044, P = 0.002, P = 0.002), group Q3 of SII (P = 0.006, P = 0.003, P = 0.005), group Q2 of NLR (P = 0.037, P = 0.025, P = 0.030) and group Q2 of MLR (P = 0.013, P = 0.004, P = 0.003) and TS-BMD were significantly different, and the relationship still existed when the covariates were increased or decreased. The Q4 group of MLR (P < 0.001, P = 0.017, P = 0.034) was significantly different from Ts-Area. Moreover, the grouping and outcome of other novel inflammatory markers also revealed significant differences, as detailed in Table 4. AIRI, SIRI, and SII were closely associated with osteoporosis.
The inflammatory system has been previously suggested to be related to osteoporosis. Neutrophils are a key component of the innate immune system42,43. In one study, neutrophils are found to reduce bone mass by inducing the expression of mediators for promoting bone absorption, including Receptor Activator for Nuclear Factor-k B (RANKL) and interleukin 6 (IL-6)44. Lymphocytes exert a dual function in bone metabolism, due to their activities in regulating the release of inflammatory factors to modulate bone formation-resorption balance44,45. The relationship between platelets and osteoporosis needs further investigation. It is suggested that inflammation stimulates platelet activation and promotes osteoclast formation46. Studies have demonstrated that platelets are vital for bone remodeling47. There is a close relationship between monocytes and osteoporosis. CCR6 and RANK distributed onto monocytes serve as the targets for modulating bone resorption in osteoporosis and rheumatoid arthritis48. Monocytes/macrophages can differentiate into osteoclasts for the regulation under appropriate stimulation conditions49. Therefore, the combination of these cytokines to form novel inflammatory markers is crucial to more precisely uncover the association with osteoporosis.
AISI, which integrates measures of NLR, PLR, and additional inflammatory markers, provides a more nuanced view of the inflammatory state than individual markers alone. A study performed by Zhang examined that AISI was vital for identifying subjects at risk of osteoporotic fractures. The results indicated that an elevated AISI was a significant predictor of future fracture risk, which was independent of traditional risk factors. In a study performed by WU, there was a strong relationship between AISI and osteoporosis, sarcopenia, and obesity16. These results suggest that AISI is important as a potential diagnostic and prognostic tool in the management of osteoporosis. By comprehensively assessing systemic inflammation, AISI may enhance our ability to identify patients at risk, monitor disease progression, and tailor therapeutic interventions to mitigate the inflammatory component of osteoporosis35.
SII, determined from neutrophils, lymphocytes, and platelet levels in peripheral blood, has been recognized as the systemic inflammation marker related to a higher fracture and osteoporosis risk. According to Tang et al., SII exhibited a strong relationship to osteoporosis among postmenopausal women27. In the cross-sectional study conducted by Zhang, II was strongly related to patients with postmenopausal osteoporosis38. However, these two studies do not cover the entire population. In another cross-sectional study performed by NI et al., SII was strongly associated with osteoporosis in middle-aged and elderly patients, believing that blood neutrophils, platelets, and lymphocytes were connected to osteoporosis34.
SIRI can be determined as the product of neutrophil/monocyte numbers divided by lymphocyte number. It reflects the balance between pro- and anti-inflammatory cells that are probably vital for the osteoporosis etiology. Chronic inflammation, characterized by increased pro-inflammatory factor levels, has been implicated in bone resorption and reduced bone formation, causing bone loss33. In a study carried out by YIN et al., there was an association between SIRI and bone turnover markers, which might indirectly influence the development of osteoporosis32.
Although many significant correlations were observed in this study, there are still many limitations. Firstly, this cross-sectional study could not determine the causality of these types of novel inflammatory markers (AIRI, SIRI, SII, ) and spine osteoporosis indices (TS-BMD, TS-BMC, TS-Area). Secondly, a weighted analysis was conducted, while the remaining sample size after screening was small. Therefore, more large-scale prospective studies are needed to confirm our results. Thirdly, in addition to the test data, some self-reported questionnaire information was included. This type of information is often subjective and may not accurately reflect the actual situation, causing biased results. Fourthly, the population information in the NHANES database is primarily from the United States population and does not provide good coverage of populations around the world, making it impossible to identify the inter-ethnic differences. We did not include certain confounders (e.g., C-reactive protein, interleukins, sex hormone levels), since they could not be fully collected from the NHANES database.
Conclusion
To conclude, there is a potentially close relationship between inflammation and senile osteoporosis. The novel inflammatory markers have the advantage of being convenient and objective in predicting low bone mineral density and the risk of osteoporosis in elderly patients. Among these markers, elderly patients with high levels of AIRI, SIRI, and SII should focus on the risk of osteoporosis. However, this study has some limitations. Moreover, we need to expand the sample size to a wider population, aiming to investigate the relationship between inflammation and osteoporosis.
Data availability
The survey data are publicly available on the internet for data users and researchers throughout the world (www.cdc.gov/nchs/nhanes/).
References
Song, S. et al. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol. Ther. 237, 108168 (2022).
Salari, N. et al. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 16 (1), 609 (2021).
Ines, F., Hans, P. D. & Barbara, O.-P. Long-term and sequential treatment for osteoporosis. Nat. Rev. Endocrinol., 19(9). (2023).
Clynes, M. A. et al. The epidemiology of osteoporosis. Br. Med. Bull. 133 (1), 105–117 (2020).
Williams, S. A., Daigle, S. G. & Weiss, R. et al. Economic burden of osteoporosis-related fractures in the US medicare population. Ann. Pharmacother. 55 (7), 821–829 (2021).
Daher, M. et al. Vertebroplasty versus kyphoplasty in the treatment of osteoporotic vertebral compression fractures: A meta-analysis. World Neurosurg. 171, 65–71 (2023).
Ulrich, S. et al. Osteoporotic fractures of the thoracic and lumbar vertebrae: Diagnosis and Conservative treatment. Dtsch. Arztebl Int., 118(40). (2021).
Bassani, J. E., Galich, F. M. & Petracchi, M. G. Osteoporotic vertebral fractures. In Slullitel, P., Rossi, L. & Camino-Willhuber, G (eds) Orthopaedics and trauma: Current concepts and best practices 691–700 (Springer, 2024).
Chen Pong, W., Linsey Utami G. & Le, R. O. Y. C. Dual-energy X-ray absorptiometry bone densitometry and pitfalls in the assessment of osteoporosis: A primer for the practicing clinician. Arch. Osteoporos., 15(1). (2020).
Nicholas, R. F., Charlotte, B. & Olivier, B, et al. Evidence-Based guideline for the management of osteoporosis in men. Nat. Rev. Rheumatol., 20(4). (2024).
Tanphiriyakun, T. & Rojanasthien, S. Bone mineral density response prediction following osteoporosis treatment using machine learning to aid personalized therapy. Sci. Rep. 11 (1), 13811 (2021).
St Jeor, J. D., Jackson, T. J. & Xiong, A. E., et al. Osteoporosis in spine surgery patients: What is the best way to diagnose osteoporosis in this population?. Neurosurg Focus 49(2), E4 (2020).
Jomrich, G. et al. High systemic Immune-Inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann. Surg. 273 (3), 532–541 (2021).
Zheng, Q. et al. Systemic Immune-Inflammation index is associated with increased urinary albumin excretion: A population-based study. Front. Immunol., 13(0). (2022).
Angelo, Z., Panagiotis, P. & Arduino, A. M. Aggregate index of systemic inflammation (AISI), disease severity, and mortality in COVID-19: A systematic review and Meta-Analysis. J. Clin. Med., 12(14). (2023).
Nie, Y. Z., Yan, Z. Q. & Yin, H. et al. Osteosarcopenic obesity and its components-osteoporosis, sarcopenia, and obesity-are associated with blood cell count-derived inflammation indices in older Chinese people. BMC Geriatr. 22 (1), 532 (2022).
Verena, F. & Melanie, H.-L. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin Cell. Dev. Biol., 123(0). (2021).
H E G. Bone and the immune system. Proc. Soc. Exp. Biol. Med., 197(3). (1991).
Zhou-Shan T et al. Protocatechualdehyde inhibits iron overload-induced bone loss by inhibiting inflammation and oxidative stress in senile rats. Int. Immunopharmacol., 141(0). (2024).
Chanhyeok, J. et al. Catechin and flavonoid glycosides from the Ulmus genus: Exploring their nutritional pharmacology and therapeutic potential in osteoporosis and inflammatory conditions. Fitoterapia, 178(0). (2024).
Shuai, C. et al. Association between inflammatory markers and bone mineral density: A cross-sectional study from NHANES 2007–2010. J. Orthop. Surg. Res., 18(1). (2023).
Jian et al. Divergent associations of inflammatory markers with bone turnover markers in elderly patients with osteoporotic fractures. Sci. Rep., 14(1). (2024).
Kurt, R. & Josef, S. S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov, 11(3). (2012).
CDC. nchs research ethics review board. (2022). https://wwwcdcgov/nchs/nhanes/irba98htm
CDC. questionnaire instruments. (2022). https://wwwncdcgov/nchs/nhanes/ContinuousNhanes/Questionnairesaspx?BeginYear=2017
Qin, Z. et al. Systemic Immune-Inflammation index is associated with increased urinary albumin excretion: A population-based study. Front. Immunol. 13, 863640 (2022).
Tang, Y. et al. Systemic immune-inflammation index and bone mineral density in postmenopausal women: A cross-sectional study of the National health and nutrition examination survey (NHANES) 2007–2018. Front. Immunol. 13, 975400 (2022).
CDC. laboratory procedures manual. (2022). https://wwwncdcgov/nchs/data/nhanes/2017-2018/manuals/2017_MEC_Laboratory_Procedures_Manualpdf. 2022.
Compston, J. E., Mcclung, M. R. & Leslie, W. D. Osteoporosis. Lancet 393 (10169), 364–376 (2019).
Leboff, M. S. et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 33 (10), 2049–2102 (2022).
CDC. module 3: Weighting. (2022). https://wwwncdcgov/nchs/nhanes/tutorials/module3aspx
Zhou, P. et al. Association between systemic inflammatory response index and bone turnover markers in Chinese patients with osteoporotic fractures: A retrospective cross-sectional study. Front. Med. (Lausanne). 11, 1404152 (2024).
Ma, H. & Cai, X. Association of systemic inflammatory response index with bone mineral density, osteoporosis, and future fracture risk in elderly hypertensive patients. Postgrad. Med. 136 (4), 406–416 (2024).
Zhang, S. & Ni, W. High systemic immune-inflammation index is relevant to osteoporosis among middle-aged and older people: A cross-sectional study. Immun. Inflamm. Dis. 11 (8), e992 (2023).
Qu, L. et al. Association of inflammatory markers with all-cause mortality and cardiovascular mortality in postmenopausal women with osteoporosis or osteopenia. BMC Womens Health. 23 (1), 487 (2023).
Briot, K. et al. Inflammatory diseases and bone fragility. Osteoporos. Int. 28 (12), 3301–3314 (2017).
Sato, K. et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 203 (12), 2673–2682 (2006).
Du, Y. N. et al. Inverse association between systemic immune-inflammation index and bone mineral density in postmenopausal women. Gynecol. Endocrinol. 37 (7), 650–654 (2021).
Hu, Q. et al. High Neutrophil-to-Lymphocyte ratio and Platelet-to-Lymphocyte ratio are associated with sarcopenia risk in hospitalized renal cell carcinoma patients. Front. Oncol. 11, 736640 (2021).
Ponzetti, M. Updates on osteoimmunology: What’s new on the cross-talk between bone and immune system. Front. Endocrinol. (Lausanne). 10, 236 (2019).
Wu, Y. et al. Effect of bifidobacterium on osteoclasts: TNF-α/NF-κB inflammatory signal pathway-mediated mechanism. Front. Endocrinol. (Lausanne). 14, 1109296 (2023).
Miller, A. P. & Feng, W. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation 110 (12), 1664–1669 (2004).
Pourafshar, S. et al. The effects of supplemental vitamin E on hematological parameters in a rat model of ovarian hormone deficiency. Menopause 25 (3), 336–342 (2018).
Fischer, V. & Haffner-Luntzer, M. Interaction between bone and immune cells: implications for postmenopausal osteoporosis. Semin Cell. Dev. Biol. 123, 14–21 (2022).
Srivastava, R. K., Dar, H. Y. & Mishra, P. Immunoporosis immunology of osteoporosis-role of T cells. Front. Immunol. 9, 657 (2018).
Koupenova, M. et al. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ. Res. 122 (2), 337–351 (2018).
Salamanna, F. et al. Platelet features and derivatives in osteoporosis: A rational and systematic review on the best evidence. Int. J. Mol. Sci., 21(5). (2020).
Nanke, Y. et al. RANK expression and osteoclastogenesis in human monocytes in peripheral blood from rheumatoid arthritis patients. Biomed. Res. Int. 2016, 4874195. (2016).
Jiang, P. et al. CD137 promotes bone metastasis of breast cancer by enhancing the migration and osteoclast differentiation of monocytes/macrophages. Theranostics 9 (10), 2950–2966 (2019).
Acknowledgements
None.
Funding
This work was supported by the Foundation of Guangdong Basic and Applied Basic Research Foundation(2022A1515140151&2023A1515140045&2022A1515140071), Medical Scientific Research Foundation of Guangdong (A2024366) and Huizhou Science Technology Project Foundation (2022CZ010423).
Author information
Authors and Affiliations
Contributions
W.Y., X.H., S.H., G.Z. and J.Z. contributed to conceptualization. W.Y., S.H., G.Z. and J.Z. contributed to methodology. W.Y., S.Z., W.L., C.C., K.H. and G.Z. contributed to software. W.Y., G.Z. and J.Z. contributed to formal analysis. W.Y., S.Z., W.L., G.Z. and J.Z. contributed to data curation. X.L., G.Z. and J.Z. contributed to writing-original draft and writing-review & editing. S.Z. and W.L. contributed to visualization. C.C. contributed to Validation. X.H. and K.G. contributed to writing-review & editing and supervision. X.H. K.G. G.Z. J.Z. contributed to funding acquisition. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by The ethics review board of the National Center for Health Statistics. The patients/participants provided their written informed consent to participate in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yin, W., Li, X., Zheng, S. et al. Association of novel inflammatory markers with osteoporosis index in older spine osteoporosis patients: NHANES 1999–2018 cross-sectional study. Sci Rep 15, 9128 (2025). https://doi.org/10.1038/s41598-025-93378-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-93378-7