Abstract
Autism spectrum disorder (ASD) is a set of heterogeneous neurodevelopmental conditions, the etiology of which remains elusive. Sialic acid (SA) is an essential nutrient for nervous system development, and previous studies reported that the levels of SA were decreased in the blood and saliva of ASD children. However, it is not clear whether SA supplementation can alleviate behavioral problems in autism. We administered SA intervention in the VPA-induced autism model rats, evaluated behavior performance, and measured the levels of Gne and St8sia2 genes, BDNF and anti-GM1. At the same time, untargeted metabolomics was used to characterize the metabolites. It was found that the stereotypical behaviors, social preference and cognitive function were improved after SA supplementation. Additionally, the number of hippocampal neurons was increased, and the shape was normalized. Moreover, 94 differentially abundant metabolites were identified between the high dose SA and VPA groups. These changes in metabolites were correlated with pyrimidine metabolism, lysine degradation metabolism, biosynthesis of amino acids, mineral absorption, protein digestion and absorption, galactose metabolism, phenylalanine, tyrosine and tryptophan biosynthesis and phenylalanine metabolism. In conclusion, SA could ameliorate ASD-like phenotypes and change metabolites in autistic animals, which suggests that it may be a therapeutic approach for ASD.
Similar content being viewed by others

Introduction
Autism spectrum disorder (ASD) is a serious neurodevelopmental disorder characterized by social communication disorders, narrow interest or activity content, and repetitive and stereotypical behavior, but the cause is still unknown1. The prevalence of ASD has increased over the past 20 years, from 1 in 150 to 1 in 36, and it is one of the most common causes of childhood disability2. ASD as a disorder with a complex etiology, is characterized by a diversity of pathogenic causes and significant heterogeneity of clinical phenotypes in children3. Therefore, carrying out basic research on the etiology and behavioral phenotype of ASD will have great theoretical significance and clinical value.
Genome sequencing studies have found more than a thousand susceptibility genes related to ASD. Many genetic variations occur in genes encoding glycosylated extracellular proteins (proteoglycans or glycoproteins) or glycosylation-related enzymes (glycosyltransferases and sulfatyltransferases). Glycosylated proteins are the key components of the extracellular matrix of nerves and participate in a majority of biological processes of brain development4. In clinical studies, it has been found that patients with congenital glycosylation disorders often have ASD-like behavior, which further suggests that abnormal glycosylation may be a risk factor for ASD occurrence5. Therefore, targeting glycoproteins to reveal the relationship between glycosylation and the occurrence of neurodevelopmental disorders can provide a powerful tool for clinical diagnosis and treatment.
Sialic acid (SA) is an essential nutrient for nervous system development and cognition, as well as a key monosaccharide unit for gangliosides and glycoproteins (polysialic nerve cell adhesion molecules) in the brain6. SA often exists in the form of glycans in the development of the nervous system and is involved in the activities of nerve cell adhesion molecules and brain-derived neurotrophic factors7. Damage to the glycan system will affect a large number of molecular activities closely related to normal brain function8. In our previous study, it was found that SA and its glycosylated protein NCAM were underexpressed in ASD children and correlated with their behavioral phenotypes. Meanwhile, the levels of GNE (SA synthetase regulatory gene) and ST8SIA2 (polysialtransferase St8siaII regulatory gene) were also reduced. Collectively, these results indicate that the glycosylation process of SA is related to ASD9,10,11. To better understand the impact of SA on ASD, we examined the effect of SA intervention on behavioral changes in a VPA-induced ASD animal model and the possible mechanism.
Materials and methods
Experimental animals
Specific pathogen free grade healthy adult Wistar rats, including 30 females and 30 males each weighing 220–240 g, were provided by the Animal Laboratory Center of Qiqihar Medical University. The rearing environment was maintained at 22 ± 2 °C, 50 ± 10% humidity, natural circadian variable light, and national standard rat growth and breeding feed. All experiments were approved by the Ethics Committee of Qiqihar Medical University and operated in strict accordance with its relevant regulations (No. QMU-AECC-2021-62).
Establishment of the VPA-induced model rat and SA intervention
Female and male rats were allowed to mate overnight. Pregnancy was determined by the presence of a vaginal plug the next morning, and the embryonic day (E0.5) was defined as noon of that day. The pregnant rats were randomly assigned to VPA (Valproic acid, Sigma Aldrich, St Louis, MO, USA), SA high dose (SAH: 100 mg/kg, ≥ 97% purity, Solarbio, China), SA medium dose (SAM: 50 mg/kg), SA low dose (SAL:25 mg/kg) and control groups. Each group had six pregnant rats, and each rat was kept in a single cage. On E12.5, the pregnant rats in VPA and SA intervention groups received a single intraperitoneal (i.p.) injection of 600 mg kg−1 VPA, and control rats obtained equal volumes of saline. Subsequently, the SA intervention groups were induced by ingestion of SA in the drinking water from E12.5 to postnatal day 21(PND), while the VPA and control groups drank water normally. The SA intervention was performed according to the body weight of the pregnant rats from E12.5 to PND21. We assessed the consumption of water in each pregnant rat, thus ensuring the accurate intake of SA. After PND21, twelve male offspring were selected from each group as subjects, and the SA intervention dose was determined according to the offspring weight. The schematic representation of the experimental procedure is shown in Fig. 1.
Marble burial test
A clean cage was prepared with 5 cm of fresh wood chip bedding material. On PND 35, a rat was placed in the cage for 15 min for habituation and then returned to their home cage. This rat was reintroduced onto bedding material containing 20 embedded marbles for 30 min, and the number of marbles buried (i.e., covered with wood chips by more than two-thirds volume) was recorded12.
Self-grooming behavior test
On PND 36, the single rat was placed into a white cage to habituate for 5 min. Then, self-grooming behavior was recorded for 10 min. The time spent by the animals in self-grooming included wiping the nose, face, ears, or head with the help of forepaws and licking the body, tail, or anogenital area.
Open field test
On PND 37, the rats were transported to the testing room and left in their home cages for 1 h before the test. To start each session, a rat was placed in a particular corner of the arena and allowed to explore for 5 min. For the tests, every rat was individually placed in the center to initiate a 10 min test. The total distance moved and resting time were used as indicators of locomotor activity.
Three-chamber social assay
On PND 38, the three-chamber test was used to evaluate the social behavior of rats by an autotracking camera system (YH-TB, YiHong, China). The experimental procedure was performed strictly according to the previous literature13,14. It included two successive tests (sociability and social preference tests). The sociability index was calculated as the ratio of time spent exploring stranger 1 over the empty cage. The social preference index was calculated as the ratio of the time spent exploring stranger 2 over that spent exploring stranger 1.
Morris water maze test
The test included four training trials (one per day) and one exploration trial. The mean escape latency on Days 1–4 was used to assess learning ability. On Day 5, each rat performed a 60 s swimming probe trial without the platform. The swimming time in the quadrant of the platform, the latency of first entry to the platform, and the number of times crossing the platform were calculated to assess memory.
Histology of the hippocampus
Hippocampal neuronal damage in the CA1 region was determined by counting the surviving neurons by Nissl staining. After rinsing with deionized water, slices were stained with 0.1% cresyl violet (Beyotime, China) at 37 °C for 20 min. After washing and dehydration, the slides were sealed with neutral balsam, examined under a light microscope, and photographed.
Measurement of anti-GM1 and BDNF levels
Blood samples were collected after behavioral tests, and all the samples were assayed in triplicate. Blood samples were allowed to clot for 2 h at room temperature and then centrifuged at 1000 × g for 15 min. Samples were divided and stored at − 80 °C to avoid protein degradation and repeated freeze–thaw cycles. The sample with hemolysis was removed. Using the anti-GM1 antibodies and BDNF ELISA kits (CUSABIO BIOTECH CO., Ltd), detection used undiluted serum samples, and the levels of BDNF and anti-GM1 were measured in accordance with the manufacturer’s instructions. The microplate reader was set to 450 nm.
RNA extraction and RT‒PCR
Total RNA was extracted from cells using TRIzol® reagent (Ambion®, Life-Technologies) and reverse transcribed using the PrimeScript® RT reagent Kit with gDNA Eraser according to the manufacturer’s instructions (TaKaRa Bio). To evaluate gene expression, cDNA was amplified with SYBR® Select Master Mix using the ABI Prism 7500 System. A total of 2 µg RNA was used for RT. The mixture was incubated for 10 min at 25 °C, then 37 °C for 120 min, and finally 5 min at 85 °C. The qPCR protocol was as follows: denaturation at 95 °C for 10 min, then 40 amplification cycles of 95 °C for 5 s and 60 °C for 60 s. The primer sequences are described in Table 1.
Untargeted metabolomics
Seven brain tissues were randomly selected from the SAH and VPA groups to carry out metabolomics experiments in both positive and negative ion modes. UHPLC‒MS/MS analyses were performed using a Vanquish UHPLC system (Thermo Fisher, Germany) coupled with an Orbitrap Q ExactiveTM HF mass spectrometer (Thermo Fisher, Germany) at Novogene Co., Ltd. (Beijing, China).The raw data files generated by UHPLC-MS/MS were processed using Compound Discoverer 3.1 (CD3.1, Thermo Fisher) to perform peak alignment, peak picking, and quantitation for each metabolite. The normalized data were used to predict the molecular formula based on additive ions, molecular ion peaks and fragment ions. Statistical analyses were performed using the statistical software R (R version R3.4.3),Python (Python 2.7.6 version) and CentOS (CentOS release 6.6).These metabolites were annotated using the KEGG database (https://www.genome.jp/kegg/pathway.html). The metabolites with VIP > 1 and p < 0.05 were considered to be differentially abundant metabolites. The functions of these metabolites and metabolic pathways were studied using the KEGG database.
Statistical analysis
All data are expressed as the mean ± SEM. Statistical analyses were performed using SPSS 18.0 software (SPSS Inc., USA). The results were statistically assessed by one-way ANOVA among different groups. Bonferroni post hoc test was applied for multiple comparisons after one-way ANOVA. The differences in escape latency in the Morris water maze test were analyzed by repeated-measures ANOVA. The statistical significance was set at p < 0.05.
Results
Effects of SA on repetitive and stereotyped behaviors of autistic model rats
One-way ANOVAs revealed statistically significant results among the groups with respect to marbles buried (F = 13.208, p < 0.01). The average number of buried marbles in the VPA group was 13.67 ± 2.15, which was more than that in the control group. The post hoc test confirmed that there were no significant differences in the number of buried marbles between the VPA and SA intervention groups (p > 0.05)(Fig. 2A).
A significant difference was found in stereotyped self-grooming behavior by one-way ANOVA (F = 145.135, p < 0.001). After the post hoc test, it was found that VPA-treated rats spent more time self-grooming than the other rats, and self-grooming behavior was significantly reduced after SAH treatment (VPA vs. SAH: p < 0.001). The difference between controls and other groups was statistically significant (p < 0.01). Thus, a high dose of SA administration could ameliorate the stereotypical behaviors of autism rats (Fig. 2B).
SA improved the locomotor activity of autistic model rats
One-way ANOVA revealed statistically significant results of locomotor activity among the different groups. Significant differences were observed in the distance movement among these groups (F = 3.282, p = 0.017), and the VPA group exhibited hyperlocomotion compared to the SAH and control groups (p < 0.001, Fig. 3A,C). In addition, the rest time of these groups was statistically significant (F = 32.27, p < 0.001). The post hoc test showed that the rest time of the SAH and SAM groups were significantly higher than that of the VPA group (p < 0.001); however, they were lower than that of the control group (p < 0.001, Fig. 3B,C).
Effect of SA intervention on the sociability of autistic model rats
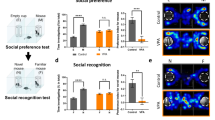
In the sociability test, the time spent in the empty cage compared to that in the unfamiliar rat’s cage was significantly decreased in the SAH and control groups (p < 0.001) (Fig. 4A). Furthermore, the sociability index of these groups was significantly different (F = 96.637, p < 0.001), and compared with the VPA and control groups, the difference in SAH group was significant by post hoc test (p < 0.001) (Fig. 4B,C). However, there was no difference among the SAL, SAM and VPA groups. In the social preference test, the time spent in the cage with the novel object was longer than that spent in the cage with the familiar object in the SAH and control groups (SAH: t = 26.667, p < 0.001; control: t = 54.58, p < 0.001, respectively) (Fig. 4D). Additionally, the social preference index was statistically significant in these groups (F = 204.885, p < 0.001) (Fig. 4E). The post hoc test results demonstrated that each dose in the SAH group might improve the social behavior of VPA rats (p < 0.001). A track plot of the rat activity of each group is shown in Fig. 4C and F.
SA improved the social behaviors of autistic model rats. (A) The time of rats stayed in the chamber. (B) The sociability index of each group. (C) Track plot of the rats’ activity in sociability stage. (D) Time spent in chamber of social preference towards stranger 1 and stranger 2. (E) Social preference index. (F) Track plot of the rats’ activity in social preference stage (n = 12). *p < 0.05.
Effects of SA on learning and memory impairment
The Morris water maze test was used to assess the effects of SA on spatial learning and memory function. In the training trials, the escape latency decreased in all of the groups, and differences were observed among groups during the same day (repeated-measures ANOVA: group effect: F = 19.504, p < 0.001; time effect: F = 94.818, p < 0.001; interaction effect between group and time: F = 2.257, p = 0.023). The post hoc test showed that SAH-treated and control rats exhibited shorter escape latencies than the VPA group (p < 0.001, Fig. 5A and C).
Effects of SA on learning and memory impairment. (A) Representation of the average escape latency in all animals in the different groups for four days. (B) The average passing times of all animals in the different groups. (C) Representative images of the escape latency and the passing times through the platform area (n = 12). *p < 0.05.
In the spatial probe test, the passing times of the platform were statistically significant among these groups (F = 20.652, p < 0.001). The results indicated that VPA-treated rats had difficulty remembering the original platform and presented fewer crossings of the invisible platform than SAH and control rats (p < 0.001). Although high doses of SAH alleviated the memory capacity of VPA model rats, the values were still lower than those of controls (p = 0.042, Fig. 5B and C).
Morphological changes in hippocampal tissue
Nissl staining revealed that VPA-induced rat hippocampal neurons in the CA1 region had a loose distribution, unclear boundaries, cell shrinkage, fewer Nissl bodies, and a lower normal cell number than the CON group. Compared to the VPA group, the SAH and SAM groups exhibited fewer injured neurons, plentiful Nissl bodies, and increased numbers of neuronal cells. However, similar changes were not detected in the SAL group. The differences in neuron number among the groups were statistically significant (F = 66.056, p < 0.05), and the SAH and SAM groups were significantly different from the VPA group, respectively (p < 0.05) (Fig. 6).
Effects of SA supplementation on the histomorphology of the hippocampal CA1 area. (A) Representative photomicrographs of Nissl staining on the hippocampal CA1 area (The bar represents 200 uM). (B) Representative photomicrographs of Nissl staining on the CA1 area (The bar represents 100 uM). (C) Quantification of neurons per visual field in the CA1 area (n = 5). *p < 0.05.
Comparison of BDNF and anti-GM1 levels in the serum from each group
The levels of anti-GM1 and BDNF in the serum of every group of rats were determined by ELISA, and the results are shown in Fig. 7. Through the Kruskal–Wallis test, there were significant differences in the expression levels of BDNF among these groups (H = 52.243, p < 0.001). Further comparisons revealed that the levels in the VPA group were different from those in the SAH, SAM and control groups (p < 0.05, Fig. 7A). In a previous study, we found that anti-GM1 levels increased in the blood of ASD children. In this study, SA intervention did not affect its level in the serum of autism model rats. There were significant differences between the control and the other groups, but no significant differences were observed between the VPA and SA intervention groups (Fig. 7B).
Changes in the expression levels of the Gne and St8sia2 genes
As shown in Fig. 8, there were significant differences in the level of Gne among these groups (F = 105.096, p < 0.001). The expression level of Gne in the SAH group was higher than that in the VPA group (p < 0.001). Additionally, statistically significant differences were observed in the expression levels of St8sia2 among the groups (F = 169.019, p < 0.01), and the St8sia2 levels of the different SA intervention groups were higher than those of the other groups (p < 0.05) (Fig. 8A,B).
The effect of SA on the expression levels of Gne and St8sia2. (A) The expression of Gne gene in these groups. (B) The expression of St8sia2 gene in these groups. (C) Correlation analysis between gene expression levels and behavioral index. (D) Correlation analysis between St8sia2 expression levels and behavioral index (n = 12). *p < 0.05.
Based on previous population experiments, the GNE and st8sia2 genes were found to be associated with behavioral problems in children with ASD. In this study, through Pearson correlation analysis, it was found that the expression level of Gne was positively correlated with rest time in the open field test (r = 0.685, p < 0.01), sociability index (r = 0.541, p < 0.01) and social preference index (r = 0.777, p < 0.01) in the social experiment; however, it was negatively related to distance movement in the open field test (r = − 0.331, p = 0.021). Meanwhile, the expression level of St8sia2 was positively correlated with rest time (r = 0.776, p < 0.01), sociability index (r = 0.501, p < 0.01) and social preference index (r = 0.689, p < 0.01) but negatively correlated with the number of buried marbles (r = − 0.313, p = 0.030) and movement distance (r = − 0.296, p = 0.041) (Fig. 8C,D).
Characteristics of the brain metabolites and KEGG pathways
Untargeted metabolomics was performed to investigate the characteristics of brain tissue metabolites in VPA-exposed offspring. Approximately sixty-five (37 metabolites were upregulated and 28 metabolites were downregulated) and twenty-nine (21 metabolites were upregulated and 8 metabolites were downregulated) metabolites differed significantly between the high dose SA (SAH) and VPA groups in the positive and negative modes, respectively (Fig. 9A,B). Analysis of PCA (Fig. 10A,B) and OPLS-DA (Fig. 10C,D) revealed that the distribution of samples was significantly different, suggesting a significant difference in metabolites between the two groups. These results indicated that the content and composition of metabolites were altered after SA treatment. Major disturbed pathways involved in pyrimidine metabolism, lysine degradation metabolism, biosynthesis of amino acids, mineral absorption, protein digestion and absorption, galactose metabolism, phenylalanine, tyrosine and tryptophan biosynthesis and phenylalanine metabolism.
The correlation between metabolites and autism model rat behaviors
Spearman’s rank correlation analysis was conducted between differentially abundant metabolites and behavioral results of autism model rats (Fig. 11). The number of buried marbles in autistic rats was negatively correlated with D-proline, dimethyl 4-hydroxyisophthalate, dehydrocholic acid, and 3-(4-hydroxyphenyl) propionic acid (p < 0.05 or p < 0.01) and was positively correlated with methyl {[(2-oxo-2H-pyran-6-yl) carbonyl]amino} methanethioate and sinapinic acid (p < 0.05). The morris passing times had a negative correlation with L-leucyl-L-alanine, 2′-deoxyinosine, H-Gly-Pro-OH, adenine, L-tyrosine, N-(1,3-benzodioxol-5-ylmethyl)-6-morpholinonicotinamide and N-acetylneuraminic acid (p < 0.05 or p < 0.01) and had a positive correlation with bilirubin, phenylacetylglycine, 1-methyl-1H-benzimidazole-2-sulfonic acid, and PC (14:0e/2:0) (p < 0.05). The active time of the open field test (OFT) was negatively related to ACar 15:0 and palmitoylcarnitine but positively related to tyrosine (p < 0.05). Meanwhile, OFT distance was negatively correlated with N1-(6-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)-4-nitrobenzamide, Biopterin and 2-(2-methyl-1H-indol-3-yl) acetonitrile and positively correlated with PC (9:0/9:0). We also found that methyl {[(2-oxo-2H-pyran-6-yl) carbonyl]amino} methanethioate and 10-nitrolinoleate had a positive relationship with social index 1. Additionally, social index 2 was positively related to 2,6-di-tert-butyl-1,4-benzoquinone, 2-amino-1,3,4-octadecanetriol, cholic acid and thiamine and negatively related to biotin and aflatoxin G1 (p < 0.05). These results suggested that differentially abundant metabolites were related to social and stereotypical behaviors in autistic rats.
Discussion
Sialic acid is an important nutrient for nervous system development. Although a decrease in SA levels in the blood and saliva of children with ASD has been confirmed in population experiments, whether sialic acid can improve the behavioral symptoms of autism remains unclear9,15. Therefore, in this study, SA was supplemented in the critical period of nervous system development in autistic model rats, and the results demonstrated that SA could alleviate behavioral problems, including abnormal activity, social disorders and cognitive problems. At the same time, it regulated the expression of SA synthetase and transferase regulatory genes and promoted the expression of BDNF in the serum. Nissl staining results indicated that SA intervention could improve the morphology of neurons in autism model rats. More importantly, SA intervention in early life affected the expression of 94 metabolites in the autism rats, which were enriched in multiple metabolic pathways associated with ASD. Based on this study, more comprehensive evidence can be provided for the association between SA and autism.
In our previous study, it was observed that the serum levels of SA in children with ASD were comparatively lower than those in typically developing children9. Additionally, other studies have reported a significant relationship between SA levels and the behavioral phenotype as well as the severity of ASD symptoms in children9,15. Based on the above, this study proposed the hypothesis of whether SA intervention can improve behavioral problems in ASD. The evaluation of behavioral performance in autism rats revealed that SA can ameliorate the core symptoms associated with autism, including stereotyped behavior, social difficulties, and cognitive impairments. One study found that the expression level of sialidase NEU1 mRNA in the peripheral blood of autistic children was positively correlated with their social affect16. Furthermore, SA levels may influence autism-like behaviors, notably stereotypes and hyperactivity in ASD children15. The open field test found that SA intervention could alleviate hyperactivity behavior in autism model rats. In the water maze experiment, the rats after SA intervention showed a shorter incubation period and more times of crossing the virtual platform, which suggests that SA might improve cognitive ability in ASD. According to current evidence, SA supplementation during early life may successfully increase the brain SA content and improve cognition development17,18. Since SA primarily affects the neurological system, it is vital to investigate the molecular biology of brain tissue.
Real-time PCR detection showed that SA treatment promoted the expression of Gen in the hippocampus of autistic model rats. Additionally, it was found that the expression level of Gne was positively correlated with rest time, sociability index and social preference index; however, it was negatively related to distance movement in the open field test. Whole exome sequencing of ASD patients revealed that the GNE gene was correlated with ASD19. Subsequently, the analysis results demonstrated that the expression level of St8sia2 was correlated with locomotor activity and social and stereotyped behaviors. St8sia2 knockout mice showed social disorders, and it is worth noting that SA intervention in early life can promote its expression and alleviate social behavior20. Both a case report and single-nucleotide polymorphism studies suggested that the st8sia2 gene may be a susceptibility gene for ASD21,22,23.
SA binds to BDNF through polymeric chains and affects the activity and function of BDNF24,25,26. A large number of ASD related studies have revealed the association between BDNF and ASD occurrence, behavioral phenotype, and treatment27. These results indicated that SA treatment promoted the expression of BDNF in the serum of VPA model rats. BDNF plays an important role in the survival, differentiation, growth and development of neurons during the development of the central nervous system. Meanwhile, it can also prevent the injury and death of neurons, improve the pathological state of neurons, and promote the regeneration and differentiation of damaged neurons and other biological effects28. Importantly, cognitive impairment is common in ASD children, and BDNF can precisely improve memory and learning function29,30. Therefore, we hypothesized that SA intervention may activate the BDNF signaling pathway and affect the performance of autistic model mice in the water maze experiment. Ganglioside M1 (GM1) is the most abundant ganglioside in the neural membranes and brain, which are engaged in neurotransmission, play an important role in axon-myelin interactions and promote axon regeneration31. Anti-GM1 antibodies have the potential to initiate immunological mechanisms that eventually damage the motor neuron or myelin sheath. They can also have an immediate effect on neuron function, resulting in the characteristic reversible blockage of nerve transmission seen in certain neuropathies32. However, in the current study, SA intervention did not affect the level of anti-GM1 antibody in autism model rats.
It is noteworthy that SA has an impact on the metabolic process of autism model rats, and 94 different metabolites were detected based on untargeted metabolomics. Through enrichment analysis, we found that the differentially abundant metabolites were mainly enriched in pyrimidine metabolism, lysine degradation metabolism, biosynthesis of amino acids, mineral absorption, protein digestion and absorption, galactose metabolism, phenylalanine, tyrosine and tryptophan biosynthesis and phenylalanine metabolism. One study reported that urinary metabolomics of young Italian autistic children supported abnormal pyrimidine metabolism33. Moreover, a metabolomic study of mid-pregnancy maternal serum of autistic children found differentially abundant metabolites in pyrimidine metabolic pathways34. There is evidence linking the incidence and core symptoms of ASD children and certain amino acids, including glutamic acid, glutamine, taurine, gamma-aminobutyric acid (GABA), glycine, tryptophan, and D-serine35,36. In line with this, our study discovered that SA had an impact on the amino acid synthesis pathway in model rats that had been exposed to VPA. Experiments based on clinical samples have confirmed the association between phenylalanine and ASD, and some studies have suggested that gut flora may play a mediating role in the association between them37,38. Interestingly, gastrointestinal problems and the disturbance of the intestinal microbiota have become a hot topic in the field of autism research39. Gastrointestinal function affects the digestion and absorption of protein and the metabolism of galactose and minerals, and SA affects only the above metabolic pathways40,41.
In addition, this study identified a variety of differentially abundant metabolites (such as L-tyrosine, tyrosine, phenylacetylglycine, biopterin, bilirubin, thiamine, and biotin) related to behavioral parameters of ASD. Numerous studies have demonstrated that the turbulence of the dopamine system plays a significant role in the development of ASD and have verified that dopamine levels can influence the behavioral characteristics of individuals with ASD42. L-tyrosine is a precursor of dopamine, and increasing its level can stimulate dopamine secretion. This study discovered that after SA intervention, the level of L-tyrosine in brain tissue rose, implying that it may influence dopamine synthesis in nerve cells. It is worth emphasizing that dopamine release and inhibition can influence social behavior, and the current study discovered that SA improved social issues in autistic model rats43,44. Phenylacetylglycine is a distinct metabolite that is discovered in the urine of children with ASD, according to a multicenter study conducted in China. Additionally, another study indicated that phenylacetylglycine may be a potential marker for ASD children with intestinal permeability issues. Inflammation plays a role in the etiology of autism spectrum disease (ASD). Pteridine metabolites are inflammatory indicators that rise in response to immune system activation. The pteridine metabolites (neopterin and biopterin) in urine served as diagnostic inflammatory markers in ASD patients, according to a large and comprehensive population investigation45. According to a different study, ASD children had higher urine biopterin levels than typical children46. Similarly, elevated levels of this metabolite were also noted in the brain tissue of autistic rats; however, SA intervention markedly decreased biopterin levels. Autism, as a neurodevelopmental disorder, may be one of the causes of autism due to damage to the formation and development of the nervous system. Bilirubinemia is a common condition in newborns. When the level of bilirubin surpasses the relevant threshold, neurotoxicity may result, and high levels of unbound bilirubin cause neuronal death. Bilirubin-induced neurotoxicity also includes cerebellar damage, reduced numbers of Purkinje cells, and disruption of the multisensory feedback loop between the cerebellum and cortical neurons, which may explain the clinical features of autism spectrum disorder47. Our findings showed that SA treatment during neurological development reduced bilirubin levels in autistic model rats, indicating that SA improved the behavioral phenotype of ASD. Thiamine (vitamin B1) is regarded as a spiritual vitamin that has a significant impact on neural tissue and mental state, can improve mental condition, and can eliminate fatigue. When it is deficient, it can induce a range of neuroinflammation48. The brainstem, cerebellum, and hypothalamus are highly sensitive to thiamine deficits, emphasizing that it is a key cause of asymmetric dysautonomia. Some studies have shown that ASD children have lower thiamine levels than normal children; however, SA intervention in animal studies reversed this phenomenon49. Based on the above, it appeared that SA intervention had a significant impact on certain metabolic compounds linked to autism, particularly reversing certain molecules that are under expressed in ASD children. Nevertheless, the role of SA in the body of ASD children is currently unclear and needs further study in the future.
This study had some limitations that must be taken into account in interpreting the results. First, the SA intervention was from E12.5 to PND35. During the period from PND1 to PND21, the autistic model rats took SA in the form of breast milk, and the amount of SA obtained was correlated with their sucking behavior, so the consistency of SA intake could not be guaranteed. Second, this study may lack some information of value due to not having evaluated SA-related enzymes (St8siaII and St8siaIV). Third, the correlation analysis was only conducted among metabolites and the open-field test, social and marble buried experiments, and the metabolites related to behavior need to be verified by further experiments. While the therapeutic use of sialic acid is inviting, extensive research is needed to further evaluate this complex regulatory pathway.
Conclusion
All ASD population trials have demonstrated the association between SA and ASD; however, it has not been documented whether SA can be employed as a viable treatment for ASD. Based on the low expression of SA in ASD children, this study focused on the critical period of neurodevelopment in autistic model rats. The supplementation of SA during this period revealed the remedy effect of SA on behavioral disorders in model rats and the molecular biological changes. In addition, we clarified the effects of SA on metabolites and the enriched metabolic pathways. The association between the differentially abundant metabolites and the behavior index parameters was deciphered using correlation analysis. Current evidence strongly suggests that SA may act as a nutrient to treat ASD and improve its behavioral problems.
Data availability
Data will be made available from the corresponding author on reasonable request.
Abbreviations
- ASD:
-
Autism spectrum disorder
- SA:
-
Sialic acid
- SAH:
-
SA high dose
- SAM:
-
SA medium dose
- SAL:
-
SA low dose
- PND:
-
Postnatal day
- VPA:
-
Valproic acid
References
Hazlett, H. C. et al. Early brain development in infants at high risk for autism spectrum disorder. Nature 542, 348–351 (2017).
Maenner, M. J. et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2020. Morb. Mortal. Wkl. Rep. Surveill. Sum. 72, 1–14 (2023).
Bhandari, R., Paliwal, J. K. & Kuhad, A. Neuropsychopathology of autism spectrum disorder: Complex interplay of genetic, epigenetic, and environmental factors. Adv. Neurobiol. 24, 97–141 (2020).
Paolillo, M. & Schinelli, S. Extracellular matrix alterations in metastatic processes. Int. J. Mol. Sci. 20, 4947 (2019).
Dwyer, C. A. & Esko, J. D. Glycan susceptibility factors in autism spectrum disorders. Mol. Asp. Med. 51, 104–114 (2016).
van Karnebeek, C. et al. NANS-mediated synthesis of sialic acid is required for brain and skeletal development. Nat. Genet. 48, 777–784 (2016).
Sato, C. & Hane, M. Mental disorders and an acidic glycan-from the perspective of polysialic acid (PSA/polySia) and the synthesizing enzyme, ST8SIA2. Glycoconj. J. 35, 353–373 (2018).
Iqbal, S., Ghanimi Fard, M., Everest-Dass, A., Packer, N. H. & Parker, L. M. Understanding cellular glycan surfaces in the central nervous system. Biochem. Soc. Trans. 47(1), 89–100 (2019).
Yang, X. et al. Sialic acid and anti-ganglioside antibody levels in children with autism spectrum disorders. Brain Res. 1678, 273–277 (2018).
Yang, X. et al. The level of GNE and its relationship with behavioral phenotypes in children with autism spectrum disorder. Medicine 99, e21013 (2020).
Yang, X. et al. The association between NCAM1 levels and behavioral phenotypes in children with autism spectrum disorder. Behav. Brain Res. 359, 234–238 (2019).
Mansouri, M., Pouretemad, H., Roghani, M., Wegener, G. & Ardalan, M. Autistic-like behaviours and associated brain structural plasticity are modulated by oxytocin in maternally separated rats. Behav. Brain Res. 393, 112756 (2020).
Singla, R. et al. Inhibition of the ERK1/2 phosphorylation by dextromethorphan protects against core autistic symptoms in VPA induced autistic rats. In silico and in vivo drug repurposition study. ACS Chem. Neurosci. 12, 1749–1767 (2021).
Wu, H. et al. Fingolimod (FTY720) attenuates social deficits, learning and memory impairments, neuronal loss and neuroinflammation in the rat model of autism. Life Sci. 173, 43–54 (2017).
Demirci, E., Guler, Y., Ozmen, S., Canpolat, M. & Kumandas, S. Levels of salivary sialic acid in children with autism spectrum disorder; Could it be related to stereotypes and hyperactivity?. Clin. Psychopharmacol. Neurosci. 17, 415–422 (2019).
Zhang, H. et al. Correlation between sialidase NEU1 mRNA expression changes in autism spectrum disorder. Front. Psychiatry 13, 870374 (2022).
Bian, D., Wang, X., Huang, J., Chen, X. & Li, H. Maternal Neu5Ac supplementation during pregnancy improves offspring learning and memory ability in rats. Front. Nutr. 8, 641027 (2021).
Oliveros, E. et al. Sialic acid and sialylated oligosaccharide supplementation during lactation improves learning and memory in rats. Nutrients 10, 1519 (2018).
Krgovic, D. et al. Impaired neurodevelopmental genes in Slovenian autistic children elucidate the comorbidity of autism with other developmental disorders. Front. Mol. Neurosci. 15, 912671 (2022).
Calandreau, L., Márquez, C., Bisaz, R., Fantin, M. & Sandi, C. Differential impact of polysialyltransferase ST8SiaII and ST8SiaIV knockout on social interaction and aggression. Genes Brain Behav. 9, 958–967 (2010).
Kamien, B. et al. Characterization of a 520 kb deletion on chromosome 15q26.1 including ST8SIA2 in a patient with behavioral disturbance, autism spectrum disorder, and epilepsy. Am. J. Med. Genet. A 164A, 782–788 (2014).
McAuley, E. Z. et al. Identification of sialyltransferase 8B as a generalized susceptibility gene for psychotic and mood disorders on chromosome 15q25-26. PloS one 7, e38172 (2012).
Hane, M., Kitajima, K. & Sato, C. Effects of intronic single nucleotide polymorphisms (iSNPs) of a polysialyltransferase, ST8SIA2 gene found in psychiatric disorders on its gene products. Biochem. Biophys. Res. Commun. 478, 1123–1129 (2016).
Sato, C. & Kitajima, K. Polysialylation and disease. Mol. Asp. Med. 79, 100892 (2021).
Hane, M. et al. Protective effects of polysialic acid on proteolytic cleavage of FGF2 and proBDNF/BDNF. Glycobiology 25, 1112–1124 (2015).
Sato, C. Releasing mechanism of neurotrophic factors via polysialic acid. Vitam. Horm. 104, 89–112 (2017).
Camuso, S., La Rosa, P., Fiorenza, M. T. & Canterini, S. Pleiotropic effects of BDNF on the cerebellum and hippocampus: Implications for neurodevelopmental disorders. Neurobiol. Dis. 163, 105606 (2022).
Wang, C. S., Kavalali, E. T. & Monteggia, L. M. BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell 185, 62–76 (2022).
Shu, C., Green Snyder, L., Shen, Y. & Chung, W. K. Imputing cognitive impairment in SPARK, a large autism cohort. Autism Res. 15, 156–170 (2022).
Sgritta, M. et al. Impaired synaptic plasticity in an animal model of autism exhibiting early hippocampal GABAergic-BDNF/TrkB signaling alterations. iScience 26, 105728 (2023).
Guo, Z. Ganglioside GM1 and the central nervous system. Int. J. Mol. Sci. 24, 9558 (2023).
Nores, G. et al. Anti-GM1 antibodies as a model of the immune response to self-glycans. Biochim. Biophys. Acta 1780, 538–545 (2008).
Gevi, F., Zolla, L., Gabriele, S. & Persico, A. M. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol. Autism 7, 47 (2016).
Ritz, B. & Yan, Q. Untargeted metabolomics screen of mid-pregnancy maternal serum and autism in offspring. Autism Res. 13, 1258–1269 (2020).
Jennings, L. & Basiri, R. Amino acids, B vitamins, and choline may independently and collaboratively influence the incidence and core symptoms of autism spectrum disorder. Nutrients 14, 2896 (2022).
Zheng, H. F., Wang, W. Q., Li, X. M., Rauw, G. & Baker, G. B. Body fluid levels of neuroactive amino acids in autism spectrum disorders: A review of the literature. Amino Acids 49, 57–65 (2017).
Randazzo, M. et al. neuroactive amino acid profile in autism spectrum disorder: Results from a clinical sample. Children (Basel, Switzerland) 10, 412 (2023).
Clayton, T. A. Metabolic differences underlying two distinct rat urinary phenotypes, a suggested role for gut microbial metabolism of phenylalanine and a possible connection to autism. FEBS Lett. 586, 956–961 (2012).
Mehra, A. et al. Gut microbiota and autism spectrum disorder: From pathogenesis to potential therapeutic perspectives. J. Trad. Complement. Med. 13, 135–149 (2023).
Mu, C. et al. Metabolic framework for the improvement of autism spectrum disorders by a modified ketogenic diet: A pilot study. J. Proteome Res. 19, 382–390 (2020).
Indika, N. R. et al. The rationale for vitamin, mineral, and cofactor treatment in the precision medical care of autism spectrum disorder. J. Personal. Med. 13, 252 (2023).
Sahin, K. et al. Therapeutic effects of a novel form of biotin on propionic acid-induced autistic features in rats. Nutrients 14, 1280 (2022).
Leung, K. Molecular Imaging and Contrast Agent Database (MICAD) (National Center for Biotechnology Information, 2004).
Dai, B. et al. Responses and functions of dopamine in nucleus accumbens core during social behaviors. Cell Rep. 40, 111246 (2022).
Oge-Enver, E. et al. Urinary neopterin and biopterin indicate that inflammation has a role in autism spectrum disorder. Metab. Brain Dis. 38, 2645–2651 (2023).
Messahel, S. et al. Urinary levels of neopterin and biopterin in autism. Neurosci. Let. 241, 17–20 (1998).
Cayabyab, R. & Ramanathan, R. High unbound bilirubin for age: A neurotoxin with major effects on the developing brain. Pediatric Res. 85, 183–190 (2019).
Anwar, A. et al. Quantitation of plasma thiamine, related metabolites and plasma protein oxidative damage markers in children with autism spectrum disorder and healthy controls. Free Radic. Res. 50, S85–S90 (2016).
Rashaid, A. H. B. et al. Profiling plasma levels of thiamine and histamine in Jordanian children with autism spectrum disorder (ASD): Potential biomarkers for evaluation of ASD therapies and diet. Nutr. Neurosci. 26, 842–849 (2023).
Acknowledgements
This work was supported by Qiqihar Medical University.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82103869) and the Postdoctoral Research Foundation of Heilongjiang (No. LBH-QY22004).
Author information
Authors and Affiliations
Contributions
X.Y. and H.L.: Conceptualization, Project administration, Writing original manuscript. H. L. and J.L.: statistical analysis. Y.Z. and G.L.: Methodology. H.W.: Methodology and supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All experiments were approved by the Ethics Committee of Qiqihar Medical University and operated in strict accordance with its relevant regulations (No. QMU-AECC-2021–62). Our study was in accordance with ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, X., Wei, H., Li, J. et al. Efficacy of sialic acid supplementation in early life in autism model rats. Sci Rep 15, 8576 (2025). https://doi.org/10.1038/s41598-025-93550-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93550-z