Abstract
We evaluated the clinical and laboratory features of decreased serum calcium (albumin corrected or uncorrected) compared to non-hypocalcemia in SLE patients. Exploring the value of serum calcium in assessing the activity and prognosis of SLE disease. Retrospective analysis and comparison of clinical and laboratory data obtained during the treatment period of SLE patients from 2018 to 2023. Both quantity and titer of serum anti-dsDNA antibodiesin SLE patients with hypocalcemia were significantly increased, and peripheral leucocytes, platelets, complement C3 or C4 reduced, while urinary cast and 24 h urine protein elevated. SLEDAI-2 K, BILAG and PGA have confirmed that SLE patients with decreased serum calcium had stronger disease activity, even without positive titers of anti-dsDNA antibodies. Multivariate analysis showed that the decreased serum calcium (OR, 0.31; 95% CI, 0.11, 0.89; P, 0.030) and positive anti-dsDNA antibodies (OR, 0.13; 95% CI, 0.04, 0.44; P, 0.001) are risk factors for increased disease activity in SLE. The Cox model showed that for newly diagnosed SLE and hypocalcemia patients, the stability time of GCs treatment may be prolonged. With the recovery of total calcium, disease activity and laboratory indicators could improve.SLE patients with decreased serum calcium have stronger disease activity and require longer treatment time for remission. Serum calcium levels may assist in assessing disease activity and predicting prognosis.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is a multi-organ autoimmune disease which severely damaging the normal operation of tissues and cells1,2,3. Correct assessment of disease activity is an important basis for formulating treatment plans and assessing clinical treatment outcomes4,5.
Decreased serum calcium concentration is a common phenomenon in clinical treatment, which has an impact on the immune system. Studies have shown that SLE patients with low serum total calcium levels have an enhanced autoimmune inflammatory response, which negatively correlated with disease activity6. This means that there may be differences in laboratory indicators related to inflammation in SLE patients with hypocalcemia6,7.
Calcium signaling is involved in the differentiation of immune cells and the processes of anti-inflammatory/pro-inflammatory responses. Similarly, it plays an important role as a second messenger in antibody production and cascade reactions8,9,10. The increase in cytoplasmic calcium concentration regulates various cellular activities, typically caused by the opening of intracellular calcium stores and calcium channels (CRACs) in the plasma membrane11,12. It means that the production of autoantibodies also requires the transmission of calcium signals13,14,15,16. Antibodies against double-stranded DNA (anti-dsDNA) have high specificity for diagnostic and prognostic test of SLE3. However, the relationship between disease activity and the levels of these autoantibodies in SLE patients with hypocalcemia is still unclear.
The course of SLE alternates between exacerbation and remission, and glucocorticoids (GCs) are still the preferred treatment drugs. It requires adjusting the level of drug therapy per the results of biomarkers and disease manifestations17,18. It is not common for newly diagnosed SLE patients to have hypocalcemia in clinical practice, and the time to reach a Low Lupus Disease Activity Stateas (LLDAS) may also vary and the influencing factors are not yet clear19. There is not much research on the relationship between blood calcium and GCs, but the proportion of hypocalcemia continues to increase during the treatment process20,21.
Therefore, analyzing the relationship between clinical manifestations, laboratory indicators, and serum calcium will provide new insights into the autoimmune status of SLE patients22. Our research is of great significance for doctors to correctly evaluate the disease activity, formulate treatment plans and judge prognosis.
Materials and methods
Subjects and general information
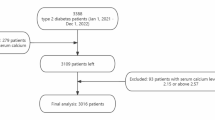
The subjects of this retrospective study were 198 cases of patients with SLE who received treatment at the first hospital of Jilin University from January 2018 to November 2023, including 37 patients were newly diagnosed with SLE. All SLE patients met the classification and diagnostic criteria revised by the American College of Rheumatology (ACR). Serum calcium levels less than 2.20 mmol/L were included in the SLE-L group (N = 99), and the SLE-N group (N = 99) has normal calcium levels. We defined Hypocalcaemia group as 45 patients from SLE-L group after an albumin-adjusted calcium. By considering compliance and comprehensiveness, we ultimately selected 15 patients whose calcium levels recovered within one year to obtain clinical evaluations and laboratory indicators recorded during follow-up. All SLE patients were treated with GCs and other medications do not affect blood calcium levels. Severe osteoporosis patients have been excluded. We also included laboratory indicators from 78 healthy individuals and all healthy people had no cancer, diabetes, thyroid diseases and other autoimmune or inflammatory diseases during the study period. According to the criteria of healthy controls and the diagnosis/exclusion of SLE, we directly obtained the qualified subjects of SLE patients who admitted to hospital and the health examinees during the period of this study.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of The First Hospital of Jilin University approval to carry out the study (Ethical Approved No. 2024 − 636). Confirms that informed consent was obtained from all participants and/or their legal guardians.
Clinical disease activity assessment
Various clinical manifestations (Rash, Alopecia, Arthritis, Mouth ulcers) of 198 patients with SLE were recorded, including the patients’ baseline clinical manifestations and those at regular follow-up visits. Disease activity was assessed using the British Isles Lupus Assessment Group (BILAG), SLE Disease Activity Index (SLEDAI-2 K) and a Physician’s Global Assessment (PGA) at clinical visit time points23,24,25. The BILAG index was used to assess response, and scores were converted to numeric values (A = 9, B = 3, C = 1, D = 0, E = 0) to enable evaluation of fluctuating global summary scores. SLEDAI-2 K > 9 is considered high disease activity. We define LLDAS as the maintenance dose of prednisone ≤ 7.5 mg/d.
Detection of laboratory indicators
Blood routine parameters were measured using Sysmex XN9000 5-classification blood-counter system (Sysmex, Japan) and urine routine tests was measured using Sysmex UF5000 (Sysmex, Japan) for urinary sediment analysis. Serum biochemical parameters were assayed by Beckman Automatic Biochemical Analyzer (Beckman Coulter, USA). Indirect immunofluorescence (IIF) method was used to know the characteristics of serum ANA fluorescence patterns and the titers of serum anti-dsDNA antibodies in SLE patients. The titer of serum ANA (sample initial dilution, 1:100) greater than or equal to 1:100 is considered a positive result, while the positive titer of serum anti-dsDNA antibodies (sample initial dilution, 1:10) is 1:10. ENA were determined with EuroLineMaster immunoblotting assay (Euroimmun, Germany). Detection of anti-β2 glycoprotein I and anti-cardiolipin antibodies using an enzyme-linked immunosorbent assay QUANTA Lyser (Werfen, Spain). All results detected by instruments were manually reviewed. Calcium concentration was corrected for serum albumin concentration using the formula: corrected calcium (mmol/L) = total calcium (mmol/L) + 0.025 × (40 − albumin [g/L]).
Statistical analysis
Data were analyzed and plotted in GraphPad Prism software version 8.0 (GraphPad, USA) and IBM SPSS Statistics for Windows version 20.0 (IBM Corp, USA). Shapiro-Wilk test was used to determine the normality. The Mean ± SD was used for the data of normal distribution, and student t-test was used for comparison among the groups. Median [IQR] was used for the data of non-normal distribution, and Mann–Whitney U test was used. Enumeration data were expressed as rate (%), Chi-square test and logistic regression was adopted. p < 0.05 was considered as a statistically significant.
Results
Comparison of clinical and laboratory index between patients with hypocalcemia and non-hypocalcemia groups in SLE patients.
Significant difference was found in disease activity between the three groups of SLE patients according to SLEDAI-2 K, PGA and BILAG (Table 1). In terms of demography, these patients are not affected by age and gender. However, steroid use and the incidence rate of skin rashes, alopecia and/or arthritis are high. Laboratory activity indicators included leucocytes, complement C3 or C4, urinary cast, 24 h urine proteins and anti-dsDNA antibodies have statistical significance, except platelets. Meanwhile, serum anti-dsDNA antibodies increased quite evident in both quantity and titer, especially high titer levels in hypocalcemia (albumin-corrected) group. For ANA, positive immunofluorescence karyotypes include nuclear speckled pattern (57.1%), nuclear homogeneous pattern (50.0%), and cytoplasmic speckled pattern (21.4%). Nuclear speckled pattern being the most common, followed by nuclear homogeneous pattern. However, compared to the SLE-N group, there was no significant difference in these fluorescence positivity rates, the same with titer (Table S1). All patients with positive ANA were tested for an ENA profile, also no surprising differences except for anti-Sm antibodies and anti-nucleosomes antibodies (Table 1 and Table S2).
Notably, the blood routine parameters, biochemical tests, and immune indicators of SLE patients was significant difference compared with healthy individuals, the changes in the SLE-L group may be more severe (Table S3). Differences in these indicators (lymphocytes, platelets, prealbumin, HDL-C) turned out the same in hypocalcemia (albumin-corrected) group. ACA-IgG and fibrinogen (FBG) may be negatively correlated with low serum calcium levels, rather than prothrombin time (PT), activated partial thromboplastin time (APTT) and thrombin time (TT) (Figure S1). In addition, we also included parathyroid hormone and 1,25 (OH) 2 D3, which did not affect the difference in serum calcium levels between the two groups (Figure S2). These results indicated that SLE patients with decreased serum calcium have stronger disease activity, with more severe laboratory indicators in the blood system, liver, and kidneys. Decreased serum calcium may be more conducive to promoting the autoimmune inflammatory response.
Disease activity and laboratory indicators of SLE patients that serum without positive titers of anti-dsDNA antibodies.
We divided SLE patients with or without positive titers of anti-dsDNA antibodies in their serum into four groups based on total calcium levels (Table 2). Obviously, whether anti-dsDNA antibodies positive or not, the disease activity scores of SLEDAI-2 K, BILAG and PGA were higher in patients with low serum calcium. Disease activity related indicators such as complement C3 or C4, ESR, lymphocytes, erythrocytes, haemoglobin and albumin levels were significantly different between the SLE-L group and the SLE-N group in positive anti-dsDNA antibodies patients. For patients without anti-dsDNA antibodies, the SLE-L group and SLE-N group also showed similar differences, except for complement C3 or C4. This means that patients with low calcium have higher disease activity than those with normal calcium. Clinical manifestations also have a high impact on disease activity scores.
Notably, these indicators were even worse in the the SLE-L group of anti-dsDNA- antibodies than that in the SLE-N group of anti-dsDNA + antibodies. As for the increased urine protein, only in the SLE-N group of anti-dsDNA- antibodies can reduce the incidence of patients. These results indicated that patients with decreased serum calcium showed differences from those with normal calcium levels, and they may have higher disease activity. Even if the anti-dsDNA antibodies is negative, attention should be paid to the serum calcium changes.
Univariate and multivariate analyses of risk factors for SLE activity
Univariate analyses demonstrated that decreased serum calcium (OR, 0.12; 95% CI, 0.06 to 0.24; P, 0.001), positive anti-dsDNA antibodies (OR, 0.15; 95% CI, 0.08 to 0.28; P, 0.001), low complement C3 or C4 (OR, 0.19; 95% CI, 0.10 to 0.35; P, 0.001), urinary cast > 3/LPH (OR, 0.05; 95% CI, 0.02 to 0.15; P, 0.001), increased urine protein (OR, 0.04; 95% CI, 0.02 to 0.09; P, 0.001), positive anti-nucleosomes antibodies (OR, 0.31; 95% CI, 0.17 to 0.57; P, 0.001), increased ESR (OR, 0.18; 95% CI, 0.09 to 0.36; P, 0.001), increased CRP (OR, 0.30; 95% CI, 0.13 to 0.71; P, 0.006), Leukopenia (OR, 0.37; 95% CI, 0.19 to 0.72; P, 0.003), Thrombocytopenia (OR, 0.45; 95% CI, 0.20 to 0.98; P, 0.045) and Belimumab uesd ever (OR, 4.22; 95% CI, 1.19 to 14.93; P, 0.025) were closely related with high diease activity (Table 3).
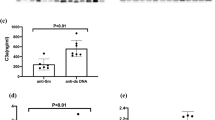
Variables that were significant on univariate analysis were added in the multivariate logistic regression model (Fig. 1). The result showed that decreased serum calcium (OR, 0.31; 95% CI, 0.11, 0.89; P, 0.030), positive anti-dsDNA antibodies (OR, 0.13; 95% CI, 0.04, 0.44; P, 0.001), increased urine protein (OR, 0.05; 95% CI, 0.02, 0.15; P, 0.001), urinary cast > 3/LPH (OR, 0.13; 95% CI, 0.03, 0.56; P, 0.006) and low complement C3 or C4 (OR, 0.24; 95% CI, 0.07, 0.76; P, 0.016) were independent risk factors for SLEDAI > 9. It means that decreased serum calcium may be a promoting factor to high diease activity.
Multivariate analyses of risk factors for SLE activity. Dependent variable = SLEDAI > 9. Belimumab, belimumab used ever. Low complement, low complement C3 or C4. Anti-dsDNA+, positive anti-dsDNA antibodies. Anti-nucleosomes +, positive anti-nucleosomes antibodies. ESR, erythrocyte sedimentation rate. CRP, C-reactive protein. OR, odds ratio. 95% CI = 95% confidence interval.
Dynamic analysis of laboratory indicators and disease activity score during the treatment process of SLE patients.
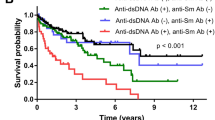
The Kaplan-Meir survival curve comparing the time to first arrival of LLDAS in newly diagnosed SLE treated with GCs between two groups was significant (Mantel-Cox χ2 = 4.471, df = 1, p < 0.05) (Fig. 2). The median time for SLE patients with hypocalcemia to reach their first outcome was 16 months, while the median time for patients with normal calcium levels was 9 months. Obviously, newly diagnosed SLE patients with hypocalcemia have a longer time to reach LLDAS after GCs treatment.
Dynamic analysis of laboratory indicators and disease activity score during the treatment process of SLE patients.
To observe the changes in disease activity of SLE patients after their serum calcium returned to normal during routine treatment, 15 SLE patients were included within one year (Fig. 3). The results showed that with the recovery of serum calcium levels, SLEDAI-2 K and BILAG obviously reduced. In laboratory indicators, the decrease in total IgG and anti-dsDNA antibodies was more significant. At the same time, albumin, complement C3 or C4, leukocytes, lymphocytes, platelets and haemoglobin have all turned around (Figure S3). These indicate that it may help reduce disease activity scores and immune inflammatory responses as serum calcium levels recover.
Two follow-up analyses of 15 SLE patients within one year. Two follow-up visits are represented by 1st and 2nd, respectively. Serum calcium levels increased during the second follow-up after treatment. 15 patients labeled as A-O and each point represents a patient. p < 0.05 was considered as a statistically significant.
Discussion
Three Disease activity assessment methods (SLEDAI-2 K, PGA and BILAG) have confirmed that SLE patients with low serum calcium have stronger disease activity. High disease activity is highly correlated with the occurrence of new injuries, while low disease activity can slowly control the development of chronic injuries26. In this research, indicators improved as serum calcium levels recovered after their one-year follow-up. This means that maintaining normal blood calcium levels may be necessary for the prognosis of SLE patients.
In our data, the incidence of hypocompletemia and the positivity rate of anti-dsDNA antibodies have exceeded half in SLE-L group and even worse in hypocalcemia (albumin-corrected) group.Studies have shown that calcium ions are involved in the development, activation, and differentiation of B cells into effector cells, as well as the production of autoantibodies26,27. And the excessive activation of complement during the inflammatory response of organs28,29,30. A continuous increase in anti-dsDNA antibodies has been shown to be associated with disease progression31,32. However, the ENA’s analysis found that the detection rate of anti-nucleosomes antibodies also relatively higher in SLE patients with uncorrected serum calcium. The possible mechanism is to accelerate progression by administering intact histones or nucleosomes from apoptotic cells, or by acetylating histone peptides after translation33,34. Our research suggests that, decreased serum calcium and anti dsDNA antibodies are independent risk factors for elevated disease activity, which may promote abnormalities in hematological and renal indicators. It means that combining the ANAs with serum calcium levels may better predict the disease activity of SLE.
GCs exert effective anti-inflammatory and immunosuppressive effects in the treatment of SLE patients35. However, newly diagnosed SLE patients with hypocalcemia at the same time require a longer time to reach LLDAS. Studies showed that GCs resistance or insensitivity is a major barrier to the treatment of SLE36,37. GCs receptors and non receptor factors (including P-glycoprotein, Macrophage migration inhibitory factor, Toll-like receptor 9, and Th17 cells) are involved in the resistance mechanism of GCs resistance37. And it has been discovered that STING can participate in the pathogenesis of autoimmune diseases by co occurring with calcium signaling38. This is a research clue for the mechanism of hormone resistance in SLE patients. We are currently conducting this basic research in independent cohort of whether enhanced immune response of SLE patients with low serum calcium related to GCs resistance and molecular mechanism and exploring the reasons and effective treatment methods for these patients.
Our research has several limitations. Firstly, we used a laboratory indicator that includes existing behaviors and demographics to test our hypothesis, which may contain unmeasurable confounding factors. As for the complex relationship between serum calcium, vitamin D and renal function needs further research. Second, our data includes albumin corrected calcium and calcium directly measured in serum, excluding ionized calcium. Calcium ions play an important role in the proliferation, differentiation, and immune processes of immune cells39. Although albumin correction can roughly reflect the level of ionized calcium in the body, the detection of ionized calcium is an important indicator for measuring cellular inflammatory response40. Finally, we found that decreased serum calcium levels affect disease prognosis, and clinical attention should be paid to actively supplementing calcium even if it is non-hypocalcemia after albumin adjusted or there is low DNA antibody titers.
In conclusion, our study suggests that SLE patients with decreased serum calcium have stronger disease activity and require longer treatment time for remission. Serum calcium levels may assist in assessing disease activity and predicting prognosis.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SLE:
-
Systemic lupus erythematosus
- Anti-dsDNA:
-
Anti-double-stranded DNA
- ANAs:
-
Anti-nuclear antibody spectrum
- ANA:
-
Anti-nuclear antibody
- ENA:
-
Anti-extractable nuclear antigen antibody
- GCs:
-
Glucocorticoids
- SLE:
-
Patients with low serum calcium group
- SLE-N:
-
SLE patients with normal serum calcium group
- BILAG:
-
British Isles lupus assessment group
- SLEDAI-2K:
-
SLE disease activity index
- PGA:
-
Physician’s global assessment
- ESR:
-
Erythrocyte sedimentation rate
- hsCRP:
-
Hypersensitive c-reactive protein
- LLDAS:
-
Low lupus disease activity state.
References
Yee, C. S. et al. The BILAG-2004 index is associated with development of new damage in SLE. Rheumatol. (Oxford). 62, 668–675 (2023).
Garcia, L. W. et al. The new EULAR/ACR 2019 SLE classification criteria: a predictor of long-term outcomes. Semin Arthritis Rheum. 57, 152103 (2022).
Tian, J., Zhang, D., Yao, X., Huang, Y. & Lu, Q. Global epidemiology of systemic lupus erythematosus: A comprehensive systematic analysis and modelling study. Ann. Rheum. Dis. 82, 351–356 (2023).
Inês, L. S. et al. What is the best instrument to measure disease activity in SLE – SLE-DAS vs easy BILAG. Autoimmun. Rev. 25, 103428 (2023).
Barber, M., Falasinnu, T., Goldman, R. R. & Clarke, A. E. The global epidemiology of SLE: narrowing the knowledge gaps. Rheumatol. (Oxford). 62, i4–i9 (2023).
Basiglio, C. L. et al. Complement activation and disease: protective effects of hyperbilirubinaemia. Clin. Sci. (Lond). 118, 99–113 (2009).
Wang, D. C. et al. Systemic lupus erythematosus with high disease activity identification based on machine learning. Inflamm. Res. 72, 1909–1918 (2023).
Vaeth, M., Kahlfuss, S. & Feske, S. CRAC channels and calcium signaling in T cell-mediated immunity. Trends Immunol. 41, 878–901 (2020).
Giri, P. S., Bharti, A. H., Begum, R. & Dwivedi, M. Calcium controlled NFATc1 activation enhances suppressive capacity of regulatory T cells isolated from generalized vitiligo patients. Immunology 167, 314–327 (2022).
Wu, B. et al. NKD2 mediates stimulation-dependent ORAI1 trafficking to augment Ca entry in T cells. Cell. Rep. 36, 109603 (2021).
Shen, Y., Thillaiappan, N. B. & Taylor, C. W. The store-operated Ca2+ entry complex comprises a small cluster of STIM1 associated with one Orai1 channel. Proc. Natl. Acad. Sci. U S A. 118, e2010789118 (2021).
Newman, R. & Tolar, P. Chronic calcium signaling in IgE+ B cells limits plasma cell differentiation and survival. Immunity 54, 2756–2771e10 (2021).
Ferri, D. M. et al. Elevated levels of Interferon-α act directly on B cells to breach multiple tolerance mechanisms promoting autoantibody production. Arthritis Rheumatol. 75, 1542–1555 (2023).
Mughales, J. A. Anti-Nuclear antibodies patterns in patients with systemic lupus erythematosus and their correlation with other diagnostic immunological parameters. Front. Immunol. 13, 850759 (2022).
Han, J. et al. Fucosylation of anti-dsDNA IgG1 correlates with disease activity of treatment-naïve systemic lupus erythematosus patients. EBioMedicine 77, 103883 (2022).
Lai, Z. W. et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet 391, 1186–1196 (2018).
Accapezzato, D. et al. Advances in the pathogenesis and treatment of systemic lupus erythematosus. Int. J. Mol. Sci. 24, 6578 (2023).
Coss, S. L. et al. The complement system and human autoimmune diseases. J. Autoimmun. 137, 102979 (2023).
Mathian, A., Arnaud, L. & Irastorza, G. R. Is it safe to withdraw low-dose glucocorticoids in SLE patients in remission. Autoimmun. Rev. 23, 103446 (2024).
Buttgereit, F. & Kvien, T. K. Controversies in rheumatology: maintenance therapy with low-dose glucocorticoids in rheumatoid arthritis. Rheumatol. (Oxford). 62, 35–41 (2022).
Pofi, R., Caratti, G., Ray, D. W. & Tomlinson, J. W. Treating the side effects of exogenous glucocorticoids; can we separate the good from the bad. Endocr. Rev. 44, 975–1011 (2023).
Lou, H., Ling, G. & Cao, X. Autoantibodies in systemic lupus erythematosus: from immunopathology to therapeutic target. J. Autoimmun. 132, 102861 (2022).
Merrill, J. T. et al. Efficacy and safety of rituximab in moderately-to-Severely active systemic lupus erythematosus. Arthritis Rheum. 62, 222–233 (2010).
Oon, S. et al. Lupus low disease activity state (LLDAS) discriminates responders in the BLISS-52 and BLISS-76 phase III trials of Belimumab in systemic lupus erythematosus. Ann. Rheum. Dis. 78, 629–633 (2019).
Parodis, I. et al. Predictors of low disease activity and clinical remission following Belimumab treatment in systemic lupus erythematosus. Rheumatol. (Oxford). 58, 2170–2176 (2019).
Samões, B., Zen, M., Aleixo, J. A., Gatto, M. & Doria, A. Caveats and pitfalls in defining low disease activity in systemic lupus erythematosus. Autoimmun. Rev. 21, 103165 (2022).
Feske, S., Wulff, H. & Skolnik, E. Y. Ion channels in innate and adaptive immunity. Annu. Rev. Immunol. 33, 291–353 (2015).
Wang, Y., Xiao, S., Xia, Y. & Wang, H. The therapeutic strategies for SLE by targeting Anti-dsDNA antibodies. Clin. Rev. Allergy Immunol. 63, 152–165 (2022).
Java, A., Atkinson, J., Hu, Z. & Pozzi, N. Mutations in atypical hemolytic uremic syndrome provide evidence for the role of calcium in complement factor I. Blood 142, 607–610 (2023).
Java, A., Atkinson, J. & Salmon, J. Defective complement inhibitory function predisposes to renal disease. Annu. Rev. Med. 2013:64307–64324 .
Ji, L. et al. Low-dose glucocorticoids withdrawn in systemic lupus erythematosus: a desirable and attainable goal. Rheumatol. (Oxford). 62, 181–189 (2022).
Grajales, C. M. et al. Serological abnormalities that predict progression to systemic autoimmune rheumatic diseases in antinuclear antibody–positive individuals. Rheumatol. (Oxford). 61, 1092–1105 (2022).
Pisetsky, D. S., Spencer, D. M., Rovin, B. & Lipsky, P. E. Role of ANA testing in the classification of patients with systemic lupus erythematosus. Ann. Rheum. Dis. 80, e124 (2021).
Datta, S. K. Harnessing tolerogenic histone peptide epitopes from nucleosomes for selective Down-Regulation of pathogenic autoimmune response in lupus (Past, present, and Future). Front. Immunol. 12, 629807 (2021).
Schaaf, M. & Meijer, O. C. Immune modulations by glucocorticoids: from molecular biology to clinical research. Cells 11, 4032 (2022).
Barnes, P. J. & Adcock, I. M. Glucocorticoid resistance in inflammatory diseases. Lancet 373, 1905–1917 (2009).
Gao, H. et al. Molecular mechanisms of glucocorticoid resistance in systemic lupus erythematosus: A review. Life Sci. 209, 383–387 (2018).
Mathavarajah, S., Salsman, J. & Dellaire, G. An emerging role for calcium signalling in innate and autoimmunity via the cGAS-STING axis. Cytokine Growth Factor. Rev. 50, 43–51. https://doi.org/10.1016/j.cytogfr.2019.04.003 (2019).
Zomot, E., Cohen, H. A., Dagan, I., Militsin, R. & Palty, R. Bidirectional regulation of calcium release-activated calcium (CRAC) channel by SARAF. J. Cell. Biol. 220, e202104007 (2021).
Wang, Z. et al. E4BP4-mediated Inhibition of T follicular helper cell differentiation is compromised in autoimmune diseases. J. Clin. Invest. 130, 3717–3733 (2020).
Acknowledgements
The authors would like to thank staff members at the Department of Clinical Laboratory, The First Hospital of Jilin University for their technical assistance.
Funding
This research project was supported by the 14th Youth Development Fund (JDYY14202329) and Norman Bethune Special Fund (20210101247JC) by The First Hospital of Jilin University, and the Science and Technology Innovation Platform of Jilin Province (YDZJ202202CXJD050).
Author information
Authors and Affiliations
Contributions
XD contributed to the conception and design of the research. Other contributions are XD, YYC and YY for the data management; XD, YY and QZ for the data statistical analysis and interpretation of the data; XD, YYC and XYZ for manuscript draft; JH for the design of the research and manuscript draft. All authors contributed for critical revision of the manuscript, approved the final version of the manuscript prior to submission and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Du, X., Che, Y., Yuan, Y. et al. High disease activity correlate with decreased serum calcium in systemic lupus erythematosus. Sci Rep 15, 9588 (2025). https://doi.org/10.1038/s41598-025-93771-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-93771-2