Abstract
Coronavirus disease-2019 (COVID-19) remains a critical global health concern. We developed a fully automated, high-throughput competition immunoassay to elucidate how epitope recognition on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike receptor-binding domain (RBD) correlates with neutralizing activity. Analysis of clinical samples from both SARS-CoV-2-infected and vaccinated individuals revealed that vaccination elicits significantly higher antibody titers across multiple S1 subunit epitopes compared to natural infection. Notably, median antibody levels against the receptor-binding motif (RBM) exceeded 50% in both cohorts, highlighting the RBM as a key target for antibody induction irrespective of immune origin. Furthermore, the strongest correlation with neutralizing activity was observed for antibodies directed against the broader S1 subunit, indicating that epitopes outside the RBM also contribute to neutralization. These findings underscore the importance of both RBM- and non-RBM-directed antibodies in effective immune defense against SARS-CoV-2. Our assay enables large-scale, reliable quantification of neutralizing antibodies and provides critical insights for developing improved diagnostic antigens and vaccine strategies aimed at eliciting robust, multi-epitope immune responses.
Similar content being viewed by others
Introduction
As of July 2024, the coronavirus disease-2019 (COVID-19) outbreak, declared a global pandemic by the World Health Organization, has reportedly accounted for over 7.54 million deaths worldwide (https://data.who.int/dashboards/covid19/cases)1,2. It is caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), an RNA virus that has undergone rapid evolution, changing its genomic sequence to acquire distinct characteristics such as increased transmissibility, enhanced severity, and ability to evade the immune system3. SARS-CoV-2 mutations resulting in variants with elevated transmission rates compared to earlier variants have been classified as variants of concern (VOCs). Five independent VOCs, namely Alpha, Beta, Gamma, Delta, and Omicron, have been identified to date4.
Vaccines play a crucial role in the combat against COVID-195,6,7. However, vaccines need to be continually modified for sustained protection due to rapid virus evolution, yielding new strains8,9. The fundamental principle of most currently available COVID-19 vaccines is to inhibit the interaction between the virus’s spike protein receptor-binding domain (RBD) and human angiotensin-converting enzyme 2 (ACE2). Therefore, immunogens capable of potently stimulating increased production of neutralizing antibodies (nAbs) against epitopes are urgently needed. Efforts to understand nAb responses to SARS-CoV-2 have significantly accelerated vaccine development10. nAbs are closely linked to the antigen-binding region11. Therefore, elucidating the relationship between the binding region of nAbs and their neutralizing activity is essential to assess their neutralizing potential accurately. However, large-scale clinical sample analysis is complex due to the lack of high-throughput analysis tools12,13.
Various quantitative- or qualitative-serologic assessments have been developed to evaluate antibody responses, each characterized by its sensitivity and performance. Enzyme-linked immunosorbent assays (ELISAs), for instance, are widely used for their high sensitivity and specificity but are limited by their lower throughput and longer processing times. Lateral flow assays (LFAs) offer rapid results and ease of use but often suffer lower sensitivity than ELISAs14. Of note, these serum antibody tests do not necessarily predict the presence of neutralizing antibodies or protection from disease15. Neutralization assays, considered the gold standard, directly assess the ability of antibodies to inhibit viral infection but are labor-intensive and unsuitable for high-throughput screening16. These limitations underscore the need for a high-throughput, automated system to assess neutralizing antibody responses accurately.
The HISCL system is a fully automated chemiluminescent sandwich-based platform that affords high sensitivity, specificity, and reproducibility17. In this study, we extended its capabilities by incorporating a competition assay with two clinically approved monoclonal antibodies, REGN10933 (Casirivimab) and REGN10987 (Imdevimab). These antibodies target complementary, non-overlapping epitopes within the receptor-binding motif (RBM), effectively covering different surfaces of the ACE2-binding interface while minimizing mutual interference (Fig. 1). By applying this system to clinical samples from virus-infected and vaccinated individuals, we aimed to elucidate how specific antibody-binding profiles correlate with neutralizing activity. Assessing naturally acquired versus vaccine-elicited immune responses under real-world conditions provides critical insights into how epitope-specific antibodies influence overall neutralization, thereby informing more effective diagnostic and therapeutic strategies.
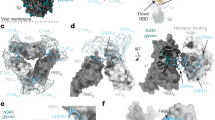
Crystal structure of the SARS-CoV-2 RBD in complex with ACE2 or neutralizing antibodies.The RBD surface is colored to highlight interacting residues identified using Discovery Studio 2022. Two distinct regions, designated as Region A and Region B, represent the non-overlapping epitopes targeted by REGN10933 (Casirivimab) and REGN10987 (Imdevimab), respectively, which cover different sides of the RBM. (A) RBD bound to human ACE2 (PDB 6M0J), with ACE2-interacting residues in orange. (B) RBD bound to two neutralizing antibodies (PDB 6XDG), with interacting residues in green and cyan. (C) Superposition of the complexes, illustrating how these regions are spatially separated.
Materials and methods
Clinical samples and ethics
This study included COVID-19 convalescent individuals18 and COVID-19 vaccinated individuals without a previous history of COVID-19 in earlier studies conducted at the National Center for Global Health and Medicine (NCGM), Tokyo, Japan. (approval nos. NCGM-G-003536 and NCGM-G-004176). This study was conducted under the Declaration of Helsinki and approved by the Medical Research Ethics Committee of NCGM and Sysmex Corporation (approval nos. NCGM-S-004403 [NCGM] and 2021 − 107 [Sysmex]). Informed consent was obtained from all patients. As per the Ethical Guidelines for Medical and Health Research Involving Human Subjects, information on this study has been publicly disclosed, and the participants were allowed to withdraw their consent at any time. Therefore, the ethics committee waived the need for written informed consent from enrolled patients.
In total, 300 clinical samples, consisting of sera from 150 COVID-19 convalescent individuals without any vaccination19,20 and 150 individuals who received 3rd booster-dose of ancestral monovalent BNT162b2 without a primary history of COVID-1921, were collected. Specimens from infected individuals were collected between June 12, 2020, and November 25, 2020. The median (IQR) duration from symptom onset to sample collection was 59 (36–101), except for 18 individuals whose onset date was missing. The convalescent group did not include reinfection cases. Specimens from vaccinated individuals were collected between December 15, 2021, and December 20, 2021. The median (IQR) duration from booster dose (3rd BNT162b2 vaccination) to sample collection was 13 (12–14). The in vitro neutralizing activity cell-based assay using live clinically-isolated SARS-CoV-2 ancestral variant was conducted as previously described as reference test17,19,20.
Production of recombinant antibodies for competition assay
Two antibody structures used in the competition assay were obtained from the Protein Data Bank (PDB). Both have resolved co-crystal structures with the receptor-binding domain (RBD) (PDB ID: 6XDG)22. Based on these structural data, we used the Analyze Protein Surface function in Discovery Studio 2022 to identify residues that interact with the antibodies (Fig. 1). The VH and VL gene sequences were also retrieved from the PDB. We then designed the competing antibodies by grafting each VH and VL sequence onto the constant region of mouse IgG2a (kappa) (accession numbers: P01863 and P01837). Human antibodies were designed as a control by grafting onto a constant region of human IgG1 (kappa) (accession: P0DOX5 and P01834). Each sequence was cloned into a pcDNA3.4 vector (Thermo Fisher Scientific, Waltham, MA, USA) and transfected into Expi293 cells (Thermo Fisher Scientific) according to the manufacturer’s protocol. Six days post-transfection, the supernatants were collected, and recombinant antibodies were purified using the HiTrap Mabselect SuRe column (Cytiva, Marlborough, MA, USA) and Superdex 200 increase 10/300 GL column (Cytiva).
Assay description
SARS-CoV-2 antibody competitive immunoassays were performed using the HISCL system. The HISCL system is a fully automated system based on the chemiluminescent sandwich method. For this assay, serum or plasma samples were mixed with a competitive antibody. This mixture was subsequently incubated with SARS-CoV-2-specific recombinant antigens bound to magnetic beads. After separating the bound and free components, the antigen-antibody complex was incubated with an alkaline phosphatase-conjugated antibody against human IgG to form a sandwich immunocomplex. Following the second separation, a luminescent substrate was added to the solution for luminescence measurements. The entire reaction was carried out at a constant temperature of 42 °C, and the luminescence intensity was measured within 17 min. An overview of this competition-based assay is illustrated in Fig. S2. If sample-derived antibodies bind near the epitope recognized by the competing antibody, the competing antibody blocks their binding, thereby reducing the signal. The antibody titer corresponding to each region was calculated using the following formula:
(titer without competitive antibodies) – (titer with competitive antibodies).
Statistical analyses
All statistical analyses were conducted using R version 4.3.2. Results are expressed as the median (range) and 95% confidence interval (CIs). The ratio of antibody titers in each region to the S1 antibody titer was calculated, limiting to samples with an S1 antibody titer ≥ 20 BAU/mL, and statistical analysis was conducted. Correlation analysis was performed using Spearman’s rank correlation test. Statistical significance was set at p < 0.05.
Results and discussion
We developed a fully automated HISCL system for high-throughput detection of binding site–specific antibodies in patient specimens. By measuring region-specific antibody titers, this system enables detailed analysis of how individual epitopes contribute to the clinical immune response. We defined two distinct regions, designated Region A and Region B, within the RBD of the SARS-CoV-2 spike protein. These regions were determined based on the binding footprints of REGN10933 and REGN10987, respectively, which predominantly target different sides of the RBM (Fig. 1). This design allows separate quantification of antibodies directed against each region, providing deeper insight into epitope-specific immune responses. We verified the competition effect by conducting a competition assay with a human monoclonal IgG antibody sharing the same variable region as the competing antibody, which produced > 80% signal reduction (Fig. S1).
Initially we evaluated region-specific antibody titers in sera from vaccinated and virus-infected individuals (Fig. 2). Overall, the vaccinated group displayed higher antibody titers across all assessed regions than the convalescent group. This result suggests that three vaccine doses cause more efficient antibody induction than antigen exposure due to a natural viral infection.
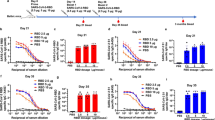
Epitope-specific antibody titers. Antibody titers were calculated as the difference between measurements obtained without and with competitive antibodies. The x-axis displays four regions: S1 (the Spike S1 subunit), Region A + B, Region A, and Region B (Regions A and B are detailed in Fig. 1). (A) Antibody titers in samples from virus-infected individuals. (B) Antibody titers in samples from vaccinated individuals.
Next, we focused on samples with an S1 antibody titer ≥ 20 BAU/mL to assess the distribution of antibodies targeting the RBD (Fig. 3). The median for both virus-infected and vaccinated individuals exceeded 50% in regions A and B (Fig. 3A), indicating that the RBM is a major target for SARS-CoV-2 antibody induction, regardless of the infection or vaccination status.
Ratio of antibody titers targeting defined regions of the S1 subunit. The y-axis in each panel represents the percentage of total S1-targeting antibodies that bind to the indicated region(s). P-values were calculated using the Wilcoxon–Mann–Whitney test with R (***p < 0.001). (A) The ratio of antibody titers targeted at A + B of the S1 subunit. (B) The ratio of antibody titers targeted at A of the S1 subunit. (C) The ratio of antibody titers targeted at B of the S1 subunit.
To evaluate the antibody titer ratio of A + B in S1 and A in S1, we revealed a trend in which infected individuals had significantly higher ratios than vaccinated individuals (Fig. 3B). This suggests that vaccination with the ancestral monovalent BNT162b2, which encodes a stabilized full-length spike protein23, might promote a broader epitope recognition compared to natural infection, resulting in more uniform coverage of both RBM and non-RBM epitopes.
We further investigated the relationship between the activity of nAbs and epitope regions targeted by the antibodies. Spearman’s rank correlation coefficient revealed the strongest correlation between the antibody levels of the S1 subunit and neutralization activity in both the virus-infected and vaccinated individuals (Fig. 4A and B). Interestingly, focusing solely on a narrow antibody-binding domain did not necessarily enhance the correlation with neutralization. While RBM-targeting antibodies directly block ACE2 binding and exhibit potent neutralizing activity11, our findings suggest that non-RBM antibodies may also contribute to neutralization through indirect mechanisms.
Correlation between the antibody titer and neutralizing activity. The red and gray areas indicate the regression lines and 95% confidence intervals, respectively. Spearman’s rank correlation coefficient was calculated using R. (A) Correlations in the infected samples. (B) Correlations in the vaccinated samples.
Although several previous studies have reported such effects24,25,26, our study provides additional evidence from clinical samples, highlighting the importance of targeting multiple epitopes for effective SARS-CoV-2 neutralization. Future studies should investigate (i) cross-neutralization, (ii) targeting the N-terminal domain (NTD), (iii) targeting the S2 subunit, (iv) preventing S1 shedding, (v) steric hindrance, and (vi) preventing conformational changes to further elucidate the roles of non-RBM antibodies. Establishing an antibody screening system using HISCL could facilitate the discovery of such antibodies, contributing to the development of next-generation therapeutics.
Currently, the ACE2 competition assay is the primary platform for determining the neutralizing activity of antibodies in clinical use as competitive virus neutralization tests (cVNTs) against SARS-CoV-227. However, this method is limited to detecting only ACE2-blocking antibodies, overlooking those targeting other key epitopes. In contrast, our system employs two monoclonal antibodies that target complementary, non-overlapping epitopes within the RBM, enabling a more refined characterization of antibody responses. This approach can reveal how specific viral mutations might diminish antibody binding to one epitope while sparing the other, providing valuable insights into immune escape mechanisms. Additionally, using clinically approved antibodies (Casirivimab and Imdevimab) links our results directly to therapeutic strategies, illustrating how serum-derived antibodies may compete with or complement these treatments in real-world scenarios.
In conclusion, we developed a novel high-throughput system using a fully automated immunoassay to detect the antibody epitopes in specimens, focusing on two regions of the SARS-CoV-2 RBD. Clinical sample analysis revealed that the vaccinated individuals exhibited higher antibody titers across all epitope regions than the virus-infected individuals. Our findings suggest that antibodies binding to regions other than RBM also indirectly contribute to the neutralizing activity. Overall, our highly automated immunological assay system can facilitate the design of novel diagnostic antigens tailored to the specific objectives of antibody testing in clinical settings.
Data availability
No publicly available repositories or databases are suitable for the current data submission. All data supporting the results of this study are available in the article. They can also be obtained from the corresponding author, YT, upon reasonable request.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- COVID-19:
-
Coronavirus disease-2019
- nAbs:
-
Neutralizing antibodies
- RBD:
-
Receptor-binding domain
- RBM:
-
Receptor-binding motif
- SARS-CoV-2:
-
Severe acute respiratory syndrome-coronavirus-2
- VOCs:
-
Variants of concern
References
Cucinotta, D., Vanelli, M. W. H. O. & Declares COVID-19 a pandemic. Acta Biomed. 91, 157–160. https://doi.org/10.23750/abm.v91i1.9397 (2020).
Msemburi, W. et al. The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature 613, 130–137. https://doi.org/10.1038/s41586-022-05522-2 (2023).
Markov, P. V. et al. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 21, 361–379. https://doi.org/10.1038/s41579-023-00878-2 (2023).
Carabelli, A. M. et al. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat. Rev. Microbiol. 21, 162–177. https://doi.org/10.1038/s41579-022-00841-7 (2023).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615. https://doi.org/10.1056/NEJMoa2034577 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl. J. Med. 384, 403–416. https://doi.org/10.1056/NEJMoa2035389 (2021).
Voysey, M. et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 397, 881–891. https://doi.org/10.1016/S0140-6736(21)00432-3 (2021).
Dai, L. & Gao, G. F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 21, 73–82. https://doi.org/10.1038/s41577-020-00480-0 (2021).
Altmann, D. M. & Boyton, R. J. COVID-19 vaccination: the road ahead. Science 375, 1127–1132. https://doi.org/10.1126/science.abn1755 (2022).
Senevirathne, T. H., Wekking, D., Swain, J. W. R., Solinas, C. & De Silva, P. COVID-19: from emerging variants to vaccination. Cytokine Growth Factor. Rev. 76, 127–141. https://doi.org/10.1016/j.cytogfr.2023.11.005 (2024).
Chen, Y. et al. Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses. Nat. Rev. Immunol. 23, 189–199. https://doi.org/10.1038/s41577-022-00784-3 (2023).
Atyeo, C. et al. Distinct early serological signatures track with SARS-CoV-2 survival. Immunity 53, 524–532 e524, (2020). https://doi.org/10.1016/j.immuni.2020.07.020
Liu, Y. et al. An infectivity-enhancing site on the SARS-CoV-2 spike protein targeted by antibodies. Cell 184, 3452–3466 e3418, (2021). https://doi.org/10.1016/j.cell.2021.05.032
Michel, M. et al. Evaluating ELISA, immunofluorescence, and lateral flow assay for SARS-CoV-2 serologic assays. Front. Microbiol. 11, 597529. https://doi.org/10.3389/fmicb.2020.597529 (2020).
Iwamoto, N. et al. High diagnostic accuracy of quantitative SARS-CoV-2 spike-binding-IgG assay and correlation with in vitro viral neutralizing activity. Heliyon 10, e24513. https://doi.org/10.1016/j.heliyon.2024.e24513 (2024).
Perera, R. A. et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro. Surveill. 25 https://doi.org/10.2807/1560-7917.ES.2020.25.16.2000421 (2020).
Noda, K. et al. A novel highly quantitative and reproducible assay for the detection of anti-SARS-CoV-2 IgG and IgM antibodies. Sci. Rep. 11, 5198. https://doi.org/10.1038/s41598-021-84387-3 (2021).
Terada, M. et al. How we secured a COVID-19 convalescent plasma procurement scheme in Japan. Transfusion 61, 1998–2007. https://doi.org/10.1111/trf.16541 (2021).
Maeda, K. et al. Neutralization of SARS-CoV-2 with IgG from COVID-19-convalescent plasma. Sci. Rep. 11, 5563. https://doi.org/10.1038/s41598-021-84733-5 (2021).
Tsuchiya, K. et al. Neutralization activity of IgG antibody in COVID–19–convalescent plasma against SARS-CoV-2 variants. Sci. Rep. 13, 1263. https://doi.org/10.1038/s41598-023-28591-3 (2023).
Amano, M. et al. BNT162b2 vaccination elicits markedly high-level SARS-CoV-2-Neutralizing antibodies in vaccinees who responded poorly to a second dose in Japan. J. Infect. Dis. 226, 2038–2039. https://doi.org/10.1093/infdis/jiac209 (2022).
Hansen, J. et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 369, 1010–1014. https://doi.org/10.1126/science.abd0827 (2020).
Lu, M. et al. SARS-CoV-2 prefusion Spike protein stabilized by six rather than two prolines is more potent for inducing antibodies that neutralize viral variants of concern. Proc. Natl. Acad. Sci. U S A. 119, e2110105119. https://doi.org/10.1073/pnas.2110105119 (2022).
Wu, N. C. et al. A natural mutation between SARS-CoV-2 and SARS-CoV determines neutralization by a cross-reactive antibody. PLoS Pathog. 16, e1009089. https://doi.org/10.1371/journal.ppat.1009089 (2020).
Rao, X. et al. Defining a de Novo non-RBM antibody as RBD-8 and its synergistic rescue of immune-evaded antibodies to neutralize Omicron SARS-CoV-2. Proc. Natl. Acad. Sci. U S A. 120, e2314193120. https://doi.org/10.1073/pnas.2314193120 (2023).
Hastie, K. M. et al. Defining variant-resistant epitopes targeted by SARS-CoV-2 antibodies: A global consortium study. Science 374, 472–478. https://doi.org/10.1126/science.abh2315 (2021).
Zhang, S. et al. The spike-ACE2 binding assay: an in vitro platform for evaluating vaccination efficacy and for screening SARS-CoV-2 inhibitors and neutralizing antibodies. J. Immunol. Methods. 503, 113244. https://doi.org/10.1016/j.jim.2022.113244 (2022).
Acknowledgements
We thank Ms. Mariko Kato for her technical assistance with this study. We would also like to thank all patients involved in the clinical trial who provided convalescent plasma samples and the staff at the Diseases Control and Prevention Center, Center for Clinical Sciences, Department of Hematology, Clinical Laboratory Department, Department of Clinical Engineering, and Nursing Department of the National Center for Global Health and Medicine Hospital.
Funding
This work was partly supported by a grant from the Japan Agency for Medical Research and Development [grant number JP20fk0108502] and the Intramural Research Program of the National Center for Global Health and Medicine [grant number 19A3001 to H. Mitsuya]. Additionally, this study was funded by Sysmex Corporation.
Author information
Authors and Affiliations
Contributions
Y.A., H.M., and Y.T. conceptualize the current research. Y.A. and J.K. prepared recombinant antibodies and established HISCL system-based SARS-CoV-2 antibody competitive immunoassay. M.A., K.T., K.M., and Y.T. conducted cell-based viral neutralizing assay. M.T., N.I., and S.S. collected and managed human samples. Y.A., J.K., and Y.T. analyzed and curated data. Y.A., Y.I., and M.Y. wrote the original draft. H.M. and Y.T. reviewed and edited the manuscript. H.M. and M.Y. aquired funding. H.M. Supervised the research. M.Y. administrated the project. All authors reviewed the final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
Y.A., K.J., Y.I., and M.Y. are salaried employees of Sysmex Co., Ltd. H.M. received a research grant from Sysmex Co., Ltd. The other authors have no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Atarashi, Y., Kim, J., Irino, Y. et al. A high-throughput, fully automated competition assay to evaluate SARS-CoV-2 neutralizing responses and epitope specificity in clinical samples. Sci Rep 15, 11589 (2025). https://doi.org/10.1038/s41598-025-94317-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94317-2