Abstract
Intensive care units (ICUs) are acknowledged for their propensity for noise, often exhibiting higher sound levels on average than other departments. This is mainly ascribed to a high concentration of medical devices and staff, creating an acoustic environment characterised by a high level of staff activity and a concoction of alarms from therapeutic and monitoring devices. Excessive noise in ICUs has been associated with adverse health effects and human factor impacts acknowledged to negatively affect both patients and healthcare providers. This study aimed to evaluate the sound levels of the Royal Liverpool University Hospital (RLUH) ICU and compare it against recommended guidelines. Prospective sound level measurements were taken from a six-bedded bay within the RLUH ICU between 15th June and 1st July 2022. This audit focussed on sound data that equal or exceeded 87 dBA, in accordance with levels in the UK Noise Regulations. The data involved 11 patients admitted to the bay within the defined timeframe. A retrospective review of the patients’ records was conducted to identify potential noisy events during the recording period. Results revealed all LAeq and LAmax measurements exceeded the recommended guidelines. Although HSE exposure limit values were not exceeded, the lowest LAmin value recorded was 44.2 dBA and only one hour from 16 days of recording (less than 1% of the time) fell below international daytime guidelines of 45 dBA. The top documented potential causes of noise were patient repositioning/personal care, medication administration and suctioning. Sound levels in the RLUH ICU considerably exceed national and international guidelines. These findings highlight the need to address the issue of noise pollution in the ICU setting. Hospital staff should consider implementing strategies and interventions for noise reduction in ICUs.
Similar content being viewed by others
Introduction
Patients and healthcare staff are consistently exposed to high levels of noise in hospital1. Since the 1960s, there has been a rise in average daytime and night-time sound levels2, which is largely attributed to the increased use of advanced medical technology3. Intensive care units (ICUs) are acknowledged for their propensity for noise, often exhibiting higher average sound levels than other areas within hospital3,4. This is mainly ascribed to a high concentration of medical devices and staff in ICUs, creating a soundscape characterised by a high level of staff activity, background noise, and an acoustic concoction of alarms from therapeutic and monitoring devices3,5.
An increasing body of research on noise pollution has reported associations with short- and long-term adverse effects on health2,6. Previous simulation studies have suggested that medical device alarms alone may not be the source of noise and that critical care itself may be noisy1. Excessive ambient sound levels in the ICU have been acknowledged as a major contributor to sleep disturbance among patients7,8,9. Noise can elicit changes in sleep depth, disrupting the sleep-wake cycle and causing a subjective deterioration in sleep quality10,11. A literature review conducted by Xie et al. suggested that noise may account for up to 76% of sleep disturbance in ICU patients12. However, the key studies included in their review were heterogenous with small sample sizes12. During sleep, excessive noise exposure triggers the inflammatory response and disrupts endothelial function, resulting in oxidative stress that can adversely affect the vasculature of vital organs and may ultimately contribute to the development of various clinical conditions13. Several studies have demonstrated the potential wide-ranging adverse effects of sleep deprivation, encompassing psychological disturbances14, immune system disruption15, an increased incidence of cardiovascular disease16 and respiratory deterioration17,18, including apnoea periods and challenges in weaning patients from ventilatory support19. Importantly, a pertinent consequence in ICU patients is the development of delirium20. Whilst in ICU, between 30% and 75% of patients experience delirium21. Patients who experience delirium have prolonged hospital admissions, higher morbidity and mortality, and may experience ongoing cognitive impairment after discharge21. In a recent meta-analysis the use of earplugs significantly decreased the risk of delirium in ICU patients22. Similarly, Van de Pol et al. found that implementing a noise reduction protocol, which included the use of earplugs, significantly reduced the incidence of delirium among ICU patients23.
In addition to its impact on patients, high noise levels in health settings can have adverse effects on healthcare staff24. Noise is the most commonly encountered performance obstacle among critical care nurses25, can negatively impact work performance26, and increases the likelihood of medical errors27. Empirical evidence has demonstrated a strong correlation between excessive noise and heightened levels of fatigue, irritation, and stress among nurses operating in ICUs28,29,30,31, with excessive noise emerging as a contributing risk factor for occupational burnout28,32. Moreover, alarm fatigue and burnout experienced by critical care staff have the potential to reduce work performance and negatively affect patient outcomes33,34. Song et al. reported that voice strain among ICU nurses correlated with excessive workplace noise35. Beyond the direct implications for staff well-being, research has also raised patient-safety concerns, given that excessive noise has also been shown to lead to miscommunication between staff and hinder concentration when performing tasks36,37,38,39. The cognitive expense of subconscious processing of distracting noise restricts the brain’s capacity to process visual and auditory information40. Self-assessment surveys conducted among ICU staff found significantly higher distraction ratings, higher stress levels, and reduced confidence in performance following noise exposure41.
The World Health Organisation (WHO) suggest A-weighted sound level (dBA) of approximately 50 to 55 dBA during daytime hours and 40 to 45 dBA overnight is acceptable to the average healthy adult6, with some variance in comfort according to individual sensitivity to noise42. Within this range, the majority of individuals would not experience significant adverse health effects or sleep disruption6. However, quantifiable effects of noise on sleep have been observed at equivalent sound pressure levels (LAeq) as low as 30 dBA, with a concomitant peak sound level (LAmax) threshold of 45 dBA6. Table 1 provides a detailed outline of the noise measurement parameters, including dBA and LAeq.
The WHO6, International Noise Council (INC)44, and the United States Environmental Protection Agency (USEPA)45 have all published guidelines pertaining to sound pressure levels in hospitals. Among them, the WHO have set the most stringent threshold standard, with a recommended limit of 35 dBA during the day and 30 dBA at night6. By contrast, the guidelines provided by the INC and USEPA propose slightly higher thresholds44,45. Table 2 provides an overview of the standard LAeq set by each organisation.
Previous research has revealed a consistent upward trend in the already elevated noise levels within ICUs, with an annual increment of 0.38 dBA during the day and 0.42 dBA at night3. Notably, the average diurnal sound levels rose from 57 dBA in 1960 to 72 dBA in 2005, while nocturnal levels increased from 42 dBA to 60 dBA over the same time period3. Consequently, sound levels observed in the ICU persistently surpass the recommended thresholds stipulated by the WHO46,47,48,49. Research conducted in daily clinical practice has shown that average sound levels in the ICU range between 51 dBA to 70 dBA3,50,51,52, which is comparable to the noise levels encountered in heavy traffic6. Additionally, studies have observed peak sound levels in the ICU exceeding 80 dBA49,53,54, generally attributable to the operation of IV infusion pumps, monitor alarms, and ventilators53. A UK study conducted across five adult ICUs demonstrated peak sound levels above 100 dBA occurring 22–28 times per hour52. Peaks exceeding 85 dBA were observed at all sites, up to 16 per hour at night and more frequently throughout the day52. Considering these effects of noise pollution in the ICU for patients and health care staff, an analysis of the sources of high noise levels could allow for increased attention and mitigating strategies.
Aims
The primary aim of this study is to comprehensively assess the sound levels in patient care areas within the ICU at royal liverpool university hospital (RLUH) and ascertain whether the measured sound levels comply with current standards and guidelines. As a secondary outcome, this study aims to evaluate the circumstances in which guidelines are breached.
Methods
This retrospective, observational study was designed to evaluate the noise levels and their potential sources on the ICU department at the RLUH. The study followed an approved audit protocol registered with the Liverpool University Hospitals Foundation Trust (LUHFT) Audit Department (project 11338). The hospital audit and research department determined that additional ethical approval was not required as this was an ongoing service improvement project.
Sound level measurements
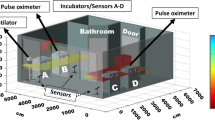
Prospective continuous sound pressure level measurements were taken in one location within a six-bedded bay of patients. Maximum, mean and minimum sound pressure levels were obtained for each one-minute period between the start of recording at 12:52 on 15th June 2022 and 18:25 on 1st July 2022, providing 23,374 min of sound pressure data. Measurements were taken using a Class 1 integrating sound level meter (Brüel & Kjær, Type 2250) calibrated to 94 dB at 1 kHz both before and after the measurements using a sound calibrator (Brüel & Kjær, Type 4230).
The sound level meter was located centrally within the open bay, adjacent to the nurses station but outside any one patient’s bed space and the microphone was positioned 0.5 m above the level of the average patient’s head. It was also placed at least 1 m away from hard surfaces.
As all sound levels were above those recommended by the World Health Organisation (WHO), USEPA and International Noise Council (see Table 2), all the potential causes of noise during the study period were assessed by retrospective review of the patients’ medical records, against pre-defined parameters using a REDCap-based electronic form55.
Patients included in the study were admitted to the designated bay at different stages in their overall ICU admission, ranging from their first to the 84th day. All patients had at least one form of organ support during their stay. Five patients received respiratory support only (45.5%). Among the five patients who required two forms of organ support, four patients received cardiovascular and respiratory support and one patient received renal replacement therapy and respiratory support. Only one patient needed support for three organs. We additionally assessed all 1-minute timepoints where sound pressure ≥ 87 dBA. These specific data were deliberately selected since they surpass the figure used in the national exposure limit value (over 8 h) set by the Health and Safety Executive (HSE) which aims to mitigate health and safety risks associated with noise exposure in occupational settings56. The exposure limit value represents the maximum permissible peak sound pressure that must not be exceeded for any employee according to HSE guidelines56. Although we investigated single time point sound when LAmax ≥ 87 dBA, this did not necessarily correlate with exceeded exposure, and for reassurance in the results, the formula below was used to calculate the actual daily exposure level (LEP) for comparison with the HSE Noise in Work (2005) regulations (upper exposure action values: daily or weekly exposure of 87 dBA, lower exposure value of 80 dBA)56.
\({\text{LEP}}\,=\,{L_{{\text{Aeq}},{\text{T}}}}+{\text{1}}0*\left( {{\text{Te}}/{\text{To}}} \right)\)
Te: the duration of the workday in seconds.
T0: 28,8000 s, representing a standard 8-hour workday.
e.g. If the LAeq is 55 dBA for 8 h, then the LEP is 55 dBA.
Data collection
To identify potential instances of noisy events during their stay, a retrospective review of the patients’ medical records was conducted by the first author. This review involved thorough examination of both handwritten ICU observation charts and Patient Electronic Notes System (PENS), the in-house electronic document management system utilised at the RLUH. For the purpose of data collection, a proforma was developed using research electronic data capture (REDCap) tools hosted at the University of Liverpool55. The synthesis of REDCap forms was informed by existing literature, ensuring their alignment to established evidence9,36,52,57,58. REDCap form A and B (see appendix) collected patient demographic data, and itemised ‘daily’ data respectively. Detailed inclusion and exclusion criteria are provided in Table 3 (also in appendix).
Data analysis
Data is reported using descriptive statistics. The software used for data analysis was Microsoft Excel, (2019) (Microsoft Corporation, USA).
Results
Patient demographic
Within the defined study period, there were a total of 11 patients admitted to the ICU bay. Patient demographic data for this study is summarised in (Table 4).
Patients included in the study were admitted to the designated bay at different stages in their overall ICU admission, ranging from their first to the 84th day. All patients had at least one form of organ support during their stay. Five patients received respiratory support only (45.5%). Among the five patients who required two forms of organ support, four patients received cardiovascular and respiratory support and one patient received renal replacement therapy and respiratory support. Only one patient needed support for three organs.
Sound pressure level measurements
The comprehensive dataset of all the sound level data is depicted in (Figs. 1 and 2). The lowest LAeq over 1 min recorded among all measurements was 46.7 dBA. The corresponding LAmax and LAmin for this recording weres 59.3 and 44.7 dBA, respectively. Additionally, the lowest LAmax recorded in a single minute among all the measurements was 50.6 dBA, with corresponding LAeq and LAmin values of 48.5 and 47.5 dBA, respectively. The lowest LAmin value over a minute recorded amongst the measurements was 44.2 dBA. Notably, only a minority of LAmin values (n = 60, 0.36%, or only 1 h from 16 days) fell below the daytime guideline of 45 dBA set by INC and USEPA.
Figure 3 presents the sound level data of primary interest in this study. This subgroup analysis of the main dataset consists of 168 1-minute recordings where the LAmax value ≥ 87.0 dBA. The highest LAmax and LAmin values recorded within this subgroup were 98.8 dBA and 59.3 dBA respectively. The lowest LAeq value recorded in this subgroup was 63.5 dBA. Conversely, the highest LAeq value observed in this subgroup was 77.9 dBA, with corresponding LAmax and LAmin values of 89.6 dBA and 52.1 dBA, respectively. LAeq values exceeded 65.0 dBA for most timepoints (n = 148, 88%). The mean LAeq from this data was calculated to be 68.6 dBA.
Scatter graph showing the loudest timepoints (LAmax ≥87.0 dBA) with reference lines to show the noise exposure limit recommended threshold daytime LAeq set by the World Health Organisation (WHO),6 international noise council (INC)43 and the United States Environmental Protection Agency (USEPA)44. The red line represents an LAmax of 87 dBA but this does not represent the HSE daily personal exposure level (LEP). See results below for further explanation. LAmax maximum sound level, LAmin minimum sound level, LAeq equivalent continuous sound level, dBA A-weighted sound level.
Potential noisy events in ICU
Among the loudest timepoints (exceeding 87 dBA), multiple sources of noise were identified for most of these louder periods. The most common potential causes of noise were patient repositioning/personal care (identified 277 times across the whole recording time), medication administration (210 times), suctioning (194 times) and procedures (194 times), as shown in (Fig. 4). Notably, most of these procedures involved taking bloods (n = 163) (which can occur during line insertions, routine reviews or a deterioration in patient condition). A noisy subset of procedures involved interventions such as extubation (n = 3), tracheostomy insertion (n = 2) and a singular occurrence of ascitic drain removal.
Figure 5 shows the frequency of the presence or absence of documented, potentially noisy events per hour for all patients within the bay during the defined study period. The frequency of at least one potential noisy event being present exceeded 70% for 18 out of 24 h. During the hours of 23:00 to 02:00 and 03:00 to 06:00, there was a comparatively lower frequency of noisy events present per hour versus daytime hours (06:00 to 21:00). The hours with the noisiest events were 6am, 10am, 6pm, 8pm and 10pm. By contrast, the hour of 1am exhibited the least noisy events (n = 30, 35.7%). Across the study period, there were noisy events in 96.4% of minutes between 6am and 7am. By contrast the hour of 1am exhibited the least noisy events (n = 30, 35.7%).
Cumulative prevalence of noisy events in the RLUH ICU
The aforementioned frequently documented potential causes of noise were patient repositioning/personal care, medication administration, procedures and suctioning. Notable rises in the frequency of patient repositioning/personal care were observed in the hours of 2am (n = 68), 6am (n = 67), and 10pm (n = 65). Similarly, surges in frequency of medication administration occurred at the hours of 6am (n = 54), 6pm (n = 46) and 10pm (n = 42). Monitoring abnormalities were observed throughout each hour with a peak seen at 2pm (n = 11). These abnormalities primarily involved patients’ blood pressure (n = 21) followed by heart rate (n = 15) and saturations (n = 15).
Cumulative personal exposure to noise (HSE guidelines)
Table 5 shows the converted LAeq values to daily personal exposure level (LEP) for the patients in this study across all 14 days of the recording. The highest LEP from the measurement is 69.0 dBA.
Discussion
Sound levels
The results of this audit reveal that sound levels in the RLUH Intensive Care Unit considerably exceeded the recommended guidelines set by the WHO6, INC44 and USEPA45. Patients in the RLUH ICU were continually exposed to a sound level which, at its quietest, was approximately just below conversation level60 and more often comparable to the sound produced by a nearby television60. No LAeq value recorded among the total sound data was compliant with the 35 dBA parameter set by the WHO6.
The findings of this study align with previous research conducted in ICU49,52,61,62,63. The highest LAmax in this study reached 98.8 dBA, which is almost 10 dBA higher than the peak LAmax level observed in a Swedish neurosurgical ICU62 and a Turkish surgical ICU61. 88% of LAeq values in this dataset exceeded 65 dBA, contrasting a study conducted across the Thames Valley, United Kingdom, which involved five ICUs, including four adult ICUs and one neurosurgical ICU, and reported their maximum LAeq as 59.9 dBA52. The observed differences highlight the presence of heterogeneity among ICUs, one possible contributing factor could be variations in specialty care among hospitals, encompassing patient demographics, architectural design, staffing levels, and visiting time restrictions. The LAmax values recorded in this study are all in excess of the WHO guideline of 40 dBA overnight6, by a margin of at least 10 dBA. A 10 dBA increase in sound level corresponds to a doubled sound intensity perceptible to the human ear60. These findings are consistent with observations reported by MacKenzie et al. in their study across two ICUs in Edinburgh46.
The HSE guideline aims to provide hearing protection for workers exposed to extremely high noise levels, such as machinery noise, so the limit value (86 dBA) is set to be very high. Clinicians have anecdotally reported misinterpreting this 86 dBA figure as LAeq or LAmax equivalent, so our findings (highest LEp 69 dBA) may be able to reasuure non-acousticians auditing their own units, that even when sound levels exceed WHO guidance, they fall far below the HSE action level.
Sound sources in the ICU
Previous literature consistently identifies alarms, clinical equipment38,64,65 and staff communication9,38,46,49,66 as prominent sources of noise in ICUs. In this study, patient repositioning/personal care, medication administration and suctioning emerged as the most frequently documented potential causes of noise. In their comprehensive analysis of noise sources in acute care units including ICUs within Edinburgh hospitals, MacKenzie et al.46. reported that while patient repositioning and care can cause bed rail clinking that produces sound levels in the 70 to 80 dBA range, the occurrence rate of this noise source was relatively low, accounting for only 79 occurrences time across their 24-hour study period46. By contrast, they identified talking between staff as one of the top sources of noise, with 486 occurrences over the same timeframe46. Although not specifically cited as an avoidable noise source in their research46, existing literature suggests that staff education can contribute to the reduction of noise levels65,67. For instance, the use of ‘quiet’ signs outside patient-care areas67 and dimming the light level in wards have shown to lead to quieter conversations among staff65. Similarly, Tegnestedt et al. reported 64% of disruptive sounds were caused by monitoring alarms and staff conversations not relating to patient care in their observational study of three rooms in an ICU68. Song et al. found that the most common noise sources in four Chinese ICUs were talking and footsteps49. Moreover, they observed that the sound exposure level and maximum sound levels from voices and talking were greater than those from other sources49. However, they did not discuss the specific clinical activities linked to these noise sources.
Darbyshire et al. conducted a study mapping sources of noise in an adult ICU and found that a substantial portion of loud noise originated at the bedside, primarily from physiological monitors and ventilators positioned near patients’ ears36. These devices generally produced minimal sound, except when alarms were activated and emitted noise levels exceeding 50 dBA36, when the frequency range was comparable to that of a human scream36. They noted that although staff had the ability to adjust the volume settings of monitors and ventilators, they rarely modified them from their default setting36.
Although our study did not identify monitoring abnormalities as the commonest potential source of noise, research has shown that a majority of monitoring alarms (85 to 99.5%)69,70,71 are clinically irrelevant, and this is mainly attributed to maladjusted vital parameter alarms leading to a high incidence of ‘false positives’ (90%)72. Therefore monitoring alarms are often perceived as unhelpful by medical staff73. Clinicians may become accustomed to the acoustic environment, such as loud noise sources and reverberations of their individual ICU and this may lead to a selective disregard for what is perceived as the background noise of care, including regular low-level alarming or noise74. This phenomenon may occur even if such noise has clinical significance75. As a result, alarm fatigue can contribute to a delayed response by clinicians, posing a potential threat to patient safety76. Reduction of false alarms can be achieved through adopting better alarm management strategies, such as personalising the selection of monitoring elements for each patient and implementing patient-specific alarm settings to detect clinically relevant events73. Given the range of devices and alarms in typical ICUs, a human, organisational and technical factors approach to this noise source should take into account input from clinicans, manufacturers and regulators77.
Strengths and limitations
This study was conducted within a bay of six patients in an adult ICU, which may limit the generalisability of the findings to other ICU departments and to ICUs with single patient rooms. The study relied on retrospective data collection from patient records. This introduces limitations predominantly due to inaccuracies in documentation. Variability in accuracy and completeness of ICU observation charts, with selective recording of abnormal observations and retrospective documentation of noisy events, when clinical workload allows, combined with subjective interpretation during analysis, may all have impacted the retrospective identification of potential noisy events.
Notably, this study assessed noise levels with national and international noise and occupational health standards for healthcare and used a site-specific Class 1 sound level meter, recording over a two-week period, representing a reliable real-world noise of care recording in an intensive care setting compared to standards seen elsewhere in the existing literature.
Recommendations for research
We recommend that future studies consider conducting prospective analysis of sound levels in multiple centres, to increase the likelihood of reliably identifiying noisy events amongst a larger sample size. This could provide more generalisable data regarding sound levels and noise sources in ICU settings, so that interventions can be developed and tested. These interventions should be codesigned with patients and professionals. Issues with causal inference and confounding for sources of noise may be better addressed by a mixed methods approach for a national audit.
Recommendations for clinical practice
The development and implementation of comprehensive noise reduction protocols could help to reduce excessive noise levels. These protocols should address specific sources of noise, such as alarm settings and staff communication. For instance, the introduction of smarter alarm algorithms that offer intuitive alerting to mitigate the escalation of overall noise levels78,79,80. Additionally, educating healthcare providers on the importance of noise reduction and providing human factors training on techniques to minimise noise can contribute to a quieter environment81. However, it is important to recognise that certain interventions may not exhibit persistent impacts or longevity, particularly in environments characterised by high staff turnover81,82. To address this, architectural or engineering solutions are recommended alongside behavioural strategies. Noise issues can be controlled by changing the source and transmission path between the source and receiver. Most engineering methods focus on the transmission path in the built environment. For instance, finishing materials can be replaced with sound absorption materials. Previous studies83,84 reported that highly absorbent ceilings are effective in reducing sound pressure levels in patient wards. Additionally, acoustic curtains or temporary enclosures can be installed during medical treatment, including like-for-like replacement of existing low-performance privacy curtains with collapsible, hydrophobic, washable, and opaque PVC coated polyester curtains, either permamently or during the noisier episodes of care this study described85. In a recent meta-analysis only 14 out of 25 studies demonstrated a statistically significant reduction (p < 0.05) in mean sound levels following an intervention81. These reductions were seen in almost all studies involving staff education, noise warning devices, or architectural changes81.
By incorporating the principles of human factors into the design process, these solutions could offer the potential for more lasting changes by reducing dependence on individual compliance81. We recommend exploration of design engineering approaches in co-production with professionals and patients in addition to behavioural noise management strategies to facilitate ongoing monitoring and noise reduction efforts in the ICU.
Conclusions
This study provides valuable insight into the noise levels and source in a multi-bed adult ICU bay. Sound measurements recorded during the study period exceeded recommended parameters in international guidance. Further research is warranted to develop evidence-based interventions that promote a quieter and more conducive environment for patient care in the ICU.
Data availability
The datasets of sound pressure levels used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Pal, J. et al. Noise pollution in intensive care unit: a hidden enemy affecting the physical and mental health of patients and caregivers. Noise Health 24 (114), 130 (2022).
West, J. E. & Busch-Vishniac, I. What do we know about noise in hospitals. J. Acoust. Soc. Am. 118 (3), 1949–1949 (2005).
Busch-Vishniac, I. J. et al. Noise levels in Johns Hopkins hospital. J. Acoust. Soc. Am. 118 (6), 3629–3645 (2005).
Konkani, A. & Oakley, B. Noise in hospital intensive care units—A critical review of a critical topic. J. Crit. Care 27 (5), 522e1–522e9 (2012).
Tegnestedt, C. et al. Levels and sources of sound in the intensive care unit—An observational study of three room types. Acta Anaesthesiol. Scand. 57 (8), 1041–1050 (2013).
Berglund, B., Lindvall, T. & Schwela, D. H. WHO Guidelines for Community Noise ( World Health Organisation (WHO), 1999).
Darbyshire, J.L. Excessive noise in intensive care units. BMJ 353, pi1956 (2016).
Freedman, N. S. et al. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am. J. Respir. Crit. Care Med. 163 (2), 451–457 (2001).
Gabor, J. Y. et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am. J. Respir. Crit Care Med. 167 (5), 708–715 (2003).
Parthasarathy, S. & Tobin, M. J. Sleep in the intensive care unit. Appl. Physiol. Intens. Care Med. 191–200. (2009).
Pugh, R. J., Jones, C. & Griffiths, R. The Impact of Noise In the Intensive Care Unit. In Intensive Care Medicine: Annual Update 2007 (Springer, 2007).
Xie, H., Kang, J. & Mills, G. H. Clinical review: the impact of noise on patients’ sleep and the effectiveness of noise reduction strategies in intensive care units. Crit. Care 13 (2), 208 (2009).
Kröller-Schön, S. et al. Crucial role for Nox2 and sleep deprivation in aircraft noise-induced vascular and cerebral oxidative stress, inflammation, and gene regulation. Eur. Heart J. 39 (38), 3528–3539 (2018).
Cristensen, M. The physiological effects of noise: considerations for intensive care. Nurs. Crit. Care 7 (6), 300–305 (2002).
Frey, D. J., Fleshner, M. & Wright, K. P. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain. Behav. Immun. 21 (8), 1050–1057 (2007).
Münzel, T. et al. Cardiovascular effects of environmental noise exposure. Eur. Heart J. 35 (13), 829–836 (2014).
Chen, H. I. & Tang, Y. R. Sleep loss impairs inspiratory muscle endurance. Am. Rev. Respir. Dis. 140 (4), 907–909 (1989).
Rault, C. et al. Impact of sleep deprivation on respiratory motor output and endurance. A physiological study. Am. J. Respir. Crit Care Med. 201 (8), 976–983 (2019).
Noureddine, S. N. Sleep apnea: a challenge in critical care. Heart Lung 25 (1), 37–42 (1996).
Figueroa-Ramos, M. I. et al. Sleep and delirium in ICU patients: a review of mechanisms and manifestations. Intens. Care Med. 35 (5), 781–795 (2009).
Salluh, J. I. et al. Delirium epidemiology in critical care (DECCA): an international study. Crit. Care 14 (6), R210 (2010).
Litton, E. et al. The efficacy of earplugs as a sleep hygiene strategy for reducing delirium in the ICU: A systematic review and meta-analysis. Crit. Care Med. 44 (5), 992–999 (2016).
van de Pol, I., van Iterson, M. & Maaskant, J. Effect of nocturnal sound reduction on the incidence of delirium in intensive care unit patients: an interrupted time series analysis. Intens. Crit. Care Nurs. 41, 18–25 (2017).
Terzi, B. et al. The effects of noise levels on nurses in intensive care units. Nurs. Crit. Care 24 (5), 299–305 (2019).
Gurses, A. P. & Carayon, P. Performance Obstacles of intensive care nurses. Nurs. Res. 56 (3), 185–194 (2007).
Kooshanfar, Z. et al. Sources of noise and their effects on nurses in intensive care units: A cross sectional study. Int. J. Afr. Nurs. Sci. 16, 100403 (2022).
Adventina, T. & Widanarko, B. Systematic literature review: physical work environment factors associated with work fatigue in hospital nurses. Natl. J. Occup. Health Saf. 2(2). (2022).
Poncet, M. C. et al. Burnout syndrome in critical care nursing staff. Am. J. Respir. Crit. Care Med. 175 (7), 698–704 (2007).
Lawson, N. et al. Sound intensity and noise evaluation in a critical care unit. Am. J. Crit. Care 19 (6), e88–98 (2010).
Myhren, H., Ekeberg, O. & Stokland, O. Job Satisfaction and burnout among intensive care unit nurses and physicians. Crit. Care Res. Pract. 786176. (2013).
Maidl-Putz, C., McAndrew, N. & Leske, J. Noise in the ICU: sound levels can be harmful. Nurs. Crit. Care 9, 29–35 (2014).
Alidosti, M. et al. Association between job burnout and noise pollution among nurses in Behbahan City, Iran. J. Fundament. Mental Health 18 (2). (2016).
Topf, M. & Dillon, E. Noise-induced stress as a predictor of burnout in critical care nurses. Heart Lung: J. Crit. Care 17 (5), 567–574 (1988).
Cho, O. M. et al. Clinical alarms in intensive care units: perceived Obstacles of alarm management and alarm fatigue in nurses. Healthc. Inf. Res. 22 (1), 46–53 (2016).
Song, Z., Lee, P. J. & Jung, H. Prevalence of voice handicap among nurses in intensive care units due to occupational noise during pandemic. Front. Public. Health 11, 1250512 (2023).
Darbyshire, J. L. et al. Mapping sources of noise in an intensive care unit. Anaesthesia 74 (8), 1018–1025 (2019).
Choiniere, D. B. The effects of hospital noise. Nurs. Adm. Q. 34 (4), 327–333 (2010).
Morrison, W. E. et al. Noise, stress, and annoyance in a pediatric intensive care unit. Crit. Care Med. 31 (1), 113–119 (2003).
Delaney, L., Litton, E. & Van Haren, F. The effectiveness of noise interventions in the ICU. Curr. Opin. Anesthesiol. 32 (2), 144–149 (2019).
Molloy, K. et al. Inattentional deafness: visual load leads to time-specific suppression of auditory evoked responses. J. Neurosci. 35 (49), 16046–16054 (2015).
Schmidt, N. et al. Effects of intensive care unit ambient sounds on healthcare professionals: results of an online survey and noise exposure in an experimental setting. Intens. Care Med. Exp. 8 (1), 1–12 (2020).
Topf, M. Personal and environmental predictors of patient disturbance due to hospital noise. J. Appl. Psychol. 70 (1), 22 (1985).
Mohamed, A. M. O., Paleologos, E. K. & Howari, F. M. Chap. 19 - Noise pollution and its impact on human health and the environment. in pollution assessment for sustainable practices in applied sciences and engineering (Eds Mohamed, A. M. O., Paleologos, E. K. & Howari, F. M.) 975–1026. (2021).
Guidelines/practice parameters committee of the american college of critical care medicine, society of critical care medicine Guidelines for intensive care unit design. Crit. Care Med. 23 (3), 582–588 (1995).
Information on levels of environmental noise requisite to protect public health and welfare with an adequate margin of safety. https://scholar.google.com/scholar?q=+Information+on+levels+of+environmental+noise+requisite+to+protect+public+health+and+welfare+with+an+adequate+safety+margin (1974).
MacKenzie, D. J. & Galbrun, L. Noise levels and noise sources in acute care hospital wards. Build. Serv. Eng. Res. Tech. 28 (2), 117–131 (2007).
Delaney, L. J. et al. The nocturnal acoustical intensity of the intensive care environment: an observational study. J. Intens. Care 5, 41 (2017).
Vreman, J. et al. Contribution of alarm noise to average sound pressure levels in the ICU: an observational cross-sectional study. Intens. Crit. Care Nurs. 61, 102901 (2020).
Song, Z., Lee, P. J. & Hampton, T. Acoustic environments of intensive care units during the COVID-19 pandemic. Appl. Acoust. 199, 109037 (2022).
Khademi, G. et al. Noise pollution in intensive care units and emergency wards. Iran. J. Otorhinolaryngol. 23 (65), 141–148 (2011).
Crawford, K. J. et al. Identifying determinants of noise in a medical intensive care unit. J. Occup. Environ. Hyg. 15 (12), 810–817 (2018).
Darbyshire, J. L. & Young, J. D. An investigation of sound levels on intensive care units with reference to the WHO guidelines. Crit. Care 17 (5), R187 (2013).
Theuerkauf, N. U., Putensen, C. & Schewe, J. C. Konzepte Zur reduktion der lärmbelastung auf der intensivstation. Intensivmedizin Up2date 18 (03), 273–287 (2022).
Stafford, A., Haverland, A. & Bridges, E. Noise in the ICU. AJN Am. J. Nurs. 114 (5), 57–63 (2014).
Harris, P. A. et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inf. 95, 103208 (2019).
The Control of Noise at Work Regulations 2005 (Noise Regulations 2005) https://www.hse.gov.uk/noise/employers.htm (2005).
Simons, K. S. et al. Noise in the intensive care unit and its influence on sleep quality: a multicenter observational study in Dutch intensive care units. Crit. Care 22 (1), 250 (2018).
Li, S. Y. et al. Efficacy of controlling night-time noise and activities to improve patients’ sleep quality in a surgical intensive care unit. J. Clin. Nurs. 20 (3–4), 396–407 (2011).
Knaus, W. A. et al. APACHE II: a severity of disease classification system. Crit. Care Med. 13 (10), 818–829 (1985).
Chepesiuk, R. Decibel Hell: the effects of living in a noisy world. Environ. Health Perspect. 113 (1), A34–41 (2005).
Akansel, N. & Kaymakçi, S. Effects of intensive care unit noise on patients: a study on coronary artery bypass graft surgery patients. J. Clin. Nurs. 17 (12), 1581–1590 (2008).
Ryherd, E. E., Waye, K. P. & Ljungkvist, L. Characterizing noise and perceived work environment in a neurological intensive care unit. J. Acoust. Soc. Am. 123 (2), 747–756 (2008).
Guisasola-Rabes, M. et al. Effectiveness of a visual noise warning system on noise levels in a surgical ICU: A quality improvement programme. Eur. J. Anaesthesiol. EJA 36(11). (2019).
Carvalho, W. B., Pedreira, M. L. & de Aguiar, M. A. Noise level in a pediatric intensive care unit. J. Pediatr. (Rio J) 81 (6), 495–498 (2005).
Hilton, B. A. Noise in acute patient care areas. Res. Nurs. Health 8 (3), 283–291 (1985).
Baker, C. F. Discomfort to environmental noise: heart rate responses of SICU patients. Crit. Care Nurs. Q. 15 (2), 75–90 (1992).
Walker, L. & Karl, C. A. The hospital (Not So) quiet zone: creating an environment for patient satisfaction through noise reduction strategies. Herd 12 (4), 197–202 (2019).
Tegnestedt, C. et al. Levels and sources of sound in the intensive care unit—An observational study of three room types. Acta Anaesthesiol. Scand. 57. (2013).
Lawless, S. T. Crying Wolf: false alarms in a pediatric intensive care unit. Crit. Care Med. 22 (6). (1994).
O’carroll, T. Survey of alarms in an intensive therapy unit. Anaesthesia 41 (7), 742–744 (1986).
Siebig, S. et al. Intensive care unit alarms—How many do we need? Crit. Care Med. 38 (2), 451–456 (2010).
Drew, B. J. et al. Insights into the problem of alarm fatigue with physiologic monitor devices: a comprehensive observational study of consecutive intensive care unit patients. PLoS One 9 (10), e110274 (2014).
Chambrin, M. C. Alarms in the intensive care unit: how can the number of false alarms be reduced? Crit. Care 5 (4), 184–188 (2001).
Rice, T. Soundselves: an acoustemology of sound and self in the Edinburgh royal infirmary. Anthropol. Today 19 (4), 4–9 (2003).
Mackrill, J., Cain, R. & Jennings, P. Experiencing the hospital ward soundscape: towards a model. J. Environ. Psychol. 36, 1–8 (2013).
Ruskin, K. J. & Hueske-Kraus, D. Alarm fatigue: impacts on patient safety. Curr. Opin. Anaesthesiol. 28 (6), 685–690 (2015).
Bach, T. A., Berglund, L. M. & Turk, E. Managing alarm systems for quality and safety in the hospital setting. BMJ Open. Qual. 7 (3), e000202 (2018).
Konkani, A., Oakley, B. & Bauld, T. J. Reducing hospital noise: a review of medical device alarm management. Biomed. Instrum. Technol. 46 (6), 478–487 (2012).
Görges, M., Markewitz, B. A. & Westenskow, D. R. Improving alarm performance in the medical intensive care unit using delays and clinical context. Anesth. Analg. 108 (5), 1546–1552 (2009).
Bingham, M. et al. Effects of altering harmonic structure on the recognition of simulated auditory arterial pressure alarms. Br. J. Anaesth., 131. (2023).
Vreman, J. et al. The effectiveness of the interventions to reduce sound levels in the ICU: A systematic review. Crit. Care Explor. 5 (4), e0885 (2023).
de Lima Andrade, E. et al. Environmental noise in hospitals: a systematic review. Environ. Sci. Pollut Res. Int. 28 (16), 19629–19642 (2021).
Hagerman I, Rasmanis G, Blomkvist V, Ulrich R, Eriksen CA, Theorell T. Influence of intensive coronary care acoustics onthe quality of care and physiological state of patients. Int J Cardiol. 98(2), 267-270. https://doi.org/10.1016/j.ijcard.2003.11.006 (2005)
Zhixiao D, Hui X, Jian. The effectiveness of acoustic treatments in general hospital wards in China. Building and Environment 244, 110728. https://doi.org/10.1016/j.buildenv.2023.110728 (2005)
Sanjay Kumar, Rui Qin Ng, Heow Pueh Lee. Experimental investigations of acoustic curtains for hospital environment noise mitigations. Applied Physics . https://doi.org/10.48550/arXiv.2008.06690 (2020)
Acknowledgements
With thanks to the research nurse team, clinical staff and patients & relatives of the Royal Liverpool ICU.
Author information
Authors and Affiliations
Contributions
TH receives grant funding from The Wellcome Trust (203919/Z/16/Z) but none of the authors report any other conflicts of interest financial or otherwise.TH, AW and PJL conceived the work, At, TH, AW, IW and PJL designed the work; AT, TH, AW and PJL were involved in the acquisition of data, AT, AW, TH and PJL were involved in the analysis of the data, AT, AW, TH, IW and PJL contributed to interpretation of data; AT drafted the manuscriptAT, AW, TH, IW and PJL edited the manuscriptAND all authors approved the submitted version and agree to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated and resolved, with the resolution documented in the literature.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Glossary
- Acoustic Environment
-
All the sound and sound-propagating qualities of a physical environment
- APACHE II
-
Acute physiology and chronic health evaluation II
- dBA
-
A-weighted sound level
- ICU
-
Intensive care unit
- INC
-
International noise council
- LAeq
-
Equivalent continuous sound level (average sound level over a specified time period)
- LAmax
-
Maximum sound level (over a specified time period)
- LAmin
-
Minimum sound level (over a specified time period)
- Noise
-
Noise suggests an element of irritation and/or harm related to sound, with many contributing factors, including psychological status, sound frequency, sound level, and context
- RLUH
-
Royal liverpool university hospital
- Soundscape
-
The acoustic environment as perceived, understood and/or experienced by people
- USEPA
-
United States environmental protection agency
- WHO
-
World health organisation
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tahvili, A., Waite, A., Hampton, T. et al. Noise and sound in the intensive care unit: a cohort study. Sci Rep 15, 10858 (2025). https://doi.org/10.1038/s41598-025-94365-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94365-8