Abstract
In this work, the response of peeled-off Gafchromic EBT2 film to argon plasma jet (APJ) was investigated. Peeled-off Gafchromic EBT2 films were exposed to APJ for different durations of varying steps from 0 to 70 s at ambient conditions. Thereafter, the films were scanned with a flatbed scanner of high spatial resolution and color depth of 16 bits per color channel. The optical properties were measured using a UV-Vis spectrophotometer. The induced chemical modification in the active layer was confirmed by ATR-FTIR spectroscopy. The pixel values correlation of each color channel of peeled-off Gafchromic EBT2 film and the exposure time by APJ at different durations were studied. The obtained results showed that the pixel values of the red and green color channels of the peeled-off Gafchromic EBT2 film exponentially decrease with increasing the exposure time to APJ, with decay constants of 0.010 ± 0.024 and 0.071 ± 0.011 for the red and green channels, respectively. The blue channel exhibits poor and anomalous responses compared to both red and green color channels. The two characteristic peaks in the UV-Vis absorption spectra at the wavelengths of 580 ± 4 nm and 632 ± 4 nm for the peeled-off Gafchromic EBT2 film go up exponentially as the exposure time increases. The ATR-FTIR spectra contain two characteristic new C = C bonds; the first is formed due to a solid-state 1,4-polymerization reaction as conjugated with neighboring C ≡ C, while the second is further stabilized by conjugation to allene structure, increasing the double bond character, while the latter is the major contributor. The obtained results of the peeled-off Gafchromic EBT2 film can be utilized in APJ diagnosis and measurements.
Similar content being viewed by others
Introduction
Glow discharge can occur either at atmospheric pressure or low pressure. It has been the subject of numerous research initiatives throughout the past century, and it is widely recognized. It is extensively utilized in numerous applications, including thin film coating, light sources, and surface treatment. However, maintaining it under low pressure is expensive. Owing to this constraint, numerous studies on glow plasma stabilization at atmospheric pressure have been conducted1,2,3,4. Recently, cold APJ have received significant interest because of their adaptability, cheap operating costs, and capacity to generate reactive chemistry at room temperature3,4. Cold APJ applications based on diverse geometries and electrode materials have been developed and investigated using the discharge plasma1. The absence of vacuum conditions, which significantly lower the cost and complexity of the glow discharge operation, is the main advantage of the application of Atmospheric Pressure Glow Discharge (APGD). This generic feature has significantly accelerated the practical applications of APGDs, such as biological decontamination and open-air surface coating2,3,4.
The argon plasma is made up of charged particles such as free electrons and positively charged argon ions (Ar+), which are produced when argon gas is ionized using a high-frequency electrical field. The generated plasma was distinguished by its energetic nature as well as the presence of reactive species such as radicals and excited atoms. Because of the way energy is distributed among its constituents, argon plasma may not have a proportionately high temperature despite its high energy content. This unusual plasma state has applications in spectroscopy for elemental analysis, material processing for etching and thin film deposition, and plasma cleaning to remove surface contaminants.
In nuclear medicine, the Gafchromic EBT2 radiochromic film is widely applied as a two-dimensional radiation dosimeter for photons and charged particles5,6,7,8,9,10. The Gafchromic EBT2 film exhibits several notable features, including tissue-equivalent material with an effective atomic number of 6.84, minimal energy dependence throughout a range of about 0.05 MeV to some MeV, and reduced susceptibility to ambient light and temperature fluctuations. The deposited energy by ionizing radiation in the active layer of Gafchromic EBT2 film polymerizes the diacetylene monomers7,11, resulting in the darkening of the EBT2 film5,11,12,13,14. The short-range particles can’t penetrate the polyester over laminate, adhesive, and topcoat layers with a total thickness of 80 μm. Nevertheless, the removal of these layers allows short-range particles to deposit their energy within the active layer to induce color change15,16,17,18,19. For the Gafchromic EBT2 film, APJ deposits energy that induces the coloration of the film20,21. The induced surface modification by APJ is limited to a few hundred angstroms of depth, leaving the bulk material properties unchanged4,9, in addition to uniformity over large planar areas10.
The work’s objectives are to investigate the response of peeled-off Gafchromic EBT2 film upon exposure to APJ at different duration times. The relationship between the exposure time and the pixel values of each color channel in peeled-off Gafchromic EBT2 films is examined. Each color channel’s sensitivity and dynamic range will be ascertained. The changes in the optical characteristics of the peeled-off Gafchromic EBT2 films caused by APJ are studied. The two characteristic absorption peaks at wavelengths of 580 ± 4 nm and 632 ± 4 nm are investigated as a function of the exposure time to APJ. The induced chemical modifications in the active layer of peeled-off Gafchromic EBT2 film are achieved and measured using ATR-FTIR.
Materials and methods
Peeled-off Gafchromic EBT2 radiochromic film
The Gafchromic EBT2 films (Lot No. A06281102B) were peeled off by eliminating the polyester over laminate, adhesive, and topcoat layers of total thickness 80 μm using a fine needle under a 60X optical microscope and then cut into\(\:\:1\:\text{c}\text{m}\:\times\:\:2\:\text{c}\text{m}\) pieces. An optical microscope with a 600X magnification was used to assure that the surface quality of the peeled-off film was maintained. The structure of the Gafchromic EBT2 film and peeled-off Gafchromic EBT2 film are shown in Fig. 1(A) and (B). Beside the optical microscope, the homogeneity of the peeled-off Gafchromic EBT2 films was confirmed by a Canon CanoScan flatbed scanner. The peeled-off Gafchromic EBT2 films were exposed to APJ for 1, 2, 5, 10, 30, 40, 60, and 70 s. The pristine and exposed peeled-off Gafchromic EBT2 films were scanned using a Canon CanoScan 9000 F Mark II flatbed scanner in transmission mode, and the images were saved in TIFF format. The scanner’s spatial resolution amounts to 9600 dpi, and the color depth is 48-bits, and 16-bits per color channel. The photos were analyzed using the free program ImageJ22. The films were positioned in the same direction in the scanner bed during scanning to offset the polarization of Gafchromic EBT2 films.
A spectrophotometer (Model Spectro dual split beam, UVS-2700) was used to evaluate the UV-Vis absorption spectra at wavelengths between 400 and 700 nm.
Plasma jet setup
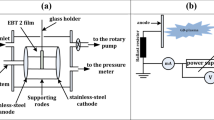
Figure 2 depicts the APJ system used to treat the surface of the peeled-off Gafchromic EBT2 film. The plasma jet is made up of two cylindrical electrodes, the inner electrode being made of stainless steel with a width of 1 mm and enclosed by a quartz tube with a diameter of 4 mm. The other electrode is covered by copper tape and connected to the ground.
Electrical measurements of the applied voltage and discharge current can be measured using a Tektronix voltage probe (model P6015A), while the current flowing in the circuit is measured using a current monitor (model 6585 Pearson Electronics, USA). The applied voltage on the two electrodes and the discharge current through the reactor are monitored using a 500 MHz four-channel digital phosphor oscilloscope type Tektronix (model DPO 4054). Argon gas is supplied at a rate of 5 standard liters per minute (SLPM) using a calibrated mass flow controller (flow meter) at a higher applied voltage of 3.5 kV. Like low-pressure glow discharges, plasma jet is characterized by relatively strong light emission near the cathode23,24.
The electrical properties (I-V characteristics of waveform, the effect of gas flow, and high applied voltage on plasma plume length) of the argon plasma jet were thoroughly investigated in ref25. It was found that the plasma plume lengths increase as the applied voltage increases until reaching saturation at higher values of applied voltages.
When 3.5 kV is applied between the two electrodes, an inert working gas (argon in this case) is ionized, gas breakdown occurs, and plasma is created throughout the channel. Because of the cross-field configuration of the generating electric field and the extremely collisional atmosphere at atmospheric pressure, the plasma recombines very close to the nozzle as it exits the device’s end and enters open air. All that remains for surface treatment is a neutral, radical-rich effluent.
In the current experiments, the APJ is pointed vertically downwards towards the active layer of peeled-off Gafchromic EBT2 film, causing the effluent to impinge on the surface. Figure 2 shows that the distance between the plasma plume and the sample surface is fixed at 0.5 cm, and the length of the plasma plume is 1.46 cm.
Results and discussion
Many techniques are applied to read out radiochromic films, including flatbed scanners, film densitometers, and UV-Vis spectrometers26,27,28,29,30,31,32,33,34. Nevertheless, for dose mapping, a flatbed scanner is successfully applied in radiotherapy as a two-dimensional readout system, either for low linear transfer radiation or for heavy ions Fig. 3.
RGB images by flatbed scanner
The peeled-off Gafchromic EBT2 films were exposed to an APJ for different exposure times: 1, 2, 5, 10, 30, 40, 60, and 70 s. After irradiation, the films continue to develop color for several hours. Radiochromic films are digitized one day after exposure to allow for post-exposure stabilization of optical density. This delay minimizes variability and ensures accurate dose measurements5. Subsequently, the films were digitized using a Canon CanoScan flatbed scanner35 and saved in TIFF format. The ImageJ free code software22 was used to analyze the images. The RGB images were decomposed in single red, green, and blue channels as presented in Fig. 3; the area of each image is (6.63 × 6.63) mm2. Qualitatively, the peeled-off Gafchromic EBT2 films exhibit the same manner for RGB images as well as the red and green channels, while the darkness increases with the increase of the exposure time as the plasma jet increases. For prolonged exposure times of 30, 40, 60, and 70 s, the films are saturated; this is simply attributed to the almost complete polymerization of diacetylene. In contrast, the blue channel shows a different performance; the darkening decreases by increasing the exposure time to 30 s; thereafter, the darkness increases by increasing the treatment time.
The line profile of the pristine Gafchromic EBT2 films that were peeled off and those that were exposed to a plasma jet for 5 s and 60 s is shown in Fig. 4 (A). The histograms of pixel values of RGB color channels are depicted in Fig. 4 (B). The red and green channels have the highest dynamic range of pixel values of 54315.0 ± 7.0 and 51921.0 ± 21.7, respectively, while the blue channel has the lowest dynamic range of 18716.0 ± 2.6. These data emphasize that the red and green channels possess the highest dynamic range and sensitivity. The exposure of the peeled-off Gafchromic EBT2 film to APJ for different durations exhibited high coloration homogeneity; for instance, the maximum exposure time of 70 s has an average variation of (1.6 ± 0.3) ×10−4 pixel values/µm.
Figure 5(A) displays the mean pixel values and associated standard deviation for peeled-off EBT2 films exposed to APJ and inspected as a function of exposure time to plasma. It indicates that the red channel has the highest pixel value of 54,421 ± 285 and a minimum pixel value of 12,809 ± 447, followed by the green channel with a value of 52,623 ± 285 and the minimum pixel value of 10,396 ± 628. Meanwhile, the blue channel has an insignificant value of 18,838 ± 464 and the minimum pixel value of 11,855 ± 480, indicating a poor response to APJ. This is because the EBT2 active layer has a yellow marker dye that absorbs the blue light without inducing a considerable contribution to the polymerization of the film. Such an observation agrees with the literature studies concerned with the response of Gafchromic EBT2 and EBT3 films to other types of radiation36,37,38,39,40. The combined image of radiochromic film is deteriorated by the presence of the blue channel; therefore, scanning the film using a monochromatic wavelength of the red and green channels would give rise to a higher dynamic range as well as higher sensitivity. The data of pixel value for the red and green channels are fitted with Eq. 1.
Where a0, a1, and a2 are fitting parameters and their values are illustrated in Table 1, a2 has the unit of s−1. The sensitivity of the peeled-off EBT2 films to APJ is calculated by the first order differentiation of Eq. (1) with respect to the exposure time.
The optical density of three separate channels (red, green, and blue) is given in Fig. 5 (B). It indicates the darkness of the red and green channels increasing with increasing exposure time to 30 s for prolonged exposure time, then the peeled-off Gafchromic EBT2 films saturate. The data of optical density for the red and green channels are fitted using Eq. 2.
Here \(\:{PV}_{unexp}\), and \(\:{PV}_{exp}\:\:\) are the pixel value of pristine and the exposed peeled-off Gafchromic EBT2 films. The red and green channel data are fitted using the following equation:
Where b0, b1, and b2 are the fitting parameters and their values are tabulated in Table 2, b2 has the unit of s−1, which represents the growth constant of the opacity of the peeled-off Gafchromic EBT2 films by plasma jet.
Optical properties of the peeled-off Gafchromic EBT2 film
The induced optical modifications of the peeled-off Gafchromic EBT2 upon exposure to APJ could be quantitatively analyzed by UV–Vis spectroscopy41,42,43,44,45,46. UV–Vis absorption spectra for the pristine peeled-off Gafchromic EBT2 film and the peeled-off Gafchromic EBT2 films exposed to APJ were measured in the wavelength range of 400–800 nm41,42, as shown in Fig. 6(A). The two characteristic peaks are observed in the absorption spectra at wavelengths of 580 ± 4 nm and 632 ± 4 nm for the peeled-off EBT2 films. These two peaks are attributed to the electronic transition between the highest occupied molecular orbitals (HOMO) and the lowest unoccupied molecular orbitals (LUMO) in the active dye layer of peeled-off EBT2 film7,10. The absorbance intensity at all wavelengths increased as the exposure time to plasma increased up to 30 s; thereafter, it tended to saturate. Figure 6 (B) shows the net absorbance spectra of the peeled-off BT2 films exposed to APJ for different exposure times, which are produced by subtraction of the pristine film from the exposed peeled-off EBT2 films. The net absorption between wavelengths of 600 and 650 nm became more complicated with the increase in exposure time that is ascribed to the saturation of the absorption starting from 30 s, which is associated with flatness in the characteristic peak at 632 ± 4 nm.
The increase in the absorbance of the peeled-off Gafchromic EBT2 films is attributed to the plasma-induced 1, 4-polymerization of diacetylene monomer in the active layer of the films. This produces a linear long-chain polymer (polyPCDA) with alternating double and triple carbon–carbon bonds in the backbone. The diacetylene monomer is of low absorbance in the visible part of the spectrum with two characteristic peaks at 580 ± 4 nm (green light) and 632 ± 4 nm (red light), while the darkened polydiacetylene absorbs light due to the extensive delocalization of π-orbital electrons along the polymer backbone37. The saturation of the peeled-off Gafchromic EBT2 films absorbance at prolonged exposure times is attributed to the complete polymerization of the diacetylene monomers in the active layer. The obtained results reveal that the absorbance of the peeled-off Gafchromic EBT2 films exposed to APJ is strongly correlated with the exposure time, showing the possibility of using the peeled-off Gafchromic EBT2 film in low-temperature plasma investigations38.
Figure 7 (A) describes the response of the peeled-off EBT2 as a function of exposure time to APJ at the two characteristic absorption peaks of wavelengths 580 ± 4 nm and 632 ± 4 nm, where the data are fitted using an exponential function as the following Eq.
Where c1, c2, and c3 are fitting parameters and their values are illustrated in Table 3, c3 has the unit of s−1. The sensitivity of the peeled-off EBT2 to APJ is calculated by the first-order differentiation of Eq. (4) with respect to the exposure time.
According to Eq. (4), the absorbance increases at the two distinctive peaks at wavelengths of 580 ± 4 nm and 632 ± 4 nm with plasma exposure time up to 30 s; beyond that, it saturates. In the peeled-off Gafchromic EBT2 films, the energetic active plasma jet species interacts with the active layer (diacetylene monomer) to cause a radical polymerization reaction and increase the optical density (darkness) of polydiacetylene. All the diacetylene monomers in the active layer convert to polymers over an extended period of exposure to APJ, and the concentration of the newly produced polymer stabilizes. Thus, the absorbance reaches saturation as shown in Fig. 7.
ATR-FTIR measurements
Infrared spectroscopy provides unique information about structural change, which helps in recording the transformation of diacetylene monomer into polymerized diacetylenes via the presence of new C = C bonds formed due to exposure of peeled-off EBT2 film to APJ. Many sampling techniques were invented, like transmission, specular reflection, diffuse reflectance, and attenuated total reflection (ATR), as the latter represents a special tool to identify inorganic ions in addition to organic functional groups quantitatively using heterogeneous samples47. The ATR-FTIR measurements were carried out using FTIR Brucker ALPHA II with a spectral resolution of 4 cm−1. The sensitive compound of the EBT2 active layer is composed of lithium pentacosa-10,12-diynoate (LiPCDA) microcrystals, which comprise the major organic content in this layer as described by the manufacturer (H = 58.3%, C = 29.6%, O = 10.8%, Li = 0.8%, Cl = 0.2%, N = 0.1%, Na = 0.1%, Br = 0.1%, including about 7.5% moisture).
The spectra were recorded after exposure to an APJ using peeled-off EBT2 films at different exposure times of 2, 5, 10, 40, and 70 s to the plasma jet in addition to an untreated sample (t = 0) from 4000 to 400 cm−1. Due to the low intensity of C ≡ C absorption (2300–2150 cm−1), we will focus only on the most informative region, which lies from 2000 to 400 cm−1. The zoomed-out data are represented in Fig. 8, which showed a characteristic absorption maxima at 1714 cm−1. Many components play a role in this absorption and appear as intermediate between them as given in Eq. 5.
Whose descriptions are:
1- Asymmetric carboxylate head group stretching band at 1620 –1600 cm−1which appears weak as a shoulder to the next absorption due to coordination to lithium ion during polymerization48.
2- The characteristic new C = C bond, which appears in two forms. The first is formed due to a solid-state 1,4- polymerization reaction as conjugated alkyne-alkene bands of C ≡ C and C = C bonds, respectively. Its stretching frequency appears in the region 1650 –1600 cm−1, as indicated graphically in Fig. 9 (polymeric form I), which is further stabilized by conjugation to allene structure (polymeric form II), increasing the double bond character and shifting the absorption maximum to a higher frequency (isolated allene strongly absorbs in 1950–1930 cm−1)49. The share of stretching vibration at 1714 cm−1 is as follow:
Comparing the data of untreated samples (t = 0 s) and other treated samples, 8 cm−1 shift to lower frequency is noticed, which is explained by a higher contribution of carboxylate ion as depicted in Fig. 8. Furthermore, the data also shows a contribution from the conjugated alkyne-alkene stretching mode even before irradiation due to the well-known fact of diacetylene self-polymerization, which was previously reported50,51,52. The reason for self-polymerization is the storage period or elevated temperatures.
In the fingerprint region, many different bands are represented as follows: symmetric carboxylate stretching bands (1288 –1198 cm−1); methylene group spacer with wagging mode (1412, 1100 cm−1 due to different spatial conformations); methylene group spacer with rocking mode called long chain band (722 cm−1) indicating more than four CH2groups49. All the aforementioned absorptions are assigned in Fig. 8.
Conclusion
The argon plasma jet (APJ) induces coloration in the active layer of the peeled-off Gafchromic EBT2 film. The exposure of the peeled-off Gafchromic EBT2 film to APJ for different durations exhibited high coloration homogeneity even for the maximum exposure time of 70 s with an average variation of (1.6 ± 0.3) ×10−4 pixel values/µm. The pixel values of the red and green channels decreased exponentially with the increase in exposure time, where the green channel has the highest dynamic range and sensitivity. The two characteristic absorption peaks in the UV-Vis spectra at 580 ± 4 nm and 632 ± 4 nm are increasing exponentially with the increase in the exposure time up to 30 s before saturating with longer exposure times. There are characteristic absorption maxima in ATR-FTIR spectra at 1714 cm−1 which confirm solid-state 1, 4- polymerization through the newly formed C = C bond. This absorption appears intermediate and is formed owing to cumulative absorptions of different conjugation forms, including allene structure, which increases the double bond character and absorption frequency. The current measurements indicate the capacity of the peeled-off Gafchromic EBT2 film as a novel and simple technique for plasma jet diagnosis. However, deep investigations of the correlation between the radiochromic films’ response and the widespread parameters of the plasma source, including gas type, gas flow rate, and discharge high voltage, are required.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Kanazawa, S., Kogoma, M., Moriwaki, T. & Okazaki, S. Stable glow plasma at atmospheric pressure. J. Phys. D. 21 (5), 838–840. https://doi.org/10.1088/0022-3727/21/5/028 (1988).
Yokoyama, T., Kogoma, M., Moriwaki, T. & Okazaki, S. The mechanism of the stabilization of glow plasma at atmospheric pressure. J. Phys. D. 23 (8), 1125–1128. https://doi.org/10.1088/0022-3727/23/8/021 (1990).
Gerlinga, T., Brandenburg, R., Christian Wilke, C. & Weltmann, K. D. Power measurement for an atmospheric pressure plasma jet at different frequencies: distribution in the core plasma and the effluent. Eur. Phys. J. Appl. Phys. 78, 10801. https://doi.org/10.1051/epjap/2017160489 (2017).
Shi, J. J., Liu, D. W. & Kong, M. G. Plasma stability control using dielectric barriers in Radio- frequency atmospheric pressure glow discharges. Appl. Phys. Lett. 89, 081502. https://doi.org/10.1063/1.2338647 (2006).
Santos, T., Ventura, T. & Lopes, M. Do C. A review on radiochromic film dosimetry for dose verification in high energy photon beams. Radiat. Phys. Chem. 179 (September 2020), 109217. https://doi.org/10.1016/j.radphyschem.2020.109217 (2021).
Arjomandy, B. et al. Energy dependence and dose response of Gafchromic EBT2 film over a wide range of photon, electron, and proton beam energies. Med. Phys. 37 (5), 1942–1947. https://doi.org/10.1118/1.3373523 (2010).
Butson, M. J., Yu, P. K. N., Cheung, T. & Alnawaf, H. Energy response of the new EBT2 radiochromic film to X-ray radiation. Radiat. Meas. 45 (7), 836–839. https://doi.org/10.1016/j.radmeas.2010.02.016 (2010).
Butson, M. J., Yu, P. K. N., Cheung, T. & Metcalfe, P. Radiochromic film for medical radiation dosimetry. Mater. Sci. Eng. R Rep. 41 (3–5), 61–120. https://doi.org/10.1016/S0927-796X(03)00034-2 (2003).
-Soares, C. G. Radiochromic film dosimetry. Radiat Meas. ;41(SUPPL. 1):100-Radiochromic film dosimetry116. (2006). https://doi.org/10.1016/j.radmeas.2007.01.007
Indra, J. Das. Radiochromic Film: Role and Applications in Radiation Dosimetry. Vol 41.; (2018). https://doi.org/10.1016/j.radmeas.2007.01.007
-Kotb, O. M., El Ghazaly, M., Mohamed, A. & Ibrahim, M. A. Effect of Gafchromic EBT2 radiochromic film’s components on the detection of UVA solar radiation selectively. Radiat. Phys. Chem. 197 (April). https://doi.org/10.1016/j.radphyschem.2022.110158 (2022).
Martín-Viera Cueto, J. A., Parra Osorio, V., Moreno Sáiz, C., Navarro Guirado, F. & Casado Villalón, F. J. Galán Montenegro P. A universal dose-response curve for radiochromic films. Med. Phys. 42 (1), 221–231. https://doi.org/10.1118/1.4903301 (2015).
El-Naggar, H. I., Ghanim, E. H., El Ghazaly, M. & Salama, T. T. On the registration of low energy alpha particle with modified Gafchromic EBT2 radiochromic film. Radiat. Phys. Chem. 191 (January 2021), 109852. https://doi.org/10.1016/j.radphyschem.2021.109852 (2022).
-Welch, D., Randers-Pehrson, G., Spotnitz, H. M. & Brenner, D. J. Unlaminated Gafchromic EBT3 film for ultraviolet radiation monitoring. Radiat. Prot. Dosimetry. 176 (4), 341–346. https://doi.org/10.1093/rpd/ncx016 (2017).
Mukherjee, B., Gholami, Y. H., Bhonsle, U., Hentschel, R. & Khachan, J. A unique alpha dosimetry technique using Gafchromic EBT3® film and feasibility study for an activity calibrator for alpha-emitting radiopharmaceuticals. Br. J. Radiol. 88 (1056). https://doi.org/10.1259/bjr.20150035 (2015).
Braithwaite, N. S. J. Introduction to gas discharges. Plasma Sources Sci. Technol. 9 (4), 517–527. https://doi.org/10.1088/0963-0252/9/4/307 (2000).
-Adamovich, I. et al. The 2022 plasma roadmap: low temperature plasma science and technology. J. Phys. D Appl. Phys. 55 (37). https://doi.org/10.1088/1361-6463/ac5e1c (2022).
Fridman, G. et al. Applied plasma medicine. Plasma Process. Polym. 5 (6), 503–533. https://doi.org/10.1002/ppap.200700154 (2008).
Laroussi, M. Low-Temperature plasmas for medicine ? IEEE Trans. Plasma Sci. 37 (6), 714–725 (2009).
-Luque-Agudo, V., Hierro-Oliva, M., Gallardo-Moreno, A. M. & González-Martín, M. L. Effect of plasma treatment on the surface properties of polylactic acid films. Polym. Test. 96. https://doi.org/10.1016/j.polymertesting.2021.107097 (2021).
Farag, O. F. et al. Response of the modified GAFCHROMIC EBT2 radiochromic film to DC glow discharge plasma. Sci. Rep. 14, 2262. https://doi.org/10.1038/s41598-024-52628-w (2024).
-Https://Imagej.Nih.Gov/Ij/. (Accessed 25 July 2022).
Lau, Y. T., Jayapalan, K. K., Pam, M. E. & Chin, O. H. Dependence of dielectric barrier discharge jet length on gas flow rate and applied voltage. J. Sci. Technol. Tropics. 10 (2), 131–138 (2014).
Okazaki, S., Kogoma, M., Uehara, M. & Kimura, Y. Appearance of stable glow discharge in air, Argon, oxygen and nitrogen at atmospheric pressure using 50 hz source. J. Phys. D. 26 (5), 889–892 (1993).
-Mohamed, A., Hassouba, Abdel-Aleam, H. & Mohamed, Hala, I. El- Naggar, Study of Some Electrical Characteristics of the Cylindrical Plasma Jet Using Argon Gas, Australian Journal of Basic and Applied Sciences, 11(15) December 2017, Pages: 26–33. https://doi.org/10.22587/aibas.2017.11.15.5
El-Sayed, N. M., Reda, F. M., Farag, O. F. & Nasrallah, D. A. Surface analysis of nitrogen plasma-treated C60/PS nanocomposite films for antibacterial activity. J. Biol. Phys. 43 (2), 211–224. https://doi.org/10.1007/s10867-017-9447-6 (2017).
Jacobs, T., Morent, R., De Geyter, N., Dubruel, P. & Leys, C. Plasma surface modification of biomedical polymers: influence on cell-material interaction. Plasma Chem. Plasma Process. 32 (5), 1039–1073. https://doi.org/10.1007/s11090-012-9394-8 (2012).
Bogaerts, A. The glow discharge: an exciting plasma! J. Anal. Spectrom. 14 (9), 1375–1384. https://doi.org/10.1039/a900772e (1999).
Farag, O. F. Comparison of the effect of plasma treatment and gamma ray irradiation on PS-Cu nanocomposite films surface. Results Phys. 9, 91–99. https://doi.org/10.1016/j.rinp.2018.02.031 (2018).
El-Sayed, N. & farag omar, Nasrallah, D. Improved surface properties of Copper/PolyMethylMethacrylate nanocomposite films using DC O2 plasma. Arab. J. Nucl. Sci. Appl. 0 (0), 0–0. https://doi.org/10.21608/ajnsa.2019.12400.1214 (2019).
Liston, E. M., Martinu, L. & Wertheimer, M. R. Plasma surface modification of polymers for improved adhesion: A critical review. J. Adhes. Sci. Technol. 7 (10), 1091–1127. https://doi.org/10.1163/156856193X00600 (1993).
-Totolin, V., Manolache, S., Rowell, R. M. & Denes, F. S. Application of cold plasma to improve properties of phenolic-bonded Aspen fiberboard. J. Nat. Fibers. 5 (2), 170–192. https://doi.org/10.1080/15440470801929671 (2008).
Donate, R., Alemán-Domínguez, M. E. & Monzón, M. On the effectiveness of oxygen plasma and alkali surface treatments to modify the properties of polylactic acid scaffolds. Polym. (Basel). 13 (10). https://doi.org/10.3390/polym13101643 (2021).
-Wang, C. X., Ren, Y. & Qiu, Y. P. Penetration depth of atmospheric pressure plasma surface modification into multiple layers of polyester fabrics. ;202:77–83. (2007). https://doi.org/10.1016/j.surfcoat.2007.04.077
https://www.canon-europe.com/scanners/flatbed-scanners/canoscan_lide_110/. (Accessed 20 July 2022).
Tagiling, N. et al. Effect of scanning parameters on dose-response of radiochromic films irradiated with photon and electron beams. Heliyon 4 (10), e00864. https://doi.org/10.1016/j.heliyon.2018.e00864 (2018).
Corp, I. S. P. Gafchromic EBT2, Self Developing Film for Radiotherapy Dosimetry ISP White Paper (ISP Corporation)., 2009).
Aydarous, A. Investigation of absorption spectra of Gafchromic EBT2 film’s components and their impact on UVR dosimetry. Radiat. Phys. Chem. 122, 55–59. https://doi.org/10.1016/j.radphyschem.2016.01.028 (2016).
Al-Sayed, A. et al. On the color channel’s effect on the dose-response of Gafchromic EBT2 and EBT3 radiochromic films to 6 mev electron beam. Rad Phys. Chem. 218, 111565. https://doi.org/10.1016/j.radphyschem.2024.111565 (2024).
Callens, M. et al. A spectroscopic study of the chromatic properties of Gafchromic™ EBT3 films. Med. Phys. 43 (3), 1156–1166. https://doi.org/10.1118/1.4941312 (2016).
Migahed, M. D. & Zidan, H. M. Influence of UV-irradiation on the structure and optical properties of polycarbonate films. Curr. Appl. Phys. 6 (1), 91–96. https://doi.org/10.1016/j.cap.2004.12.009 (2006).
El-Sayed, N. & farag omar. Optical and electrical properties of plasma surface treated polymethylmethacrylate films. Arab. J. Nucl. Sci. Appl. 0 (0), 0–0. https://doi.org/10.21608/ajnsa.2019.7116.1168 (2019).
RathoreBS, GaurMS & SinghKS Optical properties of swift heavy ion beam irradiated polycarbonate/ polystyrene composites films. Macromol. Symp. 315 (1), 169–176. https://doi.org/10.1002/masy.201250521 (2012).
El-Naggar, H. I., El Ghazaly, M., Salama, T. T. & Dawood, M. S. The response characterization of Gafchromic TM EBT2 film to ultraviolet radiation of short wavelength (UVC). Phys. Scr. 96 (11). https://doi.org/10.1088/1402-4896/ac163a (2021).
Https://Www.Labomed.Com/Dual.Htm. (Accessed 20 July 2022).
Rashidian, M. & Dorranian, D. Low-intensity UV effects on optical constants of PMMA film. J. Theor. Appl. Phys. 8 (2), 0–7. https://doi.org/10.1007/s40094-014-0121-0 (2014).
Coury, C. & Dillner, A. M. A method to quantify organic functional groups and inorganic compounds in ambient aerosols using attenuated total reflectance FTIR spectroscopy and multivariate chemometric techniques, atmospheric environment, 42, Issue 23, Pages 5923–5932, ISSN 1352–2310. (2008).
Hall Amy V. and et al. Alkali metal salts of 10,12-Pentacosadiynoic acid and their dosimetry applications. Cryst. Growth Des. 21 (4), 2416–2422 (2021).
-Fleming, I. & Williams, D. Infrared and Raman Spectra. In I. Fleming & D. Williams (Eds.), Spectroscopic methods in organic chemistry (pp. 95–117). Springer. (2019). https://doi.org/10.1007/978-3-030-18252-6_2
Hassan, M. G. et al. Ion beam dosimetry using Gafchromic EBT3 film. Appl. Phys. A. 130, 454. https://doi.org/10.1007/s00339-024-07607-3 (2024).
Roberto, D., Ortuso, Niccolò Ricardi, T., Bürgi, T. A. & Wesolowski Kaori Sugihara, the Deconvolution analysis of ATR-FTIR spectra of diacetylene during UV exposure. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 219, 23–32. https://doi.org/10.1016/j.saa.2019.04.030 (2019).
Worboys, M. Spectroscopic investigation of the photodegradation of polydiacetylene solutions. J. Mater. Chem. 8, 903–912 (1998).
Author information
Authors and Affiliations
Contributions
H.I.E.: Conceptualization, Methodology, data analysis, Writing-Reviewing and Editing. M.EG.: Conceptualization, Investigation, Methodology, Data curation, data analysis, Writing-Reviewing and Editing. M.E.E.: Conceptualization, Writing-Reviewing and Editing. A.A.: Conceptualization, data analysis, Writing-Reviewing and Editing. M.I.F.: Investigation, Methodology, Data curation, data analysis, Writing-Reviewing and Editing. E.A.: investigation, Methodology, Data curation, data analysis, Writing-Reviewing and Editing. M.A.M.A.: investigations, Methodology, Data curation, data analysis, Writing-Reviewing and Editing. E.H.G.: Conceptualization, Investigation, Methodology, data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
El-Naggar, H.I., El Ghazaly, M., Elsisi, M.E. et al. On the response of the peeled-off Gafchromic EBT2 film to argon plasma jet. Sci Rep 15, 35954 (2025). https://doi.org/10.1038/s41598-025-94573-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94573-2