Abstract
Human movements must constantly be adapted due to changing internal and external conditions in our environment. The underlying neuronal mechanisms that are responsible for motor adaptations have so far mainly been investigated in highly controlled laboratory scenarios using simple motor tasks. However, because motor adaptations in daily life and sports entail more complex processes involving several cognitive components and strategic adjustments, results from such highly controlled settings only allow restricted conclusions and do not capture neuronal processing in everyday life scenarios. Hence, we studied 56 participants using a table tennis paradigm to unravel cortical activation during motor adaptation in a sport-specific setting using functional NIRS. Furthermore, we wanted to investigate whether cortical activation during motor adaptation is influenced by the temporal order of perturbations (serial vs. randomized practice). Our findings revealed brain areas such as the dorsolateral prefrontal cortex and primary sensory cortex, left supplementary motor cortex and left primary motor cortex, as well as right superior parietal cortex and right inferior parietal cortex, exhibited dynamic alterations in their activation as motor adaptation progressed. Specifically, hemodynamic response alterations generally increased during early adaptation and decreased as motor adaptation progressed. On the other hand, no differential changes in cortical brain processing were observed with serial and randomized practice. Based on our findings, we can confirm and extent theoretical models and laboratory evidence of motor adaptation using a sport-specific motor task.
Similar content being viewed by others
Introduction
Human movements frequently require ongoing motor adaptations due to a changing environment. These adaptations represent new sensorimotor relationships and form the basis for human motor behavior. In contrast to new movement skills, adapting already learned internal models to changing physical conditions or new environmental circumstances represent fast learning processes. These adaptational processes usually require only minor changes to previously learned movements1,2,3,4,5. Underlying neural mechanisms that are responsible for such adaptations have so far mainly been investigated in highly controlled laboratory settings using EEG or fMRI. However, these methodological approaches allow only limited conclusions for everyday scenarios as motor adaptations in everyday life involve more complex, often non stationary movement patterns with various cognitive components and strategic corrections6,7. In the present study, we used a naturalistic motor adaptation paradigm8 to unravel hemodynamic response alteration, defined as cortical activation, during motor adaptation in a sport-specific table tennis setting.
Background on motor adaptation
In general, two mechanisms are involved in motor adaptation: explicit learning driven by target error and implicit learning of a forward model driven by prediction error9. During the initial phase of adaptation, predominantly explicit learning takes place in order to minimize target errors. This fast process is followed by slow implicit learning, which gradually updates the sensorimotor mapping. When perturbations disappear after a new sensorimotor mapping, transient after-effects occur, which again represent target errors in the direction opposite to the initial sensorimotor conflict. These after-effects are considered evidence for an adapted internal model that initially persists when perturbation ends10. There are two classic approaches that make motor adaptations experimentally accessible. In force-field adaptation tasks1,2,5,11,12,13,14, the execution of the movement is disturbed by an additional or altered external force. In contrast, visuomotor rotation tasks3,4,9,15,16,17,18 do not disturb the execution of the movement itself, but the subsequent movement-effect relationship. This creates a mismatch between vision and proprioception that requires adaptations such that individuals have to modify their motor output to account for the respective perturbation and resolve the sensorimotor conflict. In real life scenarios, motor adaptations are sparsely investigated. We recently investigated motor adaptation using a table tennis paradigm. Here, a table tennis robot changed its ball spin during the course of adaptation phases. These environmental changes require sensorimotor adaptations to update the movement-effect relationship while movement execution is not directly affected. In summary, we were able to show that our paradigm reflects typical motor adaptations in a sport-specific context8. Furthermore, we observed that on a behavioral level, temporal ordering of the perturbations (serial vs. random practice) had no influence on motor adaptation performance. However, temporal ordering could still induce differential brain processing due to differences in cognitive effort or motivation. Lage et al.19 suggested that, despite comparable behavioral performances between serial and random practice schedules, mental resources required for each task may differ. This perspective aligns with findings20 indicating that constant and random practice schedules elicit distinct EEG patterns. However, whether serial and random perturbations within our paradigm also produce differential neural activity remains an open question.
Brain processing during motor adaptation
Evidence from both theoretical models and empirical studies concluded that cerebellar, striatal and neocortical structures such as primary motor cortex (M1), premotor cortex (PMC), dorsolateral prefrontal cortex (DLPFC) and supplementary cortex (SMA) as well as parietal cortex show dynamic alterations during visuomotor adaptation5,17,21,22,23. The cortico-striatal circuit particularly contributes during early motor adaptation, whereas the cortico-cerebellar circuit plays a more critical role during later phases22. Among these brain structures, the posterior parietal cortex (PPC) seems to be of particular relevance for successful motor adaptation7,24. In general, brain processing increases as a response to a sudden exposure to a perturbation (e.g., visuomotor rotation). On the other hand, brain processing decreases as visuomotor adaptation progresses, and increases again when the perturbation is suddenly removed17. These neuronal mechanisms of motor adaptations have mainly been observed using functional magnetic resonance imaging25,26. However, Yeo et al.7 pointed out that motor adaptation studies using fMRI are usually performed in a supine position due to structural limitations. Since posture directly affects sensorimotor integration and adaptation due to altered somatosensory and vestibular input and only a limited amount of movement is possible during fMRI measurements, one might argue that neural correlations of motor adaptation using fMRI do not necessarily generalize to correlates of adaptations in everyday movements6,7. In a recent study, fNIRS was used to investigate changes in cortical activation during motor adaptation in a spatial orientation task7. The study showed, that fNIRS in general seems to be feasible to unravel changes in brain processing as a consequence of motor adaptation7. However, many everyday and sport-specific motor adaptations take place in non-stationary motor conditions, e.g., during walking. These result from Yeo et al. can therefore not necessarily be generalized to everyday life scenarios or a sport-specific context.
A large number of studies have shown that fNIRS is in fact suitable for investigating cortical activation during the execution of controlled everyday life and sport-specific movements while standing and walking27,28. In the present study, we used a table tennis paradigm8 to unravel cortical activation during motor adaptation in a sport-specific table tennis setting and to differentiate between temporal order of perturbations. We hypothesized that (a) cortical structures, that are important for motor adaptation such as bilateral M1, PMC, SMA, DLPFC and PPC5,7,17,21,22,23 dynamically alter their activation as motor adaptation progresses. In addition, we hypothesized that (b) hemodynamic response alterations increase during early adaptation, decrease as visuomotor adaptation progresses, and increases again when the rotation is suddenly removed17. Furthermore, we hypothesized that (c) serial and randomized practice lead to comparable motor adaptation performances8,19 with higher cortical activation during serial compared to random practice19. According to the elaboration hypothesis, serial practice demands greater working memory load and sequence integration compared to an unforeseen practice schedule during random practice19. Thus, increased cortical activation is expected mainly in the prefrontal cortex, posterior parietal cortex and supplementary motor area, since these regions are mainly involved in cognitive control, motor planning, and sequence coordination22,24,29.
Material and methods
Participants
We recruited a total of 66 participants. Five participants had to be excluded due to missing data, two participants due to pain and three participants due to issues with the robot. Thus, 56 right-handed healthy volunteers, with an average age of 22.30 ± 2.26 years, ranging from 19 to 27 years old (25 female participants) were included in the present study. The study procedure was approved by the local ethics committee of the University of Leipzig (309/17-ek). Every participant gave written, informed consent, and every procedure followed the guidelines set forth in the Declaration of Helsinki. None of the participants had a history of neurological, psychiatric, cardiovascular, or musculoskeletal diseases, nor had they taken centrally acting medications. To evaluate the effects of motor adaptation during serial and random perturbation situations, participants were randomly divided in two groups. In order to rule out any significant differences between groups with regard to potential confounders, the number of hours that each group spent on sports and fine motor training each week were quantified (see Table 1). All volunteers were found to be right-handed based on the Edinburgh Handedness Questionnaire30 with a mean handedness score of 81.65 ± 1.90. The cut-off score of ≥ 50 indicated right-handedness, ≤ − 50 showed left-handedness, and ≥ − 50 indicated ambidextrous handedness31. Furthermore, we ensured that each participant was a novice table tennis player who had not taken part in regular table tennis training before. However, participants still needed to be able to execute the required movement skill—the backhand stroke—sufficiently well in predefined, simple conditions (without perturbations). To make sure that participants satisfied this need, we employed a pretest with a predetermined target accuracy (see the following section). The amount of time spent on sports (a) and fine motor training (b) per week—such as playing video games, knitting, crocheting, or playing an instrument—was measured using a standardized questionnaire. All participants completed a visual analog scale (VAS) before and after the experiment to rate their level of attention (1 (very distracted)—10 (very attentive), fatigue (1 (sleeping)—10 (very energetic), and discomfort (1 (no discomfort)—10 (strong discomfort)) in order to account for potential psychological confounders.
Experimental procedure
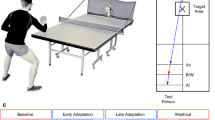
In the present study, we used a table tennis paradigm8 to unravel cortical activation during motor adaptation in a sport-specific setting (Fig. 1a). Participants performed backhand strokes (BH) cross-court against balls played by an app-controlled table tennis robot (Donic Newgy Robo-Pong 3050XL, Germany). During baseline and washout blocks participants performed BH against topspin balls. During adaptation blocks, participants performed BH against backspin balls, representing disturbances in terms of motor adaptation (see Fig. 1b,c). In contrast to our previous study8, we standardized the number of task switches for both conditions to improve the comparability of the two protocols. In the current study, we induced eight task switches in both random order and serial order condition (As-As-Al-Al-As-As, As: short ball, Al: long ball) with 16 balls per block being played. BH strokes were executed according to a block design for 20 × 32 s (jittered intertrial interval 35–40 s, 16 balls per block, see Fig. 1c,d), whereby participants were instructed to perform strokes as accurately as possible. Onsets of blocks were indicated via an auditory stimulus (short tone) and fNIRS triggers were set via Psychopy32. Motor adaptation was evaluated by means of target accuracy. 2D cinematography was used to determine the placement of participants’ balls. Target accuracy alterations due to adaptation were assessed and distances to the target were computed. Analog to our previously established procedure8, target accuracy was recorded with a high-speed video camera (GoPro Hero 9, San Mateo, US, 1920 × 1080 pixel, 200 frames per second,) and ball placement on the table tennis table was measured offline using Kinovea (v0.9.5, GPL-2.0 license).
Experimental setup and protocol. (a) Positioning of app-controlled ball robot at table tennis plate. Target marked with a cross. Participant in backhand stroke (BH) position carrying the fNIRS system. Photo editing was done using Adobe Photoshop (2024, v. 25) (b) Reference system of the racket. Translational movement in x-direction (left panel). Motor adaptation around transversal y-axis (right panel). (c) Experimental flow. Ball rotation changes (around transversal y-axis) in the course of adaptation phases. During baseline blocks participants performed BH against topspin balls (orange arrow). During adaptation blocks, participants performed BH against backspin balls (blue arrow), representing disturbances in terms of motor adaptation. In the last five blocks (washout) BH was performed against top spin balls, again. Averaging windows for the behavioral and fNIRS analysis are shown below the block diagram. (d) Each block consists of 16 trials (32 s). Blocks were separated by 35–40 s jittered intertrial intervals (ITI).
Functional near-infrared spectroscopy (fNIRS)
Hemodynamic responses were recorded using a whole-brain fNIRS system (NIRSport2, NIRX, US). Measurement requirements and fNIRS setup with 32 LED light sources and 32 avalanche photodiode detectors were used analog to Carius et al.33. In contrast to our previous study, we used additional washers for avalanche photodiodes (APD). These APD washers (diameter 2.5 cm) are intended to stabilize the APDs and prevent optodes from tilting. To ensure that washers fit under all optodes, we slightly modified the placement of the fNIRS optodes resulting in a more symmetrical configuration (106 actual measurement channels, see Fig. 2). In addition to the 106 (standard) channels, we also used eight short-distance detectors (NIRx Medical Technologies, Glen Head, NY) to exclude potential fNIRS confounders, such as changes in extracerebral blood flow. Data were acquired with a sampling frequency of 10.1725 Hz. To rule out expertise-related differences, we measured cardiac stress, operationalized via heart rate, during the execution of table tennis strokes using a Polar M430 sport watch (Polar Electro Oy, Kempele, Finland) with a sampling frequency of 1 Hz.
fNIRS configuration used during motor adaptation. Transmitters are shown as red dots and detectors as blue dots. Yellow dots represent each center of the 106 channels (inter-optode distance 35.8 ± 3.9 mm). Short-distance detectors are shown as blue circles surrounding the corresponding transmitters (8 mm distance from F3, F4, T7, T8, CP1, CP2, O1, O2). 10–20 positions for infrared sources (IR-S) and detectors (IR-D). Brain image was created with brain function mapping tool34. Respective brain regions, targeted by a 10–20 system transfer method35 and defined by the “Brodmann” Atlas (aPFC anterior prefrontal cortex, Broca broca area, dlPFC dorsolateral prefrontal cortex, IFG inferior frontal gyrus, IPC inferior parietal cortex, ITG inferior temporal gyrus, M1 primary motor cortex, MFG middle frontal gyrus, MTG middle temporal gyrus, OC occipital cortex, PMC premotor cortex, S1 primary somatosensory cortex, SMA supplementary motor cortex, SPC superior parietal cortex, STG superior temporal gyrus, TP temporal pole; L left hemisphere, R right hemisphere).
Data analyses
Behavioral data
Pythagorean theorem was used to calculate distances between hit points and target point. Distances and centroids, representing direction of deviations, were calculated for each participant and each block8. Data analysis and statistical analyses were performed using MATLAB (Version R2023a, MathWorks, Natick, MA, United States of America), RStudio (Version 2023.9.0.463, RStudio Team 2023) and JASP (Version 0.17, JASP Team 2021). Mean distances of blocks B3 to B5 (baseline), A2 to A4 (early adaptation), A8 to A10 (late adaptation), and W3 to W5 (washout) were averaged for statistical examination of adaptation-related performance changes. Mean distances of A1 indicate initial error at the beginning of the perturbation, while mean distances of W1 indicate after-effect. Direction of changes in target accuracy during baseline, perturbation, adaptation, after-effects, and washout are represented by density plots8. A 6 (time) × 2 (group) mixed ANOVA with post-hoc testing (i.e., T-tests) was used to examine target accuracy during 1) baseline, 2) initial error, 3) early adaptation, 4) late adaptation, 5) after-effect and 6) washout (see Fig. 1c) for the perturbation groups (serial vs. random). Data were spherically corrected, if needed, using the Greenhouse–Geisser adjustment. The problem of multiple comparisons was mitigated by the application of Holm adjustment.
Hemodynamics
FNIRS data analysis was performed in MATLAB using HOMER3 (version 1.80.2)36 and QT-NIRS37. We used QT-NIRS37 to estimate the fNIRS data quality. According to our previously established procedure33 (Fminmax = [0.5 2.5]; wLength = 3; sciThld = 0.7; pspThld = 0.1), 18 participants had to be excluded. The remaining participants (n = 38) showed good data quality for 81.8% of the channels. As a next step, the raw intensity signals were converted to changes in optical density36. Wavelet filtering was used to correct for motion artifacts38,39. We employed the HOMER3 hmrR_MotionCorrectWavelet filtering function (inter-quartile range 1.2240) and the approach outlined by Molavi and Dumont41. The data was slightly low-pass filtered with a low pass cutoff frequency of 3 Hz after motion-artifact correction. Since the following GLM tackles this with a polynomial drift correction, we did not employ a high pass filter. Using the modified Beer-Lambert technique (partial pathlength factor: 6.036), attenuation variations of both wavelengths (850 nm and 760 nm) were converted to concentration changes of oxy- and deoxygenated hemoglobin (HbO and HbR, respectively). As advised in the current literature42, we report both HbO and HbR (rather than just one chromophore changes). However, HbO and HbR reflect distinct neurophysiological processes related to brain activity. While HbO primarily reflects changes in blood supply, HbR is directly linked to the process of oxygen extraction from the blood43. HbO often exhibits larger signal changes and is therefore frequently the main focus of fNIRS studies. However, since HbR concentration changes (i) are less affected by systemic physiological noise44,45, (ii) have a stronger correlation with the blood oxygen level-dependent signal of the functional magnetic resonance imaging46,47, and (iii) are spatially more focused44,48, we primarily, though not solely, focus on the HbR parameter in the results and discussion section.
We used a general linear model approach (GLM) that employs ordinary least squares and a series of Gaussian functions with a standard deviation of 0.5 s and their means separated by 0.5 s over a specific regression time to model the hemodynamic response function (HRF) in order to regress extra-cerebral contaminations (measured by short-distance channels) out of the signal (used parameters in HOMER3 hrmR_GLM function: trange −2.0 to 40; glmSolveMethod 1; idxBasis 1; paramBasis 0.5 and 0.5). Additionally, we employed a third order polynomial fit to take baseline drift into consideration. The short separation channel, which exhibits the strongest correlation with the corresponding long-separation channel49,50, is used for short separation regression (SSR). Time courses of changes in HbO and HbR concentrations in each measurement channel and condition were block-averaged, and single trials were baseline corrected with respect to the two seconds before to stimulus initiation. For TFCE analysis, the complete HbO and HbR time courses were exported.
Statistical Analyses were performed using nonparametric threshold-free cluster enhancement (TFCE, Carius, et al.33) with a cluster threshold of p = 0.0551,52 and 10.0000 permutations. TFCE tests were conducted in MATLAB using the TFCE-Toolbox51. Differences in HbO and HbR concentration changes between motor adaptation phases (baseline vs. early adaptation vs. late adaptation vs. washout) and perturbation groups (serial vs. random) were tested using TFCE mixed two-way ANOVA (default TFCE parameters: E = 2/3, H = 1, Mensen and Khatami51, Smith and Nichols52). In the case of non-significant interactions, we conducted post hoc tests for the main effects time and group. For the factor time, we used dependent sample TFCE T-Tests (default parameters: E = 2/3, H = 2). For factor group we used independent sample TFCE T-Tests (default parameters: E = 2/3, H = 2).
We report cluster sizes (the number of significant channels and/or time points), corrected p-values and TFCE t-values for the peak coordinates of the resulting clusters. We temporally averaged TFCE t-values33 for all channels in the sample range 5 to 32 s in order to illustrate the task-related changes. Finally, we mapped them onto the brain surface using the brain function mapping tool34. Spatio-temporal clusters are printed as color-coded T-maps in time and space (106 channels).
Results
Behavioral data
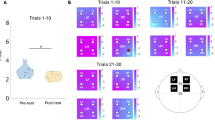
In terms of target accuracy, there was no interaction between time (baseline vs. initial error at perturbation onset vs. early vs. late adaptation vs. after-effects vs. washout) and perturbation (serial vs. random, F(2.54, 136.92) = 2.76, p = 0.054, ηp2 = 0.05). We also identified no differences between types of perturbation (F(1, 54) = 1.06, p = 0.307, ηp2 = 0.02). Thus, temporal order of perturbations did not influence motor adaptation. In contrast, we identified adaptation-dependent differences in target accuracy comparing baseline, initial error at perturbation onset, early and late adaptation, after-effects and washout across groups (F(2.54, 136.92) = 147.06, p < 0.001, ηp2 = 0.73). Post hoc tests revealed an increase in target errors at perturbation onset (baseline (B3-B5)—initial error (A1), Mdiff = −114.14, t(55) = -22.42, pholm < 0.001, d = −3.85), significant early adaptation (initial error (A1)—early adaptation (A2-A4), Mdiff = 68.18, t(55) = 13.39, pholm < 0.001, d = 2.30) and late adaptation effects (early adaptation (A2–A4)—late adaptation (A8–A10), Mdiff = 27.13, t(55) = 5.33, pholm < 0.001, d = 0.91). We observed large target errors when perturbations ends (late adaptation (A8-A10)—after-effect (W1), Mdiff = −46.53, t(55) = −9.14, pholm < 0.001, d = -1.57) and subsequently significant washout (after-effects (W1)—washout (W3-W5), Mdiff = 61.69, t(55) = 12.12, pholm < 0.001, d = 2.08). During perturbation onset, the majority of balls were played too short resulting in large target errors. In contrast, when perturbation disappears, majority of balls were played too long resulting in target errors in opposite direction (see Fig. 3a,b).
Motor adaptation assessed with table tennis paradigm. (a) Dot plot illustrates target error dispersions during initial error (block A1) at perturbation onset, final adaptation (block A10) and after effect (block W1). Display of hit points across 56 participants (896 trials per block: 56 participants × 16 trials). Dottet line represents linear trend. Intersection of dashed lines represents target. Box plots illustrate block-related dispersions in vertical and horizontal direction. Dashed lines show target in vertical and horizontal direction. During perturbation onset majority of balls were played too short resulting in large initial errors (orange). In contrast, when perturbation disappears, majority of balls were played too long resulting in target errors in opposite direction (after effect, blue). Gray box illustrates table tennis table. Hit points outside the table were calculated using data-driven linear model33. (b) Dot plots and line plots (exponential curve fit: y = a · eb · x ; y = Target error, x = Block; Random group: 139 · e−0.17 Block; R2 = 0.91; Serial group: 127 · e−0.17 Block; R2 = 0.66) showing adaptation-dependent performance changes in terms of spatial deviation from target (Euclidean distance) for random and serial group (lower panel). Double arrows below the block diagram illustrate the included data of the phase-related fNIRS block averages (upper panel).
Hemodynamics
Nonparametric cluster-based permutation analysis (TFCE) revealed no interactions between motor adaptation phases (baseline vs. early adaptation vs. late adaptation vs. washout) and type of perturbation (serial vs. random), neither for HbO (peak significance found at OCR: F(1,144)max = 5.47, pmax = 0.486, FWE TFCE-corrected), nor for HbR (ITGR: F(1,144)max = 5.07, pmax = 0.726). Additionally, we did not find significant effects for perturbation (HbO, dlPFCR: F(1,144)max = 10.18, pmax = 0.532, HbR, MTGR: F(1,144)max = 8.93, pmax = 0.799). In contrast, cluster-based permutation tests indicated differences between motor adaptation phases (HbO peak significance found at BrocaL: F(3,144)max = 17.71, pmax < 0.001, HbR peak significance found at BrocaL: F(3,144)max = 14.43, pmax < 0.001).
TFCE post hoc comparisons of HbR concentration changes indicated higher cortical activation during early adaptation compared to baseline in a widespread cluster (S1L, t(37)max = 6.09, pmax < 0.001) including S1L, dlPFCL, SMAL, STGR, S1R, SPCR, dlPFCR, BrocaR, PMCR, MTGR, STGL, OCR, MTGL, PMCL, M1L, BrocaL, IPCR, M1R & SMAR (76 channels, sorted by temporally aggregated TFCE t-values in descending order, Fig. 4a shows temporal aggregated difference maps for TFCE t-values, Range 5–32 s, and HbO & HbR grand-averages for ROIs with highest t-values). In contrast, the comparison of early and late adaptation indicated lower cortical activation during late adaptation compared to early adaptation in a widespread cluster (dlPFCR, t(37)max = −5.20, pmax < 0.001) including dlPFCL, dlPFCR, M1R, SMAL, SMAR, M1L, PMCL, SPCR, PMCR, aPFCL, S1R, IPCR, S1L, MTGR, aPFCR, IPCL & SPCL (50 channels, sorted by TFCE t-values in descending order, Fig. 4b shows grand-averages for ROIs as well as temporal aggregated difference maps for all TFCE t-values, Range 5–32 s). Finally, TFCE analysis indicated lower cortical activation during deadaptation/washout compared to late adaptation (dlPFCR, t(37)max = −4.46, pmax < 0.001, cluster including IPCR, IPCL, OCR, SPCL, S1L, aPFCR, dlPFCL, aPFCL, SPCR, M1L, SMAR, S1R, STGR, MTGR, SMAL, dlPFCR, STGL, MTGL, OCL, PMCR & PMCL (73 channels, sorted by TFCE t-values in descending order). Summarizing TFCE post hoc comparisons of HbR concentration changes (TFCE t-values summed across time phases: baseline vs. early adaptation, early vs. late adaptation, late adaptation vs. washout), largest overall adaptation-related changes were observed for dlPFCL, SMAL, dlPFCR, S1L, SPCR, M1L, S1R, IPCR,& OCR (Fig. 4c).
Adaptation-dependent hemodynamic response alterations during table tennis. Line Plots: Grand-averages (HbO & HbR) for ROIs with highest TFCE t-values across time phases. Gray shaded area represents activity phase (0–32 s). T-maps: Temporal aggregated task-related TFCE t-values (HbR). Optodes (transmitters & detectors) are shown for topographic images (created with brain function mapping tool34); colors represent mean TFCE t-values (sample range 5–32 s). Images are thresholded at p < 0.05. (a) Baseline vs. early adaptation. Red shaded areas between HbR concentration changes as well as topographic difference maps show increased HbR concentration changes during early adaptation compared to baseline indicating higher cortical activation during early adaptation (S1L, t(37)max = 6.09, pmax < .001). (b) Early adaptation vs. late adaptation. Blue shaded area between HbR concentration changes as well as topographic difference maps indicate decreased activation during late adaptation compared to early adaptation (dlPFCR, t(37)max = 5.20, pmax < .001).(c) Cortical activation across time phases (sorted by TFCE t-values). Upper panel: Baseline vs. early adaptation, Centre panel: Early adaptation vs. late adaptation, Lower panel: Summarizing TFCE post hoc comparisons of HbR concentration changes (stacked changes: baseline vs. early AD, early AD vs. late AD, late AD vs. washout), largest overall adaptation-related changes were observed for dlPFCL, SMAL, dlPFCR, S1L, SPCR, M1L, S1R, IPCR, & OCR. AD adaptation, TFCE threshold-free cluster enhancement, A anterior, P posterior, L left hemisphere, R right hemisphere.
Regarding HbO concentration changes also indicated higher cortical activation during early adaptation compared to baseline in a widespread cluster (STGL, t(37)max = 8.29, pmax < 0.001) including STGL, S1R, S1L, STGR, MTGL, MTGR, BrocaR, ITGL, OCL, dlPFCR, aPFCR, BrocaL, PMCL, dlPFCL, IPCL, M1R, OCR, ITGR, SPCR, IPCR, M1L, PMCR, aPFCL, SPCL & SMAR (85 channels, sorted by TFCE t-values in descending order). HbO also indicated lower cortical activation during late adaptation compared to early adaptation (aPFC, t(37)max = 6.07, pmax < 0.001) including dlPFCR, aPFCR, PMCR, ITGL, PMCL, BrocaL, dlPFCL, SPCR, STGL, IPCL, IPCR, OCL, SPCL, BrocaR, aPFCL, OCR, M1R, STGR, SMAR, S1L, M1L, ITGR, MTGR, MTGL & S1R (102 channels, sorted by TFCE t-values in descending order). Summarizing TFCE post hoc comparisons of HbO concentration changes (TFCE t-values summed across time phases: baseline vs. early adaptation, early vs. late adaptation, late adaptation vs. washout), largest overall adaptation-related changes were observed for STGR, MTGR, BrocaL, STGL, BrocaR, S1R, dlPFCR, S1L, aPFCR & ITGL.
Psychological and physiological confounders
Statistical analysis of questionnaires revealed a significant increase for participants’ discomfort (pre: 1.0 ± 0.0 [median ± MAD], post: 2.5 ± 0.5, Wilcoxon signed-rank tests: z = −5.28, p < 0.001). However, regarding attention (pre: 8.0 ± 1.0, post: 7.0 ± 1.0, z = 0.73, p = 0.458) and fatigue level (pre: 7.0 ± 1.0, post: 7.0 ± 1.0, z = −1.622, p = 0.098), there was no pre-post difference. There were no statistically significant differences between type of perturbation (serial vs. random) concerning pretest (Mann–Whitney U tests: attention: p = 0.062, U = 283.0, fatigue: p = 0.129, U = 300.5, discomfort: p = 0.804, U = 404.5), posttest (attention: p = 0.050, U = 276.0, fatigue: p = 0.052, U = 276.0, discomfort: p = 0.926, U = 385.5), or pre–posttest differences (attention: p = 1.00, U = 391.5, fatigue: p = 0.838, U = 379.0, discomfort: p = 0.515, U = 430.5).
Concerning cardiac stress operationalized via heart rate recordings during the execution of the table tennis strokes, there were no difference between groups (mean ± SD: Serial group: 92.50 ± 12.86 bpm, Random group: 88.32 ± 12.38 bpm; F(1, 54) = 1.60, p = 0.211, ηp2 = 0.03) and no interaction between time and group (F(1.98, 106.82) = 1.40, p = 0.250, ηp2 = 0.001). In contrast, the comparison of cardiac stress during baseline, early and late adaptation as well as deadaptation indicated lower heart rates during baseline (baseline: 87.98 ± 12.89 bpm, early adaptation: 91.13 ± 13.04 bpm, late adaptation: 91.25 ± 12.24 bpm; F(1.98, 106.82) = 12.72, p < 0.001, ηp2 = 0.01, Post Hoc baseline—early adaptation: t(55) = −4.68, pholm < 0.001, d = −0.25, early adaptation—late adaptation: t(55) = −0.15, pholm 1.000, d = −0.01). Even when heart rate is added as a covariate to the model, HbR and HbO response alterations still show a significant increase from baseline to early adaptation (fNIRS subsample, n = 38, all channels; HbR: F(1, 36) = 5.90, p = 0.020, ηp2 = 0.14; HbO: F(1, 36) = 29.36, p < 0.001, ηp2 = 0.45), indicating an effect independent from heart rate alterations. In addition, covariance analyses were conducted for those channels forming a cluster with significant HbR resp. HbO increases (see previous section). Again, the adaptation dependent increase remains after controlling for the effect of heart rate alterations (HbR: F(1, 36) = 9.32, p = 0.004, ηp2 = 0.21; HbO: F(1, 36) = 41.29, p < 0.001, ηp2 = 0.53).
Discussion
The aim of the present study was to unravel cortical activation during motor adaptation in a sport-specific table tennis setting. While earlier (neuro-)behavioral studies have explored motor adaptation and neural correlates in restricted laboratory settings21,53, we are aiming to adress everyday scenarios or sport-specific tasks that involve more complex processes, including various cognitive components and strategic corrections.
In line with our first hypothesis, we observed that (a) cortical structures, in particular, bilateral dlPFC, S1 & PMC, SMAL & M1L as well as SPCR and IPCR dynamically alter their activation as motor adaptation progresses. Specifically, in alignment with theoretical models and laboratory evidence (b) hemodynamic response alterations generally increased during early adaptation and decreases as visuomotor adaptation progresses. Contrary to our assumption, we observed further decreases during washout phase. In accordance with findings of our previous study8, we observed typical adaptation-dependent performance changes and the temporal order of perturbations did not influence the process of motor adaptation (hypothesis c). Serial and randomized practice lead to non-differential performance changes and cortical activation.
ROIs showing adaptation-related response alterations
We observed dynamical changes in cortical activation in bilateral dlPFC, bilateral S1 and bilateral PMC. Furthermore, we identified dynamical changes in SMAL and M1L as well as SPCR and IPCR throughout the adaptation process. The pronounced alterations in bilateral dlPFC and bilateral S1 corroborate the findings of Shadmehr and Holcomb5,54. These authors discovered that at the initial stage of the learning process, the capacity of individuals to adapt to a disturbing force field is linked to an elevated activation in the left putamen and bilateral dlPFC. Furthermore, according to the aforementioned study, in the subsequent stages of the adaptation process, the comparison of initial and final learning conditions revealed an activation decline in the left and right sensorimotor cortex and right putamen. We observed an activation increase in bilateral S1 and M1L during early adaptation when compared to baseline and an activation decrease during late adaptation when compared to early adaptation. Moreover, such adaptation-related changes were observed in the SMAL. Shadmehr und Holcomb5,54 posit, given the significant projections from the sensorimotor cortex to the putamen, that changes observed in these regions are likely related to large-scale reductions in motor output rather than to the acquisition of an internal model. The majority of recent empirical studies have attributed the stepwise encoding of an internal model for novel visuomotor transformations to prefrontal and PPC areas55. In this context, our results reinforce the significance of the bilateral dlPFC, where in addition to the initial increase in activation a subsequent decrease in activation was observed.
Furthermore, our investigation of a motor adaptation task in a sport-specific context provides evidence that dynamic alterations occur in PPC throughout the adaptation phase. These findings are specific to the SPCR and the IPCR. Generally speaking, the PPC, SPC in particular, facilitates sensorimotor integration by receiving multimodal input from the primary sensory cortex and output from the cerebellum. The IPC is responsible for new sensorimotor transformations and reference maps for spatial orientation and navigation7. We observed alterations in the SPCR during early and late adaptation. These findings confirm results from a recent fNIRS study7, which suggests that the right posterior parietal cortex (rPPC) may be responsible for the establishment of a visuomotor transformation in the early stages of adaptation. Moreover, lesion studies suggest that rPPC may be specialized for spatial somatosensory function. Moreover, a number of studies have demonstrated the particular importance of the rPPC in the context of visual-spatial attention56.
Finally, dPMC is also known to integrate proprioceptive and visual information17. When learning new visuomotor associations, both dPMC’s neural activity and cerebellar activation increased17 (Doyon et al., 2002). According to Tzvi et al.17, the cerebellar dPMC loop has a unique role in controlling the process of visuomotor adaptation. Thus, the dPMC seems to perform visuomotor integration, while the cerebellum most likely represents the internal model of motion. Furthermore, it has been proposed by Hardwick et al. (2015) that dPMC serves as a bridge between visuomotor control and higher cognitive functions during motor learning.
Direction of the adaptation-related response alterations
Research using fMRI has shown that cerebral activation increases in response to a sudden exposure to a visuomotor rotation, decreases as motor adaptation progresses, and increases again when the rotation is suddenly removed17,57. Furthermore, findings using fNIRS indicated that cortical activation initially increased and subsequently decreased after visuomotor adaptation occurs7,55. Taken together, empirical evidence suggest, that motor adaptations are linked to enhanced cognitive brain processing. Contrary to our original assumption, we observed decreasing activation during washout phase. This reduced cortical activation may be attributed to the fact that, in contrast to the adaptation phase, after-effects only occur for a brief period and can only be observed to a limited extent with our fNIRS methodology. As evidenced by behavioral data, the occurrence of significant errors is predominantly confined to the initial stage of the washout phase, specifically the initial block (W1). From the second washout-block onward, only minor kinematic errors were observed. However, the observed changes in cortical activation were averaged over five blocks (W1-W5). Therefore, fNIRS block averages do not only reflect a potentially activation increase in W1, but rather an overall enhancement in cortical brain processing across blocks W1-W5.
Serial vs. randomized practice
In contrast to our previous study, we standardized the number of task switches in the current study. We induced eight task switches in both the random order and the serial order group. Even with this standardized number of switches, we confirm that serial and randomized practice causes comparable contextual interference58,59,60. Also, our observations of cortical brain processing support this finding since serial and randomized practice induce similar changes in brain processing. This finding contradicts our original hypothesis, in which we suspected serial practice to induce higher cortical activation in regions responsible for working memory and sequence integration such as the PFC and SMA. However, according to the action plan reconstruction hypothesis, trial-by-trial skill variations necessitate retrieving information from long-term memory, independent from the factor predictability61. Even if participants noticed a predictability in the serial practice schedule, they never the less had to continuously build new motor action plans in each consecutive trial. This is also supported by our behavioral data, which clearly shows comparable contextual interference in both conditions. Furthermore, it might well be that participants didn´t even realized that the serial practice condition included repetitive trials.
The current study is also subject to some constraints. Even though subcortical structures are crucial for motor adaptation, with fNIRS neuroimaging only hemodynamic changes in cortical layers can be detected, making it impossible to determine if motor adaptation also affects subcortical activation (e.g., basal ganglia)21,25,57,62. Furthermore, kinematic observations may provide important additional information on the learning process. Particularly changes in hand movement (inclination of the table tennis racket) can be added to the current paradigm relatively easy and may provide important details of the actual movement adaptation. Additionally, the shift from baseline to early motor adaptation phase is not only characterized by a change from topspin (baseline) to backspin (early adaptation) but also by a shift from blocked practice (single ball velocity) to serial or random practice (changing ball velocities). Therefore, contrasting baseline and early adaptation phase in terms of cortical activation does not only portray neural adaptation to the perturbation spin and slightly changing ball trajectories due to the magnus effect but also neural adaptation to the perturbation ball length, induced by changing ball velocities. This was done to increase task complexity to ensure a slow and lasting motor adaptation process that is detectable over a long period of time using our blocked fNIRS protocol. Nevertheless, we observed that these induced complex perturbations led to typical behavioral adaptations, thus would conclude that they also induce typical neural adaptation. Also, the contrast early vs. late adaptation is not affected by this methodological limitation, since in both phases similar complex perturbations were presented. We used a ball robot to standardize the movement task as much as possible. However, this standardization also at least to some extend limits the movement task as compared to match conditions during a real table tennis game. Here, the game situation is constantly changing and the player has to anticipate which shot will be taken next. Technical and tactical information provides clues which action will be performed next (e.g., the opponent’s posture).
Finally, participants reported an increase in discomfort during the time course of the experiment. This in turn might have affected our fNIRS data as well. However, due to the dynamical nature of cortical activation during motor adaptation it seems unlikely that pain-associated brain activation might have contrasted our main findings. Nevertheless, future studies have to be designed to limit the influence of pain-related discomfort and associated brain activations.
Conclusion
Our whole-head fNIRS study was not only able to demonstrate the involvement of selected regions of interest, e.g., PPC7, during motor adaptations. Moreover, it furnished a more comprehensive representation of the continuously evolving cortical activation. In sum, our analysis of cortical brain processing during a complex sport-specific task (in a standing position) corroborates the findings of neurobehavioral studies that observed simple movement tasks under strictly controlled laboratory conditions (in a lying or sitting position).
Our findings revealed that cortical structures, particularly the bilateral dlPFC and S1, the SMAL and M1L, as well as the SPCR and IPCR, exhibited a dynamic alteration in their activation as motor adaptation progresses. In alignment with theoretical models and laboratory evidence, hemodynamic response alterations generally increased during early adaptation and decreased as visuomotor adaptation progressed. Furthermore, the temporal order of perturbations did not influence the process of motor adaptation. Serial and randomized practice led to the same performance changes. Additionally, no differential changes in cortical brain processing were observed with serial and randomized practice.
Data availability
All data that support the findings of this study are available from the corresponding author, D.C. if a formal data sharing agreement exists. Besides, all software used in the present study is open-source and as such publicly available.
References
Brashers-Krug, T., Shadmehr, R. & Bizzi, E. Consolidation in human motor memory. Nature 382, 252–255. https://doi.org/10.1038/382252a0 (1996).
Heald, J. B., Lengyel, M. & Wolpert, D. M. Contextual inference underlies the learning of sensorimotor repertoires. Nature 600, 489–493. https://doi.org/10.1038/s41586-021-04129-3 (2021).
Imamizu, H. et al. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403, 192–195. https://doi.org/10.1038/35003194 (2000).
Kording, K. P. & Wolpert, D. M. Bayesian integration in sensorimotor learning. Nature 427, 244–247. https://doi.org/10.1038/nature02169 (2004).
Shadmehr, R. & Holcomb, H. H. Neural correlates of motor memory consolidation. Science 277, 821–825 (1997).
McDougle, S. D. & Taylor, J. A. Dissociable cognitive strategies for sensorimotor learning. Nat. Commun. 10, 40. https://doi.org/10.1038/s41467-018-07941-0 (2019).
Yeo, S. S., Jang, T. S. & Yun, S. H. Sensorimotor adaptation in spatial orientation task: a fNIRS study. Sci. Rep. 13, 15160. https://doi.org/10.1038/s41598-023-42416-3 (2023).
Carius, D. et al. Quantifying motor adaptation in a sport-specific table tennis setting. Sci. Rep. 14, 601. https://doi.org/10.1038/s41598-023-50927-2 (2024).
Taylor, J. A., Krakauer, J. W. & Ivry, R. B. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J. Neurosci. 34, 3023–3032. https://doi.org/10.1523/JNEUROSCI.3619-13.2014 (2014).
Heald, J. B., Lengyel, M. & Wolpert, D. M. Contextual inference in learning and memory. Trends Cogn. Sci. 27, 43–64. https://doi.org/10.1016/j.tics.2022.10.004 (2023).
Shadmehr, R. & Mussa-Ivaldi, F. A. Adaptive representation of dynamics during learning of a motor task. J. Neurosci. 14, 3208–3224. https://doi.org/10.1523/JNEUROSCI.14-05-03208.1994 (1994).
Herzfeld, D. J., Vaswani, P. A., Marko, M. K. & Shadmehr, R. A memory of errors in sensorimotor learning. Science 345, 1349–1353. https://doi.org/10.1126/science.1253138 (2014).
Shadmehr, R. & Brashers-Krug, T. Functional stages in the formation of human long-term motor memory. J. Neurosci. 17, 409–419. https://doi.org/10.1523/JNEUROSCI.17-01-00409.1997 (1997).
Sheahan, H. R., Franklin, D. W. & Wolpert, D. M. Motor planning, not execution, separates motor memories. Neuron 92, 773–779. https://doi.org/10.1016/j.neuron.2016.10.017 (2016).
Galea, J. M., Vazquez, A., Pasricha, N., de Xivry, J. J. & Celnik, P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb. Cortex 21, 1761–1770. https://doi.org/10.1093/cercor/bhq246 (2011).
Heuer, H. & Hegele, M. Adaptation to visuomotor rotations in younger and older adults. Psychol. Aging 23, 190–202. https://doi.org/10.1037/0882-7974.23.1.190 (2008).
Tzvi, E., Koeth, F., Karabanov, A. N., Siebner, H. R. & Kramer, U. M. Cerebellar—Premotor cortex interactions underlying visuomotor adaptation. Neuroimage 220, 117142. https://doi.org/10.1016/j.neuroimage.2020.117142 (2020).
Wolpe, N. et al. Age-related reduction in motor adaptation: brain structural correlates and the role of explicit memory. Neurobiol. Aging 90, 13–23. https://doi.org/10.1016/j.neurobiolaging.2020.02.016 (2020).
Lage, G. M., Faria, L. O., Ambrósio, N. F. A., Borges, A. M. P. & Apolinário-Souza, T. What Is the level of contextual interference in serial practice? A meta-analytic review. J. Motor Learn. Dev. 10, 224–242. https://doi.org/10.1123/jmld.2021-0020 (2022).
Lelis-Torres, N., Ugrinowitsch, H., Apolinario-Souza, T., Benda, R. N. & Lage, G. M. Task engagement and mental workload involved in variation and repetition of a motor skill. Sci. Rep. 7, 14764. https://doi.org/10.1038/s41598-017-15343-3 (2017).
Tzvi, E., Loens, S. & Donchin, O. Mini-review: The role of the cerebellum in visuomotor adaptation. Cerebellum 21, 306–313. https://doi.org/10.1007/s12311-021-01281-4 (2022).
Doyon, J., Penhune, V. & Ungerleider, L. G. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 41, 252–262. https://doi.org/10.1016/s0028-3932(02)00158-6 (2003).
Krebs, H. I. et al. Robot-aided functional imaging: application to a motor learning study. Hum. Brain Mapp. 6, 59–72. https://doi.org/10.1002/(SICI)1097-0193(1998)6:1 (1998).
Gottlieb, J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron 53, 9–16. https://doi.org/10.1016/j.neuron.2006.12.009 (2007).
Bedard, P. & Sanes, J. N. Brain representations for acquiring and recalling visual-motor adaptations. Neuroimage 101, 225–235. https://doi.org/10.1016/j.neuroimage.2014.07.009 (2014).
Debas, K. et al. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc. Natl. Acad. Sci. USA 107, 17839–17844. https://doi.org/10.1073/pnas.1013176107 (2010).
Herold, F. et al. Functional near-infrared spectroscopy in movement science: a systematic review on cortical activity in postural and walking tasks. Neurophotonics 4, 041403. https://doi.org/10.1117/1.NPh.4.4.041403 (2017).
Hamacher, D., Herold, F., Wiegel, P., Hamacher, D. & Schega, L. Brain activity during walking: A systematic review. Neurosci. Biobehav. Rev. 57, 310–327. https://doi.org/10.1016/j.neubiorev.2015.08.002 (2015).
Dayan, E. & Cohen, L. G. Neuroplasticity subserving motor skill learning. Neuron 72, 443–454. https://doi.org/10.1016/j.neuron.2011.10.008 (2011).
Oldfield, R. C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9, 97–113. https://doi.org/10.1016/0028-3932(71)90067-4 (1971).
Dragovic, M. Categorization and validation of handedness using latent class analysis. Acta Neuropsychiatr. 16, 212–218. https://doi.org/10.1111/j.0924-2708.2004.00087.x (2004).
Peirce, J. W. PsychoPy–psychophysics software in python. J. Neurosci. Methods 162, 8–13. https://doi.org/10.1016/j.jneumeth.2006.11.017 (2007).
Carius, D. et al. Increased cortical activity in novices compared to experts during table tennis: A whole-brain fnirs study using threshold-free cluster enhancement analysis. Brain Topogr. https://doi.org/10.1007/s10548-023-00963-y (2023).
Wang, Y., Yan, J., Wen, J., Yu, T. & Li, X. An Intracranial electroencephalography (iEEG) brain function mapping tool with an application to epilepsy surgery evaluation. Front. Neuroinform. 10, 15. https://doi.org/10.3389/fninf.2016.00015 (2016).
ZimeoMorais, G. A., Balardin, J. B. & Sato, J. R. fNIRS optodes’ location decider (fOLD): a toolbox for probe arrangement guided by brain regions-of-interest. Sci. Rep. 8, 3341. https://doi.org/10.1038/s41598-018-21716-z (2018).
Huppert, T. J., Diamond, S. G., Franceschini, M. A. & Boas, D. A. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 48, 280–298. https://doi.org/10.1364/AO.48.00D280 (2009).
Hernandez, S. M. & Pollonini, L. NIRSplot: A tool for quality assessment of fNIRS scans. Biomed. Opt. 2020 https://doi.org/10.1364/BRAIN.2020.BM2C.5 (2020).
Brigadoi, S. et al. Motion artifacts in functional near-infrared spectroscopy: a comparison of motion correction techniques applied to real cognitive data. NeuroImage 85 (1), 181–191. https://doi.org/10.1016/j.neuroimage.2013.04.082 (2014).
Di Lorenzo, R. et al. Recommendations for motion correction of infant fNIRS data applicable to multiple data sets and acquisition systems. NeuroImage 200, 511–527. https://doi.org/10.1016/j.neuroimage.2019.06.056 (2019).
Molavi, B. & Dumont, G. A. Wavelet-based motion artifact removal for functional near-infrared spectroscopy. Physiol. Meas. 33, 259–270. https://doi.org/10.1088/0967-3334/33/2/259 (2012).
Carius, D., Hörnig, L., Ragert, P. & Kaminski, E. Characterizing cortical hemodynamic changes during climbing and its relation to climbing expertise. Neurosci. Lett. 715, 134604. https://doi.org/10.1016/j.neulet.2019.134604 (2020).
Yücel, M. A. et al. Best practices for fNIRS publications. Neurophotonics 8, 012101. https://doi.org/10.1117/1.NPh.8.1.012101 (2021).
Tachtsidis, I. & Scholkmann, F. False positives and false negatives in functional near-infrared spectroscopy: issues, challenges, and the way forward. Neurophotonics 3, 030401. https://doi.org/10.1117/1.NPh.3.3.030401 (2016).
Dravida, S., Noah, J. A., Zhang, X. & Hirsch, J. Comparison of oxyhemoglobin and deoxyhemoglobin signal reliability with and without global mean removal for digit manipulation motor tasks. Neurophotonics 5, 011006. https://doi.org/10.1117/1.NPh.5.1.011006 (2018).
Kirilina, E. et al. The physiological origin of task-evoked systemic artefacts in functional near infrared spectroscopy. NeuroImage 61, 70–81. https://doi.org/10.1016/j.neuroimage.2012.02.074 (2012).
Huppert, T. J., Hoge, R. D., Dale, A. M., Franceschini, M. A. & Boas, D. A. Quantitative spatial comparison of diffuse optical imaging with blood oxygen level-dependent and arterial spin labeling-based functional magnetic resonance imaging. J. Biomed. Opt. 11, 064018. https://doi.org/10.1117/1.2400910 (2006).
Huppert, T. J., Hoge, R. D., Diamond, S. G., Franceschini, M. A. & Boas, D. A. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. NeuroImage 29, 368–382. https://doi.org/10.1016/j.neuroimage.2005.08.065 (2006).
Plichta, M. M. et al. Event-related functional near-infrared spectroscopy (fNIRS) based on craniocerebral correlations: reproducibility of activation?. Hum. Brain Mapp. 28, 733–741. https://doi.org/10.1002/hbm.20303 (2007).
Lühmann, A., Ortega-Martinez, A., Boas, D. A. & Yücel, M. A. Using the general linear model to improve performance in fnirs single trial analysis and classification: A perspective. Front. Hum. Neurosci. 14, 30. https://doi.org/10.3389/fnhum.2020.00030 (2020).
Yücel, M. A. et al. Specificity of hemodynamic brain responses to painful stimuli: a functional near-infrared spectroscopy study. Sci. Rep. 5, 9469. https://doi.org/10.1038/srep09469 (2015).
Mensen, A. & Khatami, R. Advanced EEG analysis using threshold-free cluster-enhancement and non-parametric statistics. Neuroimage 67, 111–118. https://doi.org/10.1016/j.neuroimage.2012.10.027 (2013).
Smith, S. M. & Nichols, T. E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98. https://doi.org/10.1016/j.neuroimage.2008.03.061 (2009).
Reuter, E. M., Booms, A. & Leow, L. A. Using EEG to study sensorimotor adaptation. Neurosci. Biobehav. Rev. 134, 104520. https://doi.org/10.1016/j.neubiorev.2021.104520 (2022).
Shadmehr, R. & Holcomb, H. H. Inhibitory control of competing motor memories. Exp. Brain Res. 126, 235–251. https://doi.org/10.1007/s002210050733 (1999).
Gentili, R. J., Shewokis, P. A., Ayaz, H. & Contreras-Vidal, J. L. Functional near-infrared spectroscopy-based correlates of prefrontal cortical dynamics during a cognitive-motor executive adaptation task. Front. Hum. Neurosci. 7, 277. https://doi.org/10.3389/fnhum.2013.00277 (2013).
Sengupta, A., Banerjee, S., Ganesh, S., Grover, S. & Sridharan, D. The right posterior parietal cortex mediates spatial reorienting of attentional choice bias. Nat. Commun. 15, 6938. https://doi.org/10.1038/s41467-024-51283-z (2024).
Tzvi, E., Gajiyeva, L., Bindel, L., Hartwigsen, G. & Classen, J. Coherent theta oscillations in the cerebellum and supplementary motor area mediate visuomotor adaptation. Neuroimage 251, 118985. https://doi.org/10.1016/j.neuroimage.2022.118985 (2022).
Giuffrida, C., Shea, J. & Fairbrother, J. T. Differential transfer benefits of increased practice for constant, blocked, and serial practice schedules. J. Mot. Behav. 34, 353–365. https://doi.org/10.1080/00222890209601953 (2002).
Keller, G. J., Li, Y., Weiss, L. W. & Relyea, G. E. Contextual interference effect on acquisition and retention of pistol-shooting skills. Percept. Mot. Skills 103, 241–252. https://doi.org/10.2466/pms.103.1.241-252 (2006).
Sekiya, H. Contextual interference in implicit and explicit motor learning. Percept. Mot. Skills 103, 333–343. https://doi.org/10.2466/pms.103.2.333-343 (2006).
Lee, T. D. & Magill, R. A. Can forgetting facilitate skill acquisition?. Adv. Psychol. 27, 3–22. https://doi.org/10.1016/S0166-4115(08)62528-5 (1985).
Taylor, J. A. & Ivry, R. B. Cerebellar and prefrontal cortex contributions to adaptation, strategies, and reinforcement learning. Prog. Brain Res. 210, 217–253. https://doi.org/10.1016/B978-0-444-63356-9.00009-1 (2014).
Funding
Open Access funding enabled and organized by Projekt DEAL.
This work was supported by the Deutsche Forschungsgesellschaft (DFG, German Research Foundation), Grant Number 396169757.
Author information
Authors and Affiliations
Contributions
DC: Methodology, Conceptualization, Formal analysis, Investigation, Software, Visualization, Writing—original draft, Writing—review & editing. EK: Methodology, Conceptualization, Writing—review & editing. MC: Methodology. PR: Conceptualization, Resources, Supervision, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carius, D., Kaminski, E., Clauß, M. et al. Dynamic alterations in cortical activation during motor adaptation in table tennis using whole‑brain fNIRS. Sci Rep 15, 10399 (2025). https://doi.org/10.1038/s41598-025-94699-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94699-3