Abstract
The role of amino acids (AAs) with bone health is still controversial. We examined the association between AAs and osteoporosis in a cross-sectional study of 135 participants aged 45 years or older from the Second Hospital of Jilin University. Plasma AAs were measured with targeted quantitative methodology. We measured bone mineral density (BMD) with dual energy x-ray absorptiometry, and osteoporosis was defined as a T-score ≤ -2.5. We estimated odds ratios (OR) and corresponding 95% confidence intervals (CIs) for the associations between AAs (per 1 standard deviation increase) with osteoporosis. Approximately 18.5% of participants (n = 25) had osteoporosis. Total (adjusted β = 0.052; P = 0.002) and non-essential AA (adjusted β = 0.064; P = 0.002) levels were associated with femoral neck BMD T-scores. Greater levels of total (adjusted OR: 0.734; 95% CI: 0.655–0.821), essential (adjusted OR: 0.763; 95% CI: 0.623–0.934) and non-essential AAs (adjusted OR: 0.721; 95% CI: 0.629–0.826) were associated with lower odds of osteoporosis. Higher tryptophan (adjusted OR: 0.498; 95% CI: 0.281–0.882), cysteine (adjusted OR: 0.561; 95% CI: 0.321–0.983), glycine (adjusted OR: 0.513; 95% CI: 0.285–0.922), and ornithine levels (adjusted OR: 0.581; 95% CI: 0.345–0.978) were associated with reduced osteoporosis risk. Higher AA levels were associated with higher femoral neck BMD, and lower odds of osteoporosis.
Similar content being viewed by others
Introduction
Amino acids (AAs) are basic building blocks of proteins. The human body requires protein and non-protein AAs. There are 20 protein AAs and more than 1000 non-protein AAs. Among these AAs, essential AAs must be obtained through food (i.e., beef, eggs and plant-based foods) or supplements. AA metabolism plays a crucial role in maintaining human health and development of diseases (i.e., sarcopenia and cardiometabolic disease)1,2.
Bone mineral density (BMD), a quantitative measure of calcium and other minerals in bone, is a key measure to define osteoporosis and osteopenia in humans. A recent survey of 16,377 Chinese participants found that the age standardized prevalences of osteopenia and osteoporosis were 27.32% and 3.51%, respectively3. Osteoporosis and osteoporotic fractures are associated with increased risk of comorbidities, dependency, and even death4,5.
There is evidence suggesting that proteins or AAs modulate bone remodeling and bone mass6,7,8. Higher protein intake was associated with greater lumbar spine BMD9. After binding with specific AAs, class 3 G-protein coupled receptor family could regulate bone turnover through the activation of calcium ions6. Ding et al. found that AAs promote bone marrow stromal cell (BMSC) survival, which plays a key role for bone anabolism6. L-arginine and L-lysine are suggested to stimulate osteoblast proliferation, activation, and differentiation7.
Although there is a potential link between AAs and bone remodeling, the relationship between AAs and bone health in humans is still uncertain. Lower serum branched-chain AA levels were associated with increased risk of vertebral fractures in postmenopausal women10. A recent systematic review reported that the association between AAs with BMD differed by type of AA, such as glycine or valine11. There is also a conflicting association between leucine and BMD11. The uncertain relationship of glutamine, hydroxuproline, and taurine with osteopenia and osteoporosis have been noted in another systematic review12. Lastly, most of the existing studies used an untargeted rather than a targeted platform to measure AAs11. AAs measured with a targeted quantitative methodology is helpful to minimize the heterogeneity between studies13.
Therefore, we sought to examine the association between AAs, measured with a targeted high performance liquid chromatography coupled with mass spectrometry, with BMD, osteoporosis, and osteopenia. This research may extend our understanding about the complex relationship between AAs and bone health.
Materials and methods
Study setting and subjects
In this cross-sectional study, we identified participants from the Second Hospital of Jilin University in 2022. There was no randomization during the sample enrollment. Individuals aged 45 years or older with valid BMD and AA measurement data were included. Valid BMD values were defined as measurements with: (1) no removable or non-removable objects; (2) no excessive x-ray “noise” due to obesity; (3) no body parts out of the scan region; (4) no positioning problems; and (5) no other problems, such as participant motion or unknown artifacts. We calculated the inter-quartile ranges (IQRs) for each AA to determine whether AA measurements were valid. Valid AA values were defined as measurements within the range of 1.5 times the IQR below the first quartile and above the third quartile. We excluded individuals with current or past use of osteoporosis related medications (i.e., steroid or anti-osteoporosis medications), and those diagnosed with secondary osteoporosis (i.e., type I diabetes, osteogenesis imperfect, untreated long-term hyperthyroidism, hypogonadism or premature menopause [< 45 years old], systemic lupus erythematosus, rheumatoid arthritis, chronic liver disease, chronic malnutrition and malabsorption). We enrolled 135 participants in our study, which exceeded the minimum sample size of 64 that is required for this research question11. Written informed consent was obtained from all participants, and the study protocol was approved by the institutional review board (IRB) of the School of Public Health, Jilin University (Project #: 2022-02-02). All methods were performed in accordance with the relevant guidelines and regulations.
Blood collection and assay of AAs
To control the impact of dietary intake, we collected 8 + hour fasting blood samples from participants at their first visit before they received any treatment. The blood samples were processed and refrigerated at −80 ℃ until assay.
The details of the AA assay methodology have been described in detail in a previous study14. Briefly, following the creation of the plasma filter paper, we extracted the paper with ethanol. After centrifugation and filtration, the supernatant was moved to a 96-well plate. AA metabolite standards (Cambridge Isotope Laboratory, Tewksbury, MA, USA) and the AA quality control solution were also moved to the 96-well plate. We initially dried the plate with nitrogen, and subsequently cultured with a 1-butanol acetyl chloride mixture. This was followed with nitrogen. The test sample was mixed with mobile phase solution (80% acetonitrile aqueous solution) and measured by high performance liquid chromatography coupled with mass spectrometry. For each run, we injected 20 µL of sample. The initial flow rate was set at 0.2 mL/min. Afterwards, the speed was adjusted to 0.01 mL/min within 0.08 min (the speed was kept constant for 1.5 min). Afterwards, it returned to 0.2 mL/min within 0.01 min, and remained unchanged until 0.5 min. Curtain gas pressure was 20 psi and the ion spray voltage was 4.5 kV. The ion source gas pressure was 35 psi and the temperature of auxiliary gas was 350 ℃. Other parameters for AA measurements are published elsewhere15.
We categorized AAs into essential or non-essential. Essential AAs include leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. Non-essential AAs were alanine, asparagine, aspartic acid, arginine, citrulline, cysteine, glutamine, glutamic acid, glycine, homocysteine, histidine, ornithine, proline, serine, and tyrosine. Aromatic (e.g., phenylalanine, tyrosine, tryptophan), sulfur (methionine, cysteine and homocysteine), and branched-chain AAs (e.g., leucine and valine) were also considered in our analyses. Lastly, we calculated AA ratios such as methionine/phenylalanine and valine/phenylalanine to determine the effects of specific AA catabolism.
BMD measurement, osteopenia and osteoporosis diagnosis
We measured femoral neck, total hip, and lumbar spine BMD with the dual energy x-ray absorptiometry (DXA; Discovery Wi, Hologic, Bedford, MA, USA). A control spine phantom scan was performed each day to ensure the reliability of measurements. The coefficient of variation (CV) was < 0.5%. The BMD T-score was calculated using the following formula: BMD T-score = (BMD of an individual-referenced BMD)/(standard deviation [SD] of the referenced BMD). The referenced BMD and its SD were extracted from previous studies16,17,18. Osteoporosis and osteopenia were defined as femoral neck, total hip, or lumbar spine BMD T-scores of ≤ −2.5, and >−2.5 and <−1.0, respectively16.
Ascertainment of covariates
We considered the following covariates according to the International Osteoporosis Foundation’s (IOF’s) One-Minute Osteoporosis Risk Test19: demographics (sex and age), lifestyle factors (physical activity, smoking status, alcohol intake > 1 time/week, milk intake > 1 time/week, seafood intake > 1 time/week, calcium supplementation [yes/no]), disease history (hypertension, coronary heart disease, type 2 diabetes, and fracture), height loss of more than 3 cm after age 40 years, family history of osteoporosis, body mass index (BMI) and postmenopausal status. Body weight and height were directly measured. BMI was computed as weight divided by height2 (kg/m2). Other covariate data were collected via face-to-face interviews using a structured questionnaire. Smoking was defined as any current or past tobacco use. Physical activity (metabolic equivalent task [MET]-hours/week), including basal, sedentary, light, moderate, and heavy activity, was estimated using the Framingham questionnaire20.
Statistical analysis
We descriptively analyzed the baseline characteristics, BMD T-scores, and bone health status using frequency, percentage, mean ± SD, and median (IQR). Pearson correlation coefficients between AAs were performed. We used multivariable linear regression models to estimate β and P for the association between AAs and BMD T-score. β means each unit increase in AA leads to its corresponding change in BMD. P indicates the evidence against a null hypothesis; a smaller Psuggests stronger evidence of rejecting the null hypothesis. AA values that followed a normal distribution, as assessed by skewness and kurtosis, were scaled per 1-SD increase. For AA values that were not normally distributed, a unit increase was expressed as 1-SD increase on the logarithmic scale. After conducting the bivariate analyses between participants’ characteristics and BMD T-score, we found that the following covariates had significant associations with femoral neck, total hip, or lumbar spine BMD T-score based on alpha = 0.05: age, sex, alcohol intake > 1 time/day, BMI, physical activity, type 2 diabetes, and height loss > 3 cm. Thus, these factors were included in the final adjusted models. Multiple testing was addressed using false discovery rate (FDR) analysis, which stands for the chance of false discoveries21. We also performed multivariable logistic regression analyses to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between AA levels with osteopenia or osteoporosis. An OR shows the odds of a participant having osteoporosis when AA levels increase by one unit, while 95% CI means that we are 95% confident that the true OR will be in this range. Interaction terms between covariates and AAs (covariate*AA) in relation to osteoporosis were also tested in the models. The type II errors for the relationship between AAs and osteoporosis were also tested using an established method22. We excluded osteoporosis cases from all osteopenia analyses. Logistic regression models were adjusted for the same covariates as above. We validated the goodness-of-fit of logistic regression models using the Hosmer-Lemeshow test (P for all AAs > 0.05). Using the multivariable logistic regression or linear regression methods, we tested the association of aromatic, sulfur, and branched-chain AAs and AA ratios with BMD T-score, osteopenia, and osteoporosis. For the the random effect models, we used the R (Version: 4.1.2; R Foundation for Statistical Computing, Vienna, Austria) package “metafor” to pool the overall effects of total, essential, non-essential, and aromatic AAs on BMD, and “meta” on osteopenia and osteoporosis. Because the associations of each unit increase in AAs with BMD T-score were different, the meta-analysis approach partly addressed this issue. Descriptive and regression analyses were performed using the SPSS software (version 24.0; SPSS, Chicago, IL). We used the ‘fdrtool’ packages in the R platform to estimate the FDR. R scripts are shown in Supplementary Table 1.
Results
Descriptive analysis of the characteristics of participants
To define the study population, we conducted descriptive analysis of the baseline characteristics, BMD T-scores, and bone health status of participants. The related results are shown in Table 1. Of the 135 participants in the study, 29 (21.48%) were females. Approximately 75.9% of the females were postmenopausal. The mean age and BMI were 57.6 ± 5.0 years and 24.9 ± 3.1 kg/m2, respectively. Approximately half of the participants (51.9%) were smokers. There were 3 (2.2%) participants with coronary heart disease, 20 (14.8%) with hypertension, 10 (7.4%) with type 2 diabetes, and 13 (9.6%) with a history of fracture. Few individuals had a family history of osteoporosis (4.4%). Sixty-three (46.7%) participants had osteopenia, and 25 (18.5%) had osteoporosis.
Pearson correlations between AAs and bivariate linear regression analysis of the relationship between participants’ characteristics and BMD
We estimated the Pearson correlation coefficients between AAs, and performed bivariate linear regression analyses of the relationship between participants’ characteristics and BMD. The correlation coefficients (r) between AAs were relatively low (Pearson r mean = 0.26; SD = 0.19; Supplementary Fig. 1). In the bivariate analyses, older age was negatively associated with femoral neck BMD T-scores (Supplementary Table 2). Greater BMI and being male were positively associated with femoral neck, total hip, and lumbar spine BMD T-scores. Alcohol intake > 1 time/day was associated with greater total hip BMD T-scores. Lumbar spine BMD T-scores were higher among participants with type 2 diabetes, and lower among those with a height loss > 3 cm.
Associations between AA levels and bone health: multivariable analysis
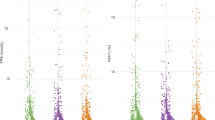
We conducted multivariable analysis of the association between AAs with BMD, osteoporosis, and osteopenia. As shown in Fig. 1, after adjusting for covariates, total (β = 0.052; P = 0.002) and non-essential AA (β = 0.064; P = 0.002) levels were positively associated with femoral neck BMD T-scores. The FDRs for total and essential AAs with femoral neck BMD were 0.025 and 0.025 in the adjusted models, respectively. Similar patterns were found for the relationship between total and non-essential AA levels with total hip and lumbar spine BMD, though these results did not reach statistical significance. However, greater levels of total (adjusted OR: 0.734; 95% CI: 0.655–0.821), essential (adjusted OR: 0.763; 95% CI: 0.623–0.934), and non-essential AAs (adjusted OR: 0.721; 95% CI: 0.629–0.826) were associated with lower odds of osteoporosis; the type II errors for these AAs were approximately 0.7. (Fig. 2). The FDRs for total, essential, and non-essential AAs were < 0.001, 0.075, and < 0.001, respectively. There was no evidence of multiplicative interaction between age, sex, alcohol intake, BMI, physical activity, type 2 diabetes, or height loss of > 3 cm with AAs in relation to osteoporosis (Supplementary Table 3). Among these AAs, higher tryptophan (adjusted OR per 1-SD increase: 0.498; 95% CI: 0.281–0.882), cysteine (adjusted OR per 1-SD increase: 0.561; 95% CI: 0.321–0.983), glycine (adjusted OR per 1-SD increase: 0.513; 95% CI: 0.285–0.922), and ornithine levels (adjusted OR per 1-SD increase: 0.581; 95% CI: 0.345–0.978) were associated with reduced odds of osteoporosis; their corresponding FDRs were 0.106, 0.154, 0.130, and 0.154, respectively. However, no association was observed between total, essential, or non-essential AAs with osteopenia after adjustment (Supplementary Fig. 2).
Multivariable linear regression analysis of the association between amino acid levels and bone mineral density (BMD) T-score. *Amino acids are per 1-SD increase in logarithmic scale; other amino acids are per 1-SD increase. **Models were adjusted for age, sex, alcohol intake > 1 time/day, body mass index, physical activity, type 2 diabetes, and height loss > 3 cm. FDR = false discovery rate.
Multivariable logistic regression analysis of the association between amino acid levels and osteoporosis. *Amino acids were per 1-SD increase on logarithmic scale; other amino acids are per 1-SD increase. Models were adjusted for age, sex, alcohol intake > 1 time/day, body mass index, physical activity, type 2 diabetes, and height loss > 3 cm.
Associations of AA ratio, aromatic, sulfur, and branched-chain AAs with bone health: multivariable analysis
We performed analyses to determine the associations between AA ratios, aromatic, sulfur, and branched-chain AAs with bone health. We found that none of AA ratios were associated with BMD T-scores, osteopenia, or osteoporosis (Supplementary Tables 4–5 and Supplementary Figs. 3–4). In addition, higher levels of aromatic and branched-chain AAs were not significantly associated with BMD T-scores, osteopenia, nor osteoporosis (Supplementary Tables 6 and Supplementary Figs. 5–6). Greater total sulfur AA levels were not associated with BMD T-scores or osteopenia, but with lower odds of osteoporosis (Supplementary Tables 6 and Supplementary Figs. 5–6).
Discussion
In this cross-sectional study, we found that higher levels of total and non-essential AAs were associated with increased femoral neck BMD. Greater levels of total, essential, and non-essential AAs were associated with lower odds of osteoporosis. Tryptophan, cysteine, glycine, and ornithine were of particular importance in the negative association. Higher sulfur AA levels were associated with lower risk of osteoporosis. However, there was no significant association between AA ratios, aromatic AAs, or branched-chain AAs with BMD, osteopenia or osteoporosis.
To the best of our knowledge, no study to date has investigated the effect of total AAs on bone health. Previous studies on the association between AAs and osteoporosis mainly focused on individual AAs examined in isolation12. Various AAs were suggested to have different associations with BMD23. We hypothesized that analyzing the effects of total AAs may help to resolve the discordant relationship between individual AAs and bone health. The negative association between total AAs and osteoporosis in our study supports our hypothesis.
The relationship of cysteine and glycine levels with BMD and/or osteoporosis is partly in line with the previous two meta-analyses11,12, in which cysteine and glycine levels were correlated with BMD or osteoporosis. The negative relationship between sulfur AAs and osteoporosis in our study was primarily attributed to the beneficial effect of cysteine. The null association between aromatic AAs and bone health in our study is partly in line with a previous longitudinal study, in which aromatic AAs (except for tryptophan) had low predictive value for BMD decline and 10-year fracture risk24. A previous case-control study found a positive correlation between tryptophan and BMD25, which is concordant with the negative association between tryptophan and osteoporosis in our study. Whether branched-chain AAs are associated with bone health remains unclear, though a previous study found that branched-chain AAs were associated with BMD decline24. Aside from our study, no other study has reported a negative relationship between ornithine levels and osteoporosis. However, ornithine metabolism is involved in the oxidation and protein production, which play a role in bone remodeling9,26,27. Furthermore, AAs, including ornithine, participate in skeletal development and homeostasis28.
Homocysteine levels were not significantly associated with BMD or osteoporosis in our study. A two-sample Mendelian randomization study found that serum homocysteine levels had causal effects on BMD, but not osteoporosis29. The Hordaland Homocysteine Study suggested that elevated plasma homocysteine levels were associated with reduced BMD in women, but not in men30. Despite the valuable findings of these studies, there is great heterogeneity regarding blood samples (serum vs. plasma), bone health measures (BMD vs. osteoporosis), and sex.
The negative relationship between AAs and osteoporosis may be explained by several underlying mechanisms. First, BMSCs are involved in both intracellular and extracellular nutrient-sensing pathways for AAs6. Second, AA receptors and transporters are expressed in osteoblasts, supporting the role of AAs on bone formation31. Third, AAs increase the production of insulin-like growth factor-1 (IGF-1), which promotes osteoblast proliferation and bone mineralization, and suppresses osteoclast differentiation and bone resorption32. Lastly, there is evidence suggesting that AAs enhance intestinal calcium absorption, promote Alk Phos secretion, and decrease the interleukin-6 levels. The elevated Alk Phos and decreased interleukin-6 levels may lead to greater bone collagen synthesis and bone formation, and lower bone resorption32.
Our study had several limitations. Our sample size is moderately small, possibly limiting our ability to detect significant effects of some AAs on bone health measures. However, the sample size is likely sufficient to estimate the overall significant results for the relationship between total AAs and osteoporosis. Data on some factors such as education level, vitamin D intake, marital status, and phosphate were not collected. We cannot exclude the concern of residual confounding. Consistent with all cross-sectional studies, we cannot establish temporality and a causal inference between AAs and bone health cannot be made. Only 21.5% of the study sample were females. A small proportion of females may limit the generalizability of our results to females, especially to postmenopausal females. Using a meta-analysis method may be limited for examining total AA effects considering the correlations between AAs. A better method is still warranted. Lastly, the participants were identified from a hospital, and there was no randomization during sample collection. Although we excluded individuals who were currently taking osteoporosis-related medications and had secondary osteoporosis, our results may not be generalizable to other populations, and we cannot exclude the potential of selection bias. Future research with bigger sample sizes and those using a longitudinal study design is warranted to address these limitations.
Conclusions
In this study, higher levels of AAs were associated with higher femoral neck BMD, and lower odds of osteoporosis. Among these AAs, cysteine, tryptophan, glycine, and ornithine levels were negatively associated with the odds of osteoporosis. The beneficial effects of AAs on bone health beyond muscle and kidney health warrant further confirmation33,34.
Data availability
The datasets used and/or analysed in this study are available on request from the corresponding author.
References
Le Couteur, D. G. et al. Branched chain amino acids, aging and age-related health. Ageing Res. Rev. 64 https://doi.org/10.1016/J.Arr.2020.101198 (2020).
Ling, Z. N. et al. Amino acid metabolism in health and disease. Signal. Transduct. Target. Ther. 8 https://doi.org/10.1038/s41392-023-01569-3 (2023).
Fan, Y. et al. Sex- and Age-Specific Prevalence of Osteopenia and Osteoporosis: Sampling Survey. Jmir Public. Health Surveillance. 10 https://doi.org/10.2196/48947 (2024).
Shen, Y. et al. The Global Burden of Osteoporosis, Low Bone Mass, and Its Related Fracture in 204 Countries and Territories, 1990–2019. Front. Endocrinol. 13 https://doi.org/10.3389/fendo.2022.882241 (2022).
Hopman, W. M. et al. Longitudinal assessment of health-related quality of life in osteoporosis: data from the population-based Canadian Multicentre Osteoporosis Study. Osteoporos. Int. 30, 1635–1644. https://doi.org/10.1007/s00198-019-05000-y (2019).
Ding, K. H. et al. Amino acids as signaling molecules modulating bone turnover. Bone 115, 15–24. https://doi.org/10.1016/j.bone.2018.02.028 (2018).
Torricelli, P., Fini, M., Giavaresi, G. & Giardino, R. Human osteopenic bone-derived osteoblasts: Essential amino acids treatment effects. Artif. Cell. Blood Sub. 31, 35–46. https://doi.org/10.1081/Bio-120018002 (2003).
El Refaey, M. et al. Kynurenine, a tryptophan metabolite that accumulates with age, induces bone loss. J. Bone Min. Res. 32, 2182–2193. https://doi.org/10.1002/jbmr.3224 (2017).
Shams-White, M. M. et al. Dietary protein and bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. Am. J. Clin. Nutr. 105, 1528–1543. https://doi.org/10.3945/ajcn.116.145110 (2017).
Urano, T., Kuroda, T., Uenishi, K. & Shiraki, M. Serum branched-chain amino acid levels are associated with fracture risk in Japanese women. Geriatr. Gerontol. Int. 24, 603–608. https://doi.org/10.1111/ggi.14896 (2024).
Lau, K. T., Krishnamoorthy, S., Sing, C. W. & Cheung, C. L. Metabolomics of osteoporosis in humans: a systematic review. Curr. Osteoporos. Rep. 21, 278–288. https://doi.org/10.1007/s11914-023-00785-8 (2023).
Wang, Y. H. et al. Distinct metabolites in osteopenia and osteoporosis: a systematic review and meta-analysis. Nutrients 15 https://doi.org/10.3390/Nu15234895 (2023).
Panahi, N. et al. Metabolomic biomarkers of low BMD: a systematic review. Osteoporos. Int. 32, 2407–2431. https://doi.org/10.1007/s00198-021-06037-8 (2021).
Liang, B. et al. Association between amino acids and recent osteoporotic fracture: a matched incident case-control study. Front. Nutr. 11 https://doi.org/10.3389/Fnut.2024.1360959 (2024).
Jing, F. et al. Discriminating gastric cancer and gastric ulcer using human plasma amino acid metabolic profile. Iubmb Life. 70, 553–562. https://doi.org/10.1002/iub.1748 (2018).
Kanis, J. A. et al. A reference standard for the description of osteoporosis. Bone 42, 467–475. https://doi.org/10.1016/j.bone.2007.11.001 (2008).
Kelly, T. L. Bone mineral density reference databases for American men and women. J. Bone Min. Res. 5, S249 (1990).
Looker, A. C. et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos. Int. 8, 468–489. https://doi.org/10.1007/s001980050093 (1998).
Zhang, H. M. et al. Clinical value of self-assessment risk of osteoporosis in Chinese. Open Med. 11, 190–195. https://doi.org/10.1515/med-2016-0036 (2016).
Kannel, W. B. & Sorlie, P. Some health benefits of physical activity. The Framingham Study. Arch. Intern. Med. 139, 857–861 (1978).
Chen, X., Robinson, D. G. & Storey, J. D. The functional false discovery rate with applications to genomics. Biostatistics 22, 68–81. https://doi.org/10.1093/biostatistics/kxz010 (2021).
Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval Health Prof. 18, 1–12 (2021).
Cui, Z., Feng, H., He, B., He, J. & Tian, Y. Relationship between serum amino acid levels and bone mineral density: A Mendelian randomization study. Front. Endocrinol. 12 https://doi.org/10.3389/fendo.2021.763538 (2021).
Su, Y. et al. Circulating amino acids are associated with bone mineral density decline and ten-year major osteoporotic fracture risk in older community-dwelling adults. Bone 129 https://doi.org/10.1016/j.bone.2019.115082 (2019).
Pernow, Y. et al. Associations between amino acids and bone mineral density in men with idiopathic osteoporosis. Bone 47, 959–965. https://doi.org/10.1016/j.bone.2010.08.017 (2010).
Zhang, J. Y. et al. Ornithine and breast cancer: a matched case-control study. Sci. Rep-Uk. 10 https://doi.org/10.1038/S41598-020-72699-9 (2020).
Kimball, J. S., Johnson, J. P. & Carlson, D. A. Oxidative Stress and Osteoporosis. J. Bone Joint Surg. Am. 103, 1451–1461. https://doi.org/10.2106/Jbjs.20.00989 (2021).
Suzuki, A. & Iwata, J. Amino acid metabolism and autophagy in skeletal development and homeostasis. Bone 146, 115881. https://doi.org/10.1016/J.Bone.2021.115881 (2021).
Wang, C., Zhang, X. & Qiu, B. Genetically predicted circulating serum homocysteine levels on osteoporosis: a two-sample mendelian randomization study. Sci. Rep-Uk. 13 https://doi.org/10.1038/s41598-023-35472-2 (2023).
Gjesdal, C. G. et al. Plasma total homocysteine level and bone mineral density - The Hordaland homocysteine study. Arch. Intern. Med. 166, 88–94. https://doi.org/10.1001/archinte.166.1.88 (2006).
Dirckx, N., Moorer, M. C., Clemens, T. L. & Riddle, R. C. The role of osteoblasts in energy homeostasis. Nat. Rev. Endocrinol. 15, 651–665. https://doi.org/10.1038/s41574-019-0246-y (2019).
Bihuniak, J. D. & Insogna, K. L. The effects of dietary protein and amino acids on skeletal metabolism. Mol. Cell. Endocrinol. 410, 78–86. https://doi.org/10.1016/j.mce.2015.03.024 (2015).
Posey, E. A., Bazer, F. W. & Wu, G. Y. Amino acids and their metabolites for improving human exercising performance. Adv. Exp. Med. Biol. 1332, 151–166. https://doi.org/10.1007/978-3-030-74180-8_9 (2021).
Li, X. Y., Zheng, S. X. & Wu, G. Y. Amino acid metabolism in the kidneys: nutritional and physiological significance. Adv. Exp. Med. Biol. 1265, 71–95. https://doi.org/10.1007/978-3-030-45328-2_5 (2020).
Acknowledgements
We are especially grateful to the participants involved in this study.
Funding
This research was supported by the Funding from the Science and Technology Department of Jilin Province (Grant Number: 20210101197JC) and Jilin Provincial Department of Finance (2024WSZXB05).
Author information
Authors and Affiliations
Contributions
Conception, design, and analysis (SY and QH); data collection and interpretation of data (all authors); drafting the article (SY); funding acquisition (JL and QH); critically revising the article for important intellectual content (all authors); final approval of the version to be published (all authors); and agreement to be accountable for all aspects of the work (all authors).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review boards (IRBs) of the School of Public Health, Jilin University (Project #: 2022-02-02). All participants provided their written informed consent to participate in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, S., Liu, T., Wang, X. et al. Plasma levels of amino acids and osteoporosis: a cross-sectional study. Sci Rep 15, 9811 (2025). https://doi.org/10.1038/s41598-025-94766-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94766-9