Abstract
This study examined the effects of biochar and nitrogen fertilizer application on CO2 emissions, microbial communities, and soil organic carbon (SOC) in irrigated wheat fields through a 3-year field experiment. Eight treatment groups were established for this study: (1) CK, without fertilizer or biochar, (2) N1 group, with nitrogen fertilizer application (300 kg/ha), (3) B group, with biochar application (20 t/ha), (4) BN1 group, with nitrogen fertilizer and biochar application, (5) N2, with a 15% reduction in nitrogen fertilizer (255 kg/ha), (6) BN2, with a 15% reduction in nitrogen fertilizer + biochar. (7) N3, with a 30% reduction in nitrogen fertilizer (210 kg/ha); and (8) BN3, with a 30% reduction in nitrogen fertilizer + biochar. The results revealed an increase in active organic carbon (AOC) and SOC contents of soil after the addition of biochar and N fertilizer, particularly with their combined application. In the BN2 treatment, SOC and AOC contents reached 27.48 g/kg and 1.47 g/kg, representing increases of 3.04% and 30.91%, respectively, compared to N1. In comparison to CK, cumulative CO2 emissions increased by 9–48% with the addition of both biochar and nitrogen fertilizer, possibly due to biochar’s influence on the composition and functional diversity of soil microbial communities. The functional diversity of soil microbes in the BN1 group differed significantly from that in CK (p < 0.01). In the B group, soil microbial attributes were lower than those in BN1, BN2, and BN3 groups. Furthermore, the bulk density of biochar-amended soil was 0.19 g/cm3 lower than that of untreated soil in CK. Overall, the combination of biochar application and a nitrogen dose of 255 kg/ha emerged as the most effective strategy for irrigated wheat fields in northern Xinjiang, enhancing SOC content while reducing carbon emissions. However, further research is required to assess the long-term effects of this approach on soil health and sustainability.
Similar content being viewed by others
Introduction
Fertilizers are introduced into the soil to provide nutrients for plant growth. Chemical fertilizers are widely used due to their efficiency and convenience, significantly increasing crop yields and ensuring stable agricultural production1. However, extensive application of chemical fertilizers causes a nutrient imbalance in agricultural soil, leading to a lower content of organic matter2. These changes negatively affect the physiochemical and biological characteristics of soil, resulting in lower soil fertility3. Thus, continuous application of chemical fertilizers affects crop yield by degrading soil quality4. Biochar application to soil is considered a crucial strategy to resolve the environmental issues associated with current agronomic practices, including the overuse of chemical fertilizers5. Various research studies recommend biochar application as an effective approach to improving soil quality and enhancing crop production6,7. Biochar not only promotes the sequestration of carbon but also enhances the soil fertility. Studies have reported that the combined application of biochar and nitrogen fertilizer or a nitrogen-containing nutrient can improve crop productivity and enhance nitrogen-use efficiency7,8,9. Biochar contributes to carbon stabilization by influencing soil biological properties, including enzymatic activities, microbial communities, and respiration10, which significantly influence the dynamics of soil organic carbon (SOC). Biochar application may also alter the chemical characteristics of soil, such as pH11 and cation exchange capacity (CEC), thereby enhancing nutrient retention12,13,14. However, soil microbes, including fungi and bacteria, are highly sensitive to environmental changes caused by biochar15, making it essential to assess its impact on microbial communities due to their crucial role in soil processes16. For instance, soil microbes are required for complete decomposition of organic matter in soil17. The role of biochar in regulating soil carbon storage, including microbial biomass carbon (MBC) and soil organic carbon (SOC), along with its influence on CO2 emissions, remains a topic of debate. Moreover, determining its effectiveness in enhancing SOC sequestration is essential. However, previous studies have demonstrated both adverse and positive impacts of biochar on SOC storage18,19. Therefore, a thorough understanding of its influence on SOC mineralization is essential before considering its widespread use as a soil additive.
Based on the findings derived from previous studies, a 3-year field experiment was carried out in northern Xinjiang, China, to assess the effects of biochar and nitrogen fertilizer on soil dynamics. The study aimed to (1) investigate the changes in soil respiration rate after biochar application with varying concentrations of nitrogen fertilizers; and (2) determine the effect of the interaction between nitrogen fertilizer and biochar on microbial community, SOC content, and soil characteristics. Further, CO2 emission levels were determined at different application rates of biochar and nitrogen fertilizer. This study hypothesized that the combined application of biochar and nitrogen fertilizers alters soil microbial dynamics and respiration processes, with the extent of these effects depending on the application rates. If biochar application is found to increase CO2 emissions while simultaneously enhancing SOC levels, its potential as a soil management strategy could be more significant than previously anticipated. The results of this study could contribute to the development of effective agronomic measures to reduce CO2 emissions while improving the N consumption efficiency in irrigated croplands.
Material and methodology
Experimental site
The field experiment was conducted at the Qitai Wheat Test Station in Xinjiang (89° 13′ to 91° 22′ E; 42° 25′ to 45° 29′ N) (Fig. 1). This region has a continental temperate climate, with an annual average temperature of 5.5 °C. In July and January, the average temperatures are 22.6 °C and − 18.9 °C, respectively, while the yearly maximum can reach approximately 39 °C. The region has a mean annual relative humidity of 60% and an average of 153 frost-free days, ranging from late April to early October. The annual average rainfall is 269.4 mm. The test site has sandy loam soil, with 15.15 g/kg organic matter in the 0–20 cm soil layer. The basic properties of soil were as follows: pH 8.2; SOC content of 8.79 g/kg; total nitrogen content of 0.93 g/kg; available phosphorus content of 7.10 mg/kg; and available potassium content of 35.1 mg/kg.
Experiment and treatment groups
A total of 24 plots, each measuring 35 m2, were established at the research station following a completely randomized factorial design. The experiment consisted of 8 treatment groups: (1) CK, control group without fertilizer or biochar; (2) N1 group, with nitrogen fertilizer application (300 kg/ha); (3) B group, with biochar application (20 t/ha); (4) BN1 group, with nitrogen fertilizer and biochar application; (5) N2, with 15% reduction in nitrogen fertilizer (255 kg/ha), (6) BN2, with biochar + 15% less nitrogen fertilizer; (7) N3, with 30% reduction in nitrogen fertilizer (210 kg/ha and (8) BN3, with biochar + 30% less nitrogen fertilizer. Each group had three replicates. Urea served as the nitrogen fertilizer, with a recommended application rate of 300 kg N/ha for the region. Initially, biochar was manually spread over the designated plots in a single application before sowing. It was then thoroughly integrated into the 0–30 cm soil layer using a rotary cultivator to ensure uniform distribution. Biochar was applied only once at the start of the experiment and was not reapplied in the next 2 years. Urea application (46% pure nitrogen) was performed once in the designated plots. Strip sowing was carried out at a density of 4.5 million plants per hectare, with a row spacing of 20 cm. All other agricultural practices followed the standard protocols for high-yield farmland in the region.
Biochar application to the soil was performed by Jinhefu Shenyang Agricultural Technology Development Corporation, China. The biochar used in this study was prepared through the pyrolysis of corn straw for 4 h at 450 °C, in the absence of oxygen. Its key properties included a surface area of 0.8 m2 g−1; mean particle diameter of 0.004–3.7 mm; ash content of 45%; total carbon content of 59.84%; C/N ratio of 38.59; and pH of 9.3. Before application, biochar was sieved using a 2-mm sieve.
Soil sampling
After harvesting in July, five soil cores were randomly collected from each plot to a depth of 30 cm. The samples were immediately placed in a cooler and stored at 4 °C until further processing. Subsampling of moist soil was performed for all assessments. The bulk density of the soil was calculated as the dry mass per unit volume of the cylinder. Since the soil contained no stones, the total bulk density is reported in this study20. The pH of the soil samples was measured using a 2:1 water-to-soil mixture21. CEC was determined by using ammonium acetate extract, while the sum of cations was expressed as the base saturation22. A field kit was employed to determine the active organic carbon (AOC) content in soil using a modified permanganate method23,24. Hach colorimeter (Hach Company, Boulder, CO) was used to measure the absorbance of samples at 550 nm. The decrease in the purple permanganate color reflected the oxidizable carbon content in the soil samples, with greater color loss indicating a higher oxidizable carbon concentration25. The total SOC content was measured following the method described by Jones and Willett26. Briefly, soil samples were extracted with distilled water (soil-to-water ratio: 1:5) for 30 min under agitation at 230 rpm. The mixture was centrifuged at 4000 rpm, filtered through a 0.45-μm membrane, and analyzed using a multi-N/C analyzer (Analytik Jena 3100, Germany) to quantify the total SOC content27.
Bacterial and fungal isolation and cultivation
The drop plate process was employed to determine the bacterial colony forming units (CFUs)28. The soil suspensions were diluted tenfold to prepare three samples, and five drops (10 μL each) were placed on TSB agar plates (3 g/L). Two plates were prepared for each soil sample, dried, inverted, and incubated at 15 °C. After 36 h and 48 h of incubation, bacterial colonies were counted by Leica MZ6 modular stereomicroscope while the viable fungi were quantified by inoculating soil suspensions on wort agar plates, supplemented with streptomycin29,30.
Analysis of the functional diversity of soil microbes
To assess the functional diversity of microbes in soil samples, BIOLOG ECO well plates (Hayward, USA) were used31. Soil (2.0 g) was added to 18 mL of disinfected NaCl solution (0.87% w/v) and allowed to shake for 20 min. Aliquots (150 µL) of this solution were then introduced into the wells of a BIOLOG ECO plate and incubated at 15 °C in the absence of light. Absorbance in each well was measured at 590 nm using a plate reader (EL808, BioTek) over 8 days at regular intervals (18, 44, 68, 89, 115, 138, 182, and 206 h).
Determination of CO2 emissions from the soil
Three days before the first emission measurement, three polyvinyl chloride (PVC) collars (10 cm diameter, 5 cm height) were vertically inserted 5 cm into the soil between plant rows in each plot. To prevent the leakage of gas, the soil around each collar was pressed tightly. CO2 emissions from the PVC collars were measured weekly between 9:00 and 11:00 a.m. using an automated CO2 flux system (LI-8100, LI-COR Inc., USA) throughout the wheat growing season. During CO2 flux measurements, stem thermometers were placed near the PVC collars to measure the temperature of soil at a depth of 5 cm.
The total CO2 emissions over the growth period were obtained by integrating daily fluxes. For days without measurements, fluxes were interpolated using the mean of the two nearest measurement days and scaled by the respective time duration. The yield-wise CO2 emissions were determined by dividing the cumulative CO2 emissions by the yield of crop32.
Calculations
The average well color development (AWCD) of BIOLOG ECO plates represents the microbial capacity to metabolize carbon sources, serving as a key indicator of functional diversity and activity in the soil. AWCD values were calculated as follows:
where C represents the absorbance of soil suspension in each well of plate, while R denotes the absorbance observed for the control well.
AWCD values reflect the microbial activity in the soil samples, while diversity indices describe microbial community composition, species distribution, and functional diversity. The commonly used diversity indices include the Simpson, McIntosh, and Shannon indices.
Mclntosh index (U), which reflects the homogeneity of the microbial community, was calculated as follows:
Simpson index (D) reflects the dominant species in the microbial community which can be calculated by using the following formula:
Shannon index (H) represents the richness of species in a microbial community and can be calculated as follows:
where P is the ratio of absorbance in i well to the total absorbance across all wells.
Statistical analysis
Statistical analyses of data were performed using SPSS 21.0 (SPSS Incorporation, USA). Data were expressed as means ± standard error (SE) based on triplicate measurements for each treatment group. The differences between the measured parameters of treatment groups were assessed by one-way analysis of variance (ANOVA). The significance of variations was examined through least significant difference (LSD) analysis at a confidence level of 95%.
Results
Soil respiration and SOC content
Biochar application influenced the soil respiration rate, with most treatments demonstrating a sharp decrease in CO2 release during the first 7 days, followed by a stabilized release (Fig. 2b). Biochar and nitrogen fertilizer application significantly affected the cumulative C mineralization (p < 0.01). Cumulative emissions were higher in BN1 and N3 upon comparison with CK and B groups (Fig. 2a). The combined application of biochar and nitrogen fertilizer increased cumulative CO2 emissions by 9–48% relative to CK (Fig. 2a). Similarly, the effect size distribution for AOC and SOC showed a consistent pattern across all observations. The changes in SOC content were consistent, showing a significant increase after biochar application (Fig. 3). The combined influence of nitrogen fertilizer and biochar application was more pronounced for SOC and AOC (p < 0.01).
Composition of the microbial community in soil
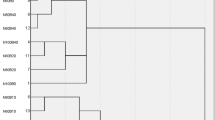
The application of biochar significantly influenced microbial functional diversity (Fig. 4). Compared to CK, BN1 showed higher AWCD values (p < 0.01), followed by BN3, BN2 and B groups. These differences became more pronounced in the later stages of incubation (after 72 h). Furthermore, Shannon and McIntosh indices were significantly affected by the application of biochar (p < 0.01) (Table 1). The interaction between nitrogen fertilizer and biochar played a key role in determining bacterial abundance and the bacteria-to-fungi ratio in the soil microbial community (Fig. 5). Compared to the groups with combined application of biochar and nitrogen fertilizer, microbial community attributes were lower when only biochar was applied (Fig. 4, Table 1).
Effects of treatments on the soil properties
Electrical conductivity, bulk density, and pH of soil under various treatments are presented in Table 2. Biochar application reduced soil bulk density, with a consistent decrease of 0.19 g/cm3 compared to CK3. Soil bulk density increased when biochar was applied alongside nitrogen fertilization (BN1, BN2, BN3). Similar to bulk density, soil electrical conductivity also showed an increasing trend. Moreover, biochar application led to a slight change in soil pH compared to CK.
Discussion
Soil CO2 emission and SOC content were substantially influenced by the combined application of N fertilizer and biochar (Figs. 2 and 3). The increase in CO2 emissions following biochar application may be due to the expansion of variable SOC pools and the mineralization of indigenous SOC, leading to enhanced production of CO233,34,35. The decrease in soil CO2 emissions after biochar application may be due to biochar adsorbing rhizodeposits and enzymes, which inhibited the activity of carbon-degrading microbes and reduced the priming effect on native SOC36,37,38. Maucieri et al.39 suggested that the decrease in CO2 emissions observed after biochar application may be associated with the decreased available N in soil due to microbial biomass and immobilization. According to Xiao et al.40, variations in CO2 emissions following biochar and nitrogen fertilizer application depend on the nitrogen fertilizer application rate. The observed decrease in CO2 emissions following biochar application suggested SOC immobilization and decreased microbial activity due to nutrient adsorption on the biochar surface. Buss et al.41 also reported the decrease in CO2 emissions attributing it to the adsorption of DOC on the biochar surface or the formation of soil aggregates that protect SOM from decomposition41. Other possible reasons include the mineralization and immobilization of N, and variation in soil microbial populations. Furthermore, the increase in CO2 emissions induced by biochar application is likely associated with a reduction in soil bulk density and enhanced microbial activity. Microorganisms play a critical role in the cycling of materials and energy flow in the soil. In addition to influencing biochemical cycles42, they also contribute to biochar stabilization in the soil43. Biochar can directly promote microbial growth and activity by providing favorable conditions15 and indirectly by enhancing soil aeration, moisture retention44, and chemical characteristics45. Moreover, biochar influences microbial biomass and activity, modifies the fungal-to-bacterial ratio, alters enzyme activity, and restructures the soil microbial community. Due to its aromatic structure, biochar is resistant to microbial decomposition, though certain biochar types can serve as a carbon source for microbial growth. Biochar is generally recalcitrant to microbial degradation due to higher C/N compared to the feedstock. The limited nitrogen availability further restricts the microbial decomposition of biochar. Therefore, biochar application contributed to an increase in the SOC pool and enhanced soil carbon sequestration. Bacteria and fungi prefer different carbon sources and exhibit varying levels of tolerance for changes in environmental conditions, such as pH and moisture content. Compared to bacteria, fungi can colonize larger soil aggregates, with higher C/N and total N, providing a survival advantage for fungi. Under similar environmental conditions, biochar application favored fungal growth by promoting the formation of larger soil aggregates. The observed changes in the fungal-to-bacterial ratio in this study may be associated with alterations in soil C/N following biochar addition or the initial C/N ratio of untreated soil. These findings suggested that nutrients within biochar, including both inherent nutrients and those absorbed from the soil, support microbial growth and activity. Further, the fungal hyphae and bacterial cells colonized into the inner and outer pores of biochar contribute to spatial heterogeneity. With the increase of biochar aging, the decline in its pH may enhance fungal adaptation and colonization, influencing microbial community composition in the soil. Soil bulk density influences its structural stability, water and nutrient transport, and aeration46,47. Several studies have revealed the decline in the bulk density of soil is associated with biochar application48,49,50. Niu51 and Kang52 observed a decrease in the bulk density of upland soil after biochar application. The findings of this study align with those of Zhang53, who reported a significant reduction in soil bulk density, ranging from 0.02 to 0.17 t/m3 under conventional fertilization and 0.03–0.20 t/m3 under balanced fertilization, following biochar application at rates of 0, 20, and 40 t/ha in a maize field. Azeem et al.54 reported that biochar addition (0, 5, and 10 t/ha) decreased the soil bulk density in a wheat-mash bean crop system, with no significant influence on fertilization.
CEC and pH are crucial soil factors affecting nutrient availability and retention. Studies have shown that biochar application increases soil pH and CEC, enhancing nutrient availability and supporting stable crop productivity55,56,57. Major et al.58 reported higher soil pH after biochar treatment with an application rate of 20 t/ha at 30 cm depth, as compared to 0 and 8 t/ha rates. Hailegnaw et al.59 and Zhang et al.60 also reported an increase in soil pH with the increase in the application rate of biochar. Due to its inherently high pH, biochar application generally increases soil pH. However, the extent of this change varied depending on field conditions, such as soil properties and seasonal variations. Wu et al.61 reported no significant change in CO2 emission in the alkaline soil after the application of olive biochar, while acidic soil was significantly influenced. These trends were associated with the presence of labile compounds in olive biochar, which undergo rapid mineralization in acidic soil. While biochar had a minimal effect on soil pH, it significantly increased CEC, likely due to its high specific surface area62,63.
When combined with external fertilizers, biochar enhances carbon fixation and limits carbon transfer, contributing to greater soil carbon retention64. The combined application of biochar and fertilizers (e.g., organic or P/N fertilizers) increased nutrient availability in the soil, thereby enhancing plant growth65. Lou et al.66 reported that combining biochar with soil fertilization not only enhanced carbon sequestration and reduced carbon emissions but also promoted crop growth by increasing soluble organic matter production and nitrogen mineralization. Meanwhile, biochar affects the soil microbes, which are key regulators of organic matter conversion and nutrient cycling in soil67. Biochar influences microbial communities both directly and indirectly by modifying abiotic factors such as C availability and pH of soil68,69. Furthermore, it alters soil pore size distribution, which in turn affects microbial composition and habitat conditions. Elbasiouny et al.70 reported that biochar application gradually released nitrogen and carbon due to its high content of recalcitrant organic carbon, with the release rate influenced by soil conditions regulating microbial biomass. Therefore, optimizing biochar application rates based on environmental factors is essential for enhancing soil fertility and crop productivity.
Conclusions
In this study, a 3-year field trial was performed to study the effects of applying biochar to soil, in conjunction with N fertilizer. The combined application of biochar and nitrogen fertilizer enhanced SOC content, while in the irrigated wheat fields of North Xinjiang, applying biochar (20 t/ha) with a lower nitrogen dose effectively increased SOC levels and decreased the emissions of CO2. These results provide valuable insights regarding sustainable agricultural practices, particularly the benefits of biochar application.
Data availability
Data is provided within the Related files. All data generated or analysed during this study are included in this published article.
References
Srivastava, R. K., Panda, R. K., Chakraborty, A. & Halder, D. Enhancing grain yield, biomass and nitrogen use efficiency of maize by varying sowing dates and nitrogen rate under rainfed and irrigated conditions. Field Crop Res. 221, 339–349. https://doi.org/10.1016/j.fcr.2017.06.019 (2018).
Li, G. et al. Controlled-release urea combining with optimal irrigation improved grain yield, nitrogen uptake, and growth of maize. Agric. Water Manag. 227, 105834. https://doi.org/10.1016/j.agwat.2019.105834 (2020).
Lian, H. et al. A synergistic increase in water and nitrogen use efficiencies in winter wheat cultivars released between the 1940s and the 2010s for cultivation in the drylands of the shaanxi Province in China. Agric. Water Manag. 240, 106308. https://doi.org/10.1016/j.agwat.2020.106308 (2020).
Ahmed, M., Rauf, M., Mukhtar, Z. & Saeed, N. A. Excessive use of nitrogenous fertilizers: An unawareness causing serious threats to environment and human health. Environ. Sci. Pollut. Res. 24, 26983–26987. https://doi.org/10.1007/s11356-017-0589-7 (2017).
Su, Z., Liu, X., Wang, Z. & Wang, J. Biochar effects on salt-affected soil properties and plant productivity: A global meta-analysis. J. Environ. Manag. 366, 121653. https://doi.org/10.1016/j.jenvman.2024.121653 (2024).
Kang, S. W. et al. Effect of barley straw biochar application on greenhouse gas emissions from upland soil for Chinese cabbage cultivation in short-term laboratory experi-ment. J. Mt. Sci. 13, 693–702. https://doi.org/10.1007/s11629-014-3428-z (2016).
Lan, Z. M. et al. Stoichiometric ratio of dissolved organic carbon to nitrate regulates nitrous oxide emission from the biochar-amended soils. Sci. Total Environ. 576, 559–571. https://doi.org/10.1016/j.scitotenv.2016.10.119 (2017).
Weldon, S. et al. The effect of a biochar temperature series on denitrification: Which biochar properties matter?. Soil Biol. Biochem. 35, 173–183. https://doi.org/10.1016/j.soilbio.2019.04.018 (2019).
Senbayram, M. et al. Effect of biochar origin and soil type on the greenhouse gas emission and the bacterial community structure in N fertilised acidic sandy and alkaline clay soil. Sci. Total Environ. 660, 69–79. https://doi.org/10.1016/j.scitotenv.2018.12.300 (2019).
Li, S. et al. Interactions between biochar and nitrogen impact soil carbon mineralization and the microbial community. Soil Tillage Res. 196, 104437. https://doi.org/10.1016/j.still.2019.104437 (2020).
Zhang, Y., Wang, J. & Feng, Y. The effects of biochar addition on soil physicochemical properties: A review. Catena 202, 105284. https://doi.org/10.1016/j.catena.2021.105284 (2021).
Dey, S. et al. Enhancing cation and anion exchange capacity of rice straw biochar by chemical modification for increased plant nutrient retention. Sci. Total Environ. 886, 163681. https://doi.org/10.1016/j.scitotenv.2023.163681 (2023).
Antonangelo, J. A., Culman, S. & Zhang, H. Comparative analysis and prediction of cation exchange capacity via summation: Influence of biochar type and nutrient ratios. Front. Soil Sci. 4, 1371777. https://doi.org/10.3389/fsoil.2024.1371777 (2024).
Dai, Z. et al. Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 3, 239–254. https://doi.org/10.1007/s42773-021-00099-x (2021).
Palansooriya, K. N. et al. Response of microbial communities to biochar-amended soils: A critical review. Biochar 1, 3–22. https://doi.org/10.1007/s42773-019-00009-2 (2019).
Li, Y. F. et al. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: A review. J. Soils Sediments 18(2), 546–563. https://doi.org/10.1007/s11368-017-1906-y (2018).
Zhan, C. Microbial decomposition and soil health: Mechanisms and ecological implications. Mol. Soil Biol. 15(2), 59–70. https://doi.org/10.5376/msb.2024.15.0007 (2024).
Li, B. et al. Global integrative meta-analysis of the responses in soil organic carbon stock to biochar amendment. J. Environ. Manag. 351, 119745. https://doi.org/10.1016/j.jenvman.2023.119745 (2024).
Ding, F. et al. A meta-analysis and critical evaluation of influencing factors on soil carbon priming following biochar amendment. J. Soils Sediments 18, 1507–1517. https://doi.org/10.1007/s11368-017-1899-6 (2018).
Patton, N. R., Lohse, K. A., Seyfried, M., Will, R. & Benner, S. G. Lithology and coarse fraction adjusted bulk density estimates for determining total organic carbon stocks in dryland soils. Geoderma 337, 844–852. https://doi.org/10.1016/j.geoderma.2018.10.036 (2019).
Mylavarapu, R., Bergeron, J., Wilkinson, N. & Hanlon, E. A. Soil pH and electrical conductivity: A county extension soil laboratory manual: CIR1081/SS118, rev. 1/2020. Edis https://doi.org/10.32473/edis-ss118-2020 (2020).
Sumner, M. E. & Miller, W. P. Cation exchange capacity and exchange coefficients. Methods Soil Anal. Part 3 Chem. Methods 5, 1201–1229 (1996).
Tatzber, M. et al. KMnO4 determination of active carbon for laboratory routines: Three long-term field experiments in Austria. Soil Res. 53(2), 190–204. https://doi.org/10.1071/SR14200 (2015).
Lã, O. R. et al. A green and reliable titrimetric method for total organic carbon determination with potassium permanganate. Química Nova 46(2), 143–149. https://doi.org/10.21577/0100-4042.20170961 (2023).
Ola, S. A., Usifoh, O. A. & Nnochiri, E. S. The effects of potassium permanganate on the geotechnical properties of soils. Acta Polytech. 63(4), 260–267. https://doi.org/10.14311/ap.2023.63.0260 (2023).
Jones, D. L. & Willett, V. B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 38(5), 991–999. https://doi.org/10.1016/j.soilbio.2005.08.012 (2006).
Yang, X. et al. Labile organic carbon fractions and carbon pool management index in a 3-year field study with biochar amendment. J. Soils Sediments 18, 1569–1578. https://doi.org/10.1007/s11368-017-1874-2 (2018).
Salvo, L. P. D. et al. The drop plate method as an alternative for Azospirillum spp viable cell enumeration within the consensus protocol of the REDCAI network. Rev. Argent. Microbiol. 54(2), 61–70. https://doi.org/10.1016/j.ram.2021.05.002 (2022).
Olsen, R. A. & Hovland, J. Fungal Flora and Activity in Norway Spruce Needle Litter. Report. Department of Microbiology, Agricultural University of Norway. 41, 41 (1985).
Domsh, K., Gams, W. & Anderson, T. Compendium of Soil Fungi 809 (Academic Press, 1980).
Huang, Z. et al. Analysis on metabolic functions of rhizosphere microbial communities of Pinus massoniana provenances with different carbon storage by Biolog Eco microplates. Front. Microbiol. 15, 1365111. https://doi.org/10.3389/fmicb.2024.1365111 (2024).
Mehmood, F. et al. Impacts of irrigation managements on soil CO2 emission and soil CH4 uptake of winter wheat field in the North China plain. Water 13(15), 2052. https://doi.org/10.3390/w13152052 (2021).
Bekchanova, M., Kuppens, T., Cuypers, A., Jozefczak, M. & Malina, R. Biochar’s effect on the soil carbon cycle: A rapid review and meta-analysis. Biochar 6(1), 1–19. https://doi.org/10.1007/s42773-024-00381-8 (2024).
Tian, L., Shao, G., Gao, Y., Song, E. & Lu, J. Effects of biochar on soil organic carbon in relation to soil nutrient contents, climate zones and cropping systems: A Chinese meta-analysis. Land 13(10), 1608. https://doi.org/10.3390/land13101608 (2024).
Reed, E. Y., Chadwick, D. R., Hill, P. W. & Jones, D. L. Critical comparison of the impact of biochar and wood ash on soil organic matter cycling and grassland productivity. Soil Biol. Biochem. 110, 134–142. https://doi.org/10.1016/j.soilbio.2017.03.012 (2017).
Feng, J. et al. Trade-offs in carbon-degrading enzyme activities limit long-term soil carbon sequestration with biochar addition. Biol. Rev. 98(4), 1184–1199. https://doi.org/10.1111/brv.12949 (2023).
He, C. et al. Decoupled fungal and bacterial functional responses to biochar amendment drive rhizosphere priming effect on soil organic carbon mineralization. Biochar 6(1), 84. https://doi.org/10.1007/s42773-024-00376-5 (2024).
Weng, Z. et al. Biochar built carbon over a decade by stabilizing rhizodeposits. Nat. Clim. Change 7, 371–376. https://doi.org/10.1038/nclimate3276 (2017).
Maucieri, C. et al. Short-term effects of biochar and salinity on soil greenhouse gas emissions from a semi-arid Australian soil after re-wetting. Geoderma 307, 267–276. https://doi.org/10.1016/j.geoderma.2017.07.028 (2017).
Xiao, Y. et al. Effects of biochar, N fertilizer, and crop residues on greenhouse gas emissions from acidic soils. CLEAN Soil Air Water 46(7), 1700346. https://doi.org/10.1002/clen.201700346 (2018).
Buss, W., Hasemer, H., Sokol, N. W., Rohling, E. J. & Borevitz, J. Applying minerals to soil to draw down atmospheric carbon dioxide through synergistic organic and inorganic pathways. Commun. Earth Environ. 5(1), 602. https://doi.org/10.1038/s43247-024-01771-3 (2024).
Zhao, Y. et al. Advances in the effects of biochar on microbial ecological function in soil and crop quality. Sustainability 14(16), 10411. https://doi.org/10.3390/su141610411 (2022).
Zi, H. B. et al. Responses of soil bacterial community and enzyme activity to experimental warming of an alpine meadow. Eur. J. Soil Sci. 69(3), 429–438. https://doi.org/10.1111/ejss.12547 (2018).
Adhikari, S., Timms, W. & Mahmud, M. P. Optimising water holding capacity and hydrophobicity of biochar for soil amendment—A review. Sci. Total Environ. 851, 158043. https://doi.org/10.1016/j.scitotenv.2022.158043 (2022).
Singh, H., Northup, B. K., Rice, C. W. & Prasad, P. V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 4(1), 8. https://doi.org/10.1007/s42773-022-00138-1 (2022).
Tan, Z., Lin, C. S. K., Ji, X. & Rainey, T. J. Returning biochar to fields: A review. Appl. Soil Ecol. 116, 1–11. https://doi.org/10.1016/j.apsoil.2017.03.017 (2017).
Amoakwah, E., Frimpong, K. A., Okae-Anti, D. & Arthur, E. Soil water retention, air flow and pore structure characteristics after corn cob biochar application to a tropical sandy loam. Geoderma 307, 189–197. https://doi.org/10.1016/j.geoderma.2017.08.025 (2017).
Verheijen, F. G. et al. The influence of biochar particle size and concentration on bulk density and maximum water holding capacity of sandy vs sandy loam soil in a column experiment. Geoderma 347, 194–202. https://doi.org/10.1016/j.geoderma.2019.03.044 (2019).
Yan, Q. et al. Effects of maize straw-derived biochar application on soil temperature, water conditions and growth of winter wheat. Eur. J. Soil Sci. 70(6), 1280–1289. https://doi.org/10.1111/ejss.12863 (2019).
Rogovska, N., Laird, D. A. & Karlen, D. L. Corn and soil response to biochar application and stover harvest. Field Crop Res. 187, 96–106. https://doi.org/10.1016/j.fcr.2015.12.013 (2016).
Niu, Y. et al. Yield-scaled N2O emissions were effectively reduced by biochar amendment of sandy loam soil under maize—Wheat rotation in the North China Plain. Atmos. Environ. 170, 58–70. https://doi.org/10.1016/j.atmosenv.2017.09.050 (2017).
Kang, S. W. et al. Effect of biochar derived from barley straw on soil physicochemical properties, crop growth, and nitrous oxide emission in an upland field in South Korea. Environ. Sci. Pollut. Res. 25, 25813–25821. https://doi.org/10.1007/s11356-018-1888-3 (2018).
Zhang, D. et al. Biochar helps enhance maize productivity and reduce greenhouse gas emissions under balanced fertilization in a rainfed low fertility inceptisol. Chemosphere 142, 106–113. https://doi.org/10.1016/j.chemosphere.2015.04.088 (2016).
Azeem, M. et al. Biochar improves soil quality and N2-fixation and reduces net ecosystem CO2 exchange in a dryland legume-cereal cropping system. Soil Tillage Res. 186, 172–182. https://doi.org/10.1016/j.still.2018.10.007 (2019).
Sandhu, S. S. et al. Analyzing the impacts of three types of biochar on soil carbon fractions and physiochemical properties in a corn-soybean rotation. Chemosphere 184, 473–481. https://doi.org/10.1016/j.chemosphere.2017.05.165 (2017).
Agegnehu, G., Bass, A. M., Nelson, P. N. & Bird, M. I. Benefits of biochar, compost and biochar-compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 543, 295–306. https://doi.org/10.1016/j.scitotenv.2015.11.054 (2016).
Haile, M., Birhane, E., Gebresamuel, G., Adaramola, M. S. & Rannestad, M. M. Application of biochar derived from expansive shrubs and limestone improved acidic soil characteristics. Carbon Manag. 15(1), 2364784. https://doi.org/10.1080/17583004.2024.2364784 (2024).
Major, J. et al. Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 333, 117–128. https://doi.org/10.1007/s11104-010-0327-0 (2010).
Hailegnaw, N. S., Mercl, F., Pračke, K., Száková, J. & Tlustoš, P. Mutual relationships of biochar and soil pH, CEC, and exchangeable base cations in a model laboratory experiment. J Soils Sediments 19, 2405–2416. https://doi.org/10.1007/s11368-019-02264-z (2019).
Zhang, Y., Miao, S., Song, Y., Wang, X. & Jin, F. Biochar application reduces saline–alkali stress by improving soil functions and regulating the diversity and abundance of soil bacterial community in highly saline–alkali paddy field. Sustainability 16(3), 1001. https://doi.org/10.3390/su16031001 (2024).
Wu, D. et al. Effect of biochar origin and soil pH on greenhouse gas emissions from sandy and clay soils. Appl. Soil Ecol. 129, 121–127. https://doi.org/10.1016/j.apsoil.2018.05.009 (2018).
Jin, F. et al. Long-term effects of biochar one-off application on soil physicochemical properties, salt concentration, nutrient availability, enzyme activity, and rice yield of highly saline-alkali paddy soils: Based on a 6-year field experiment. Biochar 6(1), 1–22. https://doi.org/10.1007/s42773-024-00332-3 (2024).
Martinsen, V. et al. pH effects of the addition of three biochars to acidic Indonesian mineral soils. Soil Sci. Plant Nutr. 61, 821–834. https://doi.org/10.1080/00380768.2015.1052985 (2015).
Ngo, P. T. et al. Mixing of biochar with organic amendments reduces carbon removal after field exposure under tropical conditions. Ecol. Eng. 91, 378–380. https://doi.org/10.1016/j.ecoleng.2016.01.011 (2016).
Fahad, S. et al. A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol. Biochem. 103, 191–198. https://doi.org/10.1016/j.plaphy.2016.03.001 (2016).
Luo, X. et al. Biochar addition reduced net N mineralization of a coastal wetland soil in the Yellow River Delta, China. Geoderma 282, 120–128. https://doi.org/10.1016/j.geoderma.2016.07.015 (2016).
Bhattacharyya, S. S. & Furtak, K. Soil–plant–microbe interactions determine soil biological fertility by altering rhizospheric nutrient cycling and biocrust formation. Sustainability 15(1), 625. https://doi.org/10.3390/su15010625 (2022).
Li, Q. et al. Biochar affects organic carbon composition and stability in highly acidic tea plantation soil. J. Environ. Manag. 370, 122803. https://doi.org/10.1016/j.jenvman.2024.122803 (2024).
Khadem, A. & Raiesi, F. Responses of microbial performance and community to corn biochar in calcareous sandy and clayey soils. Appl. Soil Ecol. 114, 16–27. https://doi.org/10.1016/j.apsoil.2017.02.018 (2017).
Elbasiouny, H., Elbehiry, F., El-Ramady, H. & Hasanuzzaman, M. Contradictory results of soil greenhouse gas emissions as affected by biochar application: Special focus on alkaline soils. Int. J. Environ. Res. 15, 903–920. https://doi.org/10.1007/s41742-021-00358-6 (2021).
Funding
This project was financially supported by the Tianshan Talent Training Program (2023TSYCCX0085), the Regional Science Fund Project of the Natural Science Foundation of China (32260326), and the earmarked fund for the Xinjiang Agriculture Research System (XJARS-01).
Author information
Authors and Affiliations
Contributions
The present report was conducted by ZLY, WZ under the guidance and supervision of YWJ. The studies were carefully selected, and the data was subjected to analysis by YWJ, ZLY, WZ, and ZJS. YWJ, ZLY drafted the report and subsequently evaluated and reviewed it by YWJ, LPY, and SLL. All the authors approved the final version of this study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, W., Zhang, L., Wang, Z. et al. Effects of biochar and nitrogen fertilizer on microbial communities, CO2 emissions, and organic carbon content in soil. Sci Rep 15, 9789 (2025). https://doi.org/10.1038/s41598-025-94784-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94784-7
Keywords
This article is cited by
-
Dynamic modulation of rhizosphere microbial diversity and function across tobacco growth stages by biochar
Biology and Fertility of Soils (2026)
-
Use of biomass-derived biochar as a sustainable material for carbon sequestration in soil: recent advancements and future perspectives
npj Materials Sustainability (2025)
-
Comprehensive evaluation of biochar influence on soil microbial diversity, nutrient cycling and contaminant remediation using systematic review and meta-analysis
Discover Sustainability (2025)
-
Evaluating Soil Reclamation and Growth Enhancing Potential of Various Types of Biochar with Respect To Rice Crop Cultivated in Saline Sodic Soil
Journal of Soil Science and Plant Nutrition (2025)