Abstract
Anxiety symptoms occur more frequently during adolescence and early adulthood, increasing the risk of future anxiety disorders. Neuroscientific research on anxiety has primarily focused on adulthood, employing mostly univariate approaches, discounting large-scale alterations of the brain. Indeed adolescents with trait anxiety may display similar abnormalities shown by adults in brain regions ascribed to the default mode network, associated with self-referential thinking and rumination-related processes. The present study aims to explore resting-state connectivity patterns associated with trait anxiety in a large sample of young individuals. We analyzed the rs-fMRI images of 1263 adolescents (mean age 20.55 years) and their scores on anxiety trait. A significant association between trait anxiety and resting-state functional connectivity in two networks was found, with some regions overlapping with the default mode network, such as the cingulate gyrus, the middle temporal gyri and the precuneus. Of note, the higher the trait anxiety, the lower the connectivity within both networks, suggesting abnormal self-referential processing, awareness, and emotion regulation abilities in adolescents with high anxiety trait. These findings provided a better understanding of the association between trait anxiety and brain rs-functional connectivity, and may pave the way for the development of potential biomarkers in adolescents with anxiety.
Similar content being viewed by others
Introduction
Experiencing anxiety is a common condition that almost everyone comes across during the lifetime. Nevertheless, this condition can largely vary in terms of intensity level (going from mild manifestations to clinical disorders), symptomatology (behavioural and psychological indices), frequency and occurrence. Some periods or conditions are indeed more likely to present with higher anxiety level, such as early adolescence and young adulthood1, which are characterized by distinctive brain developmental and organizational features2,3.
Moreover, high levels of anxiety experienced during these periods can indeed represent a risk factor for the future development of anxiety disorders4.
In non-clinical settings anxiety is usually measured and identified through trait anxiety, a general measure of the individual propensity of perceiving stress and worry in everyday life5. High trait anxiety scores can point to the presence or the risk for future development of clinical anxiety disorders6,7,8.
For this reason, the investigation of trait anxiety has been successfully employed in several studies concerning non-clinical population, analysing both structural and functional features related to it, highlighting the role of the prefrontal, the cingulate and the insular cortex, along with the amygdala9,10,11,12. The advantages of studying brain features associated with trait anxiety, instead of a fully diagnosed disorder, ranges from the exploration of anxiety in its subclinical forms to the exclusion of the effects of psychotropic drugs potentially taken by diagnosed patients. In this way it is possible to identify early markers of clinical disorders, studying neural predisposition factors that could impact the aetiology of anxiety.
Functional studies on trait anxiety reported impaired connectivity in brain regions associated to attentional control, such as the dorsolateral prefrontal cortex and the cingulate cortex13,14, and altered fronto-limbic circuits, such as impaired amygdala–prefrontal cortex connections15.
In children and adolescents few studies have been conducted so far, finding increased activation of the right dorsolateral prefrontal cortex during a task measuring attention bias for angry faces16.
Moreover, a study comparing adults and adolescents’ response in threat-safety discriminations during threat reappraisal reported a decreased activity of the subgenual anterior cingulate cortex in both anxious adults and adolescents, while the ventromedial prefrontal cortex exhibited a different pattern, in particular a reduced activity in anxious adults compared to controls and an increased activity in anxious adolescents compared to controls17.
Despite the efforts made so far in identifying neural underpinnings of trait anxiety, most studies have employed univariate approaches including region-of-interest analyses, with a priori selection of specific brain regions.
However, a network approach to neuroscience may allow the identification of widespread aberrations happening in relation to a specific disorder, considering that dysfunctions in such disorders rely on distributed regions across the brain18.
Thus, a system neuroscience perspective can help examining how brain networks are impacted in different aberrant mental states18,19, investigating resting-state spontaneous fluctuations20. In addition, the role of brain networks in describing mental states such as anxiety is relevant also in the context of structural studies (i.e. gray and white matter) for both clinical and subclinical population21,22.
Anxiety in adults has been shown to influence the connectivity patterns within and between several large-scale neural networks, such as the default mode (DMN), the salience (SN) and the central executive (CEN) (sometime also substituted by the Frontoparietal, FPN), that are considered important centres for perceptual, behavioural and emotional processing10,23. These three macro-networks interact with each other, balancing internal self-reflection processes and external task focusing. In particular, the DMN is active during internally focused activities, the CEN during cognitive functions such as problem-solving, working memory and decision-making, while the SN is thought to mediate the switch between the CEN and the DMN, guiding the transition between different brain states18,23.
Of these networks, the DMN seems to play a fundamental role in anxiety. Several studies have reported functional alterations of the DMN in association with anxiety, particularly a modified functional activity during auditory tasks24 or emotion regulation tasks25, and alterations in resting-state connectivity26,27,28. In this context, Sylvester et al.19 introduced a new functional network model of anxiety, proposing that anxiety behaviors and disorders result in abnormal functional connectivity pattern where increased activity in the SN is linked to decreased regulation by the DMN. This model although interesting is limited to the observation of the brain of adults. One important question is whether the functional alterations inside the DMN may apply to adolescents with anxiety as well. The DMN is a collection of functionally related regions such as the posterior cingulate, the temporal poles, the frontal and posterior medial cortices including the precuneus.
In particular, the precuneus has been repetitively linked to anxiety29,30,31, specifically to self-referential processing rumination32,33, which consist in the persistent focus on past negative experiences, with the constant inspection of the potential causes and consequences of those negative experiences32,34. Moreover, also the posterior cingulate cortex has been shown to mediate anxious symptoms, being involved in attention-dependent processing and having a modulatory influence on the amygdala35,36.
A confirmation of altered connectivity inside these regions in adolescents with trait anxiety, is crucial for advancing research in this field. Although anxiety has been a recurrent topic in the recent neuroscientific research, leading to the development of different neural hypotheses, two main limitations can be identified. First, most of the research has been conducted in small groups that are not strictly homogeneous for what concerns ages, with a net prevalence of adult subjects, making it difficult to associate anxiety features to specific sensitive periods, in particular adolescence. Since anxiety prevalence and symptomatology can vary according to the brain developmental phase, creating neural models comprising a mix of age ranges might result in an imprecise framework. Second, the general approach used to study anxiety typically involves the analysis of single, specifically localized and a priori selected brain regions, with the use of univariate methods, discounting the interpretation of cognitive and affective states through an extensive analysis of the brain’s organization. Machine learning, multivariate in nature, can help to unravel the complexity of large-scale brain features related to functional activation.
Indeed, a promising alternative to seed-based correlation concerns decomposition methods such as independent component analysis (ICA), that is able to explore different resting-state networks properties for individual or group-level analyses20. Group ICA is a widely used strategy in resting-state analyses, given the fact that it can separate spatially or temporally independent sources, allowing for group inferences37, without starting from a priori spatial restraints.
Moreover, Group ICA can be useful to find potential functional network biomarkers, identifying patterns of functional activity or connectivity related to a specific condition/state38, thus resulting in a powerful method that in the future could be integrated with standard diagnosis approaches, which rely mostly on qualitative methods.
Given these considerations, our study aims to employ a large-scale investigation of resting-state connectivity in relation to trait anxiety in a sample of 1263 adolescents, to test the hypothesis that the default mode network may be associated with trait anxiety following previous observations on adult individuals.
Methods
Participants
Neuroimaging data for this study was selected from the MRi-Share database39, a multi-modal brain MRI database acquired in a sample of 1870 young healthy adults (18–35 years old), undergoing university-level education (https://www.i-share.fr/). Informed consent was obtained from all participants and/or their legal guardians, and all the research was performed in accordance with relevant guidelines and regulations, in accordance with the Declaration of Helsinki.
The study protocol was approved by the Comite de Protection des Personnes Sud-Ouest et Outre-Mer (local ethics committee CPP SOOMIII) with agreement nr 2015-A00850-49.
The original MRi-Share database contains structural (T1 and FLAIR), diffusion (multispectral), susceptibility-weighted (SWI), and resting-state functional imaging modalities and is related to the i-Share cohort study, launched in February 2013 with the approval of the local ethics committee (CPP2015-A00850-49).
The exclusion criteria used for the MRi-Share database consists in (1) age over 35 years; (2) pregnancy or nursing; (3) claustrophobia; and (4) contraindications for head MRI, while the additional exclusion criteria used for the present study were the following: (1) diagnosis of bulimia/anorexia/obsessive compulsive disorder/depression; (2) daily alcohol consumption; and (3) drug consumption more than 10 times in life (cocaine, mdma, heroine etc..).
After the removal of subjects’ data with incidental findings requiring medical referral, and following the additional exclusion criteria for the present study, the final sample, comprising individuals from 18 to 24 years old, resulted to be 1263 participants (M = 341; F = 922; mean age = 20.55; SD = 2.25).
The sample characteristics are summarized in Table 1.
Questionnaires
The questionnaires that participants had to fill were web-based and they were related to general physical health, lifestyle habits and mental health (see list of questionnaires at https://research.i-share.fr/data/). For the purpose of our study, the French version of the State-Trait Anxiety Inventory5,40 (Inventaire d’Anxiété Trait-État, Forme Y) was considered, in particular the Trait Anxiety score. The State-Trait Anxiety Inventory (STAI) is a well-validated questionnaire that measures the level of state and trait anxiety with 20 items per each, rated on a 4-point Likert scale, going from 1 “Almost Never” to 4 “Almost Always”. Moreover, it has internal consistency coefficients ranging from 0.86 to 0.95 and test–retest reliability coefficients ranging from 0.65 to 0.75 over a 2-month interval40.
MRI acquisition
Structural and functional data was acquired with a 3T Siemens Prisma scanner with a 64-channels head coil (gradients: 80 mT/m–200 T/m/s). T1-weighted images were acquired in a time duration of 4 min and 54 s, with a voxel size of 1.0 × 1.0 × 1.0 mm3 (192 × 256 × 256) and key parameters 3D MPRAGE, sagittal, R = 2, TR/TE/TI = 2000/2.0/880 ms, while resting state data was acquired in a time duration of 14 min and 58 s, with a voxel size of 2.4 × 2.4 × 2.4 mm3 (88 × 88 × 66) and key parameters 2D axial, EPI, MB = 6, TR/TE = 850/35.0 ms, flip angle = 56°, fat sat39. Before the rs-fMRI acquisition, participants were asked to “keep their eyes closed, to relax, to refrain from moving, to stay awake, and to let their thoughts come and go”39.
Pre-processing
Resting state fMRI data was pre-processed through CONN Toolbox41 (RRID:SCR_009550) release 21.a. Functional and anatomical data were preprocessed using a flexible preprocessing pipeline42 including realignment with correction of susceptibility distortion interactions, slice timing correction, outlier detection, direct segmentation and MNI-space normalization, and smoothing. Functional data were realigned using SPM realign and unwarp procedure43, where all scans were coregistered to a reference image, and resampled using b-spline interpolation to correct for motion and magnetic susceptibility interactions. Potential outlier scans were identified using ART (Artifact detection Tools)44, and a reference BOLD (Blood Oxygenation Level Dependent) image was computed for each subject by averaging all scans excluding outliers. Potential outlier scans are identified from the observed global BOLD signal and the amount of subject-motion in the scanner. Acquisitions with framewise displacement (FD) above 0.9 mm or global BOLD signal changes above 5 s.d. are flagged as potential outliers42. Functional and anatomical data were normalized into standard MNI space, segmented into grey matter, white matter, and CSF (Cerebrospinal Fluid) tissue classes, and resampled to 2 mm isotropic voxels following a direct normalization procedure45,46. Last, functional data were smoothed using spatial convolution with a Gaussian kernel of 4 mm full width half maximum (FWHM)47.
In addition, functional data were denoised using a standard denoising pipeline42 including the regression of potential confounding effects characterized by CSF, motion parameters and their first order derivatives, outlier scans, white matter timeseries, and linear trends within each functional run, followed by bandpass frequency filtering of the BOLD timeseries48 between 0.008 and 0.09 Hz.
Group ICA analysis
In the first-level analysis, Group ICA (independent component analysis) estimated 20 temporally coherent networks from the rs-fMRI data combined across all subjects. This number was chosen according to previous studies and CONN default suggestion. The resulting networks were then visually inspected for noise control49.
Group ICA analysis is able to compute a series of spatial maps characterizing the BOLD response across a set of independent spatial components, using Calhoun’s group-level ICA approach37, with variance normalization pre-conditioning, optional subject-level dimensionality reduction, subject/condition concatenation of BOLD signal data along temporal dimension, group-level dimensionality reduction (to the target number of dimensions/components), fastICA for estimation of independent spatial components, and GICA3 backprojection for individual subject-level spatial map estimation42 (see Fig. 1 for a visual representation of the Group ICA steps).
Group ICA steps. Group ICA steps automatically performed by CONN include: (1) variance normalization to adjust the variance of the time series, aiming at rendering data more comparable across subjects; optional subject-level dimensionality reduction (here not represented); (2) subject/condition concatenation of BOLD signal data along temporal dimension, in order to combine together BOLD signal data of different subjects/conditions along the time axis; (3) group-level dimensionality reduction, which refers to the reduction of the data complexity in order to extract common features or patterns; (4) fastICA for the estimation of the independent components from the mixed data matrix; (5) GICA3 backprojection, consisting in the estimation of individual subject-level spatial map starting from group-level spatial map. For the purpose of the image, here only three subjects and two components are represented. A basic template was taken from Surf Ice software for the creation of this image (version 6-October-2021 v1.0.20211006 https://github.com/neurolabusc/surf-ice).
The components thus represent an estimation of independent hemodynamic sources, and have a specific contribution for each voxel signal37. In the second-level analysis, for each component a GLM contrast was performed in order to calculate how trait anxiety influences resting state connectivity, controlling for sex. The contrast was set with a voxel level p < 0.001 uncorrected and a cluster level p < 0.05 pFDR corrected. Significant results were plotted in Surf Ice (version 6-October-2021 v1.0.20211006 https://github.com/neurolabusc/surf-ice) and BrainNet Viewer50 for visualization.
The anatomical specification of the significant areas was derived from MRIcroGL Toolbox51, with the integrated AAL atlas function, and CONN automatic atlas (Harvard–Oxford atlas).
Results
Group ICA analysis
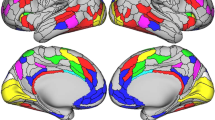
Of the twenty estimated components, the connectivity between two networks, e.g. IC 8 and IC 10, and specific brain regions resulted to be significantly associated with trait anxiety (see Figs. 2 and 3 for the visual representation). In particular, the higher the trait anxiety, the lower the connectivity of these two networks with specific brain regions (see Figs. 4 and 5, Tables 2 and 3 for the visual and anatomical specification of the significant areas resulting from the 2nd level contrast). IC 8 included the superior temporal gyrus, the inferior frontal gyrus, the middle occipital gyrus, the precuneus, the postcentral gyrus, the middle temporal gyrus, the fusiform gyrus, and the hippocampus. This network showed a decreased resting-state connectivity with the posterior cingulate gyrus, and the middle temporal gyrus. IC 10 included the cerebellum (vermis), the inferior and middle occipital gyrus, the precuneus, the cuneus, the calcarine sulcus, and the lingual gyrus. This network showed a decreased resting-state connectivity with the precuneus.
Visual representation of the component of interest IC 8. Components IC 8. The colours refer to the z score derived from the statistical values obtained by the analysis, for each brain area. Figures were created with Surf Ice software, version 6-October-2021 v1.0.20211006 https://github.com/neurolabusc/surf-ice.
Visual representation of the component of interest IC 10. Components IC 10. The colours refer to the z score derived from the statistical values obtained by the analysis, for each brain area. On top, cortical and subcortical areas. At the bottom, posterior cerebellar view (left) and superior cerebellar view (right). Figures were created with Surf Ice software, version 6-October-2021 v1.0.20211006 https://github.com/neurolabusc/surf-ice.
Visual representation of the areas showing decreased resting-state connectivity with IC8, in association with trait anxiety. Significant areas resulted from the 2nd level contrast. The coloured regions represent areas showing a decreased resting-state connectivity with IC8, associated with increase trait anxiety. Figures were created with Surf Ice software, version 6-October-2021 v1.0.20211006 https://github.com/neurolabusc/surf-ice.
Visual representation of the areas showing decreased resting-state connectivity with IC10, in association with trait anxiety. Significant areas resulted from the 2nd level contrast. The coloured regions represent areas showing a decreased resting-state connectivity with IC10, associated with increase trait anxiety. Figures were created with Surf Ice software, version 6-October-2021 v1.0.20211006 https://github.com/neurolabusc/surf-ice.
Discussion
Our study aimed to explore the relationship between trait anxiety and resting-state functional connectivity in a large cohort of young participants, investigating this relationship in association with the default mode network, using Group ICA. The analysis revealed that two independent components, IC8 and IC10, showed significant associations between trait anxiety and decreased connectivity in several key brain regions.
In the following sections we will explain in detail such results.
A temporo-parietal network
IC8 encompassed regions associated with higher order cognitive and sensory processes that are integral to several networks and showed a decreased resting state connectivity with regions ascribable to the posterior cingulate gyrus and the middle temporal gyrus.
Indeed some of the regions comprising the network, such as the precuneus, the superior, and middle temporal gyrus, are regions of the default mode network, involved in social cognition and working memory, and previous research have shown a decreased functional connectivity of them in highly anxious individuals, also at rest10. Moreover, an alteration of the DMN has been shown to be a common feature of anxiety disorders10,24,52, in particular a reduced connectivity of it during rest might be associated to deficits in its associated cognitive functions, such as self-referential processes10,53. The postcentral gyrus contributes to somatosensory processing10, whereas the hippocampus has been suggested to have a role also in contextual anxiety and memory, being strongly connected with other brain regions involved in emotion regulation, including the amygdala, the prefrontal cortex and the hypothalamus54,55,56. Together, the regions included in IC8 suggest a network that might be involved in the integration of sensory information with cognitive processes. Notably, this network showed decreased connectivity in the middle temporal gyrus, as well as the posterior cingulate gyrus. These regions are also integral to the DMN, implicated in self-referential thinking and emotional processes57,58,59,60.
The middle temporal gyrus is indeed involved in mind wandering, autobiographical memory retrieval, semantic memory and social cognition61,62; moreover, the left middle temporal gyrus have been found impaired in young patients with social anxiety disorders63.
The posterior cingulate cortex has been associated with internally directed cognition, self-referential processes, focus of attention regulation and environmental monitoring for threatening stimuli64,65,66, being a key region that interacts also with different neural networks, such as the fronto-parietal, the sensorimotor and the dorsal attention networks67. Additionally, abnormalities in functional connectivity of the posterior cingulate cortex have been documented in several psychiatric and neurological conditions, including attention deficit hyperactive disorder, autism spectrum disorder and schizophrenia67.
Therefore, we hypothesize that a decreased resting-state connectivity of IC8 network with the middle temporal gyrus and posterior cingulate gyrus might be related to disrupted self-referential processes such as self-focus, and maladaptive rumination, often observed in anxiety disorders, as both the posterior cingulate cortex and the precuneus are involved in ruminative processes32,68.
An occipito-cerebellar network
On the other side, IC10 encompassed regions associated with sensory integration, emotion regulation, and visual processing including the cerebellar vermis, inferior and middle occipital gyrus, precuneus, cuneus, calcarine sulcus, and lingual gyrus. The cerebellum, beyond its well-known role in motor coordination, plays a key role in various cognitive, affective and social functions69, and has been increasingly linked to anxiety-related processes70. Specifically, the cerebellar vermis, often referred to as the “limbic cerebellum”, is critical for emotional regulation due to its strong connections with key limbic system regions, such as periaqueductal gray and dorsal raphe nuclei, both of which are associated with anxiety modulation and stress responses70. Moreover, cerebellar structure was associated with social abilities in a sample of children and teenagers, providing support for the social and cognitive role of cerebellum71. Regions within the occipital lobe, such as inferior and middle occipital gyri, cuneus, and calcarine sulcus, are primarily involved in visual perception and processing of sensory stimuli, particularly under conditions of uncertainty72,73. Disruptions in functional connectivity within these areas have been associated with anxiety disorders, possibly due to impaired sensory integration and heightened sensitivity to uncertain stimuli, as demonstrated by a decreased functional connectivity in visual processing areas such as the lingual and the inferior occipital gyrus in patients with social anxiety disorders, suggesting perceptual impairments74. Overall, this network, involved in visuo-sensory and affective processes, showed decreased connectivity in the precuneus, the key default mode region involved in self-processing, consciousness and episodic memory retrieval75.
The posterior precuneus has many structural pathways connected to the visual and dorsal attention networks, suggesting its role as a connector among multiple large-scale networks76.
Studies in patients with social anxiety disorder have revealed decreased connectivity between the left precuneus and some areas such as posterior lobe of cerebellum, inferior temporal gyrus, parahippocampal gyrus and medial prefrontal cortex29. These disruptions may contribute to the social and emotional processing difficulties seen in such disorder. Similarly, panic disorder patients showed altered regional homogeneity in the precuneus30. In addition, high trait anxious individuals displayed decreased activation in the precuneus, during anticipation of uncertain threat compared to the certain condition77.
We thus hypothesize that a decreased resting-state connectivity of IC10 network with the precuneus might be involved in disruptions in the ability to process internal thoughts, self-awareness and visual perceptual processing, as trait anxiety could lead to cognitive perturbations in areas related to visual-sensory integration and emotion regulation.
Conclusions and limitations
In conclusion, this study provided new evidence of the association between trait anxiety and decreased resting-state functional connectivity in two key independent networks, including areas ascribable to the default mode network, using a large sample of adolescents. Our findings underscore the importance of large-scale brain networks in understanding the neural correlates of anxiety and point toward disruptions in self-referential and sensory integration processes as key mechanisms underlying anxiety-related cognitive dysfunctions. From a methodological point of view, this study aligns with the recent trend in system neuroscience to study affective and pathological states at a network level18. To do this we took advantage of an unsupervised machine learning algorithm known as Group ICA, instead of the classically used methods such as ROI to ROI or seed-based connectivity. This method has the advantage of being multivariate in nature and does not require an a priori selection of brain areas. As such it allows greater sensitivity to detect distributed network-level functional changes.
However, some limitations must be acknowledged. First, the findings should be extended with caution to proper anxiety disorders, as the current study focuses on trait anxiety rather than clinical diagnoses, although it has been largely demonstrated that high levels of trait anxiety are associated with clinical anxiety disorders and stress-induced depression8,78,79. Indeed, trait anxiety can account for a substantial portion of the variation in how individuals respond to stress and their likelihood of developing stress-related psychopathologies80.
Second, the current study does not include state anxiety data, which prevents us from exploring how transient, context-dependent anxiety states may influence resting-state connectivity patterns. As trait and state anxiety are typically highly correlated, the inclusion of state anxiety as a covariate could have provided additional insights into the dynamic interplay between stable, trait-like anxiety tendencies and the more fluctuating, situation-specific experiences of anxiety.
Finally, controlling for other demographic variables, such as socioeconomic status or ethnicity, would ensure that the results are not confounded by these factors. Addressing these limitations will enhance the generalizability and robustness of our findings in understanding the neural underpinnings of anxiety in adolescents.
Data availability
Due to French regulations regarding sharing of the medical imaging data, individual raw data used for this study cannot be shared through a public repository. Rather, to have access to i-Share and MRi-Share de-identified data, please contact Christophe Tzourio. A request has to be submitted to the i-Share Scientific Collaborations Coordinator (ilaria.montagni@u-bordeaux.fr) with a letter of intent (explaining the rationale and objectives of the research proposal), and a brief summary of the planned means and options for funding. The i-Share Steering Committee will assess this request, and provide a response (principle agreement, request to reformulate the application or for further information, refusal with reasons). If positive, applicants will have to complete and return an application package that will be reviewed by the principal investigator, the Steering Committee, and the operational staff. Reviews are based on criteria such as the regulatory framework and adherence to regulations (access to data, confidentiality), the scientific and methodological quality of the project, the relevance of the project in relation to the overall consistency of the cohort in the long term, the complementarity/competition with projects planned or currently underway, ethical aspects. Both de-identified raw and processed data (and data dictionaries) will be shared after (1) final approval of the application, and (2) formalization of the specifics of the collaboration.
References
de Lijster, J. M. et al. The age of onset of anxiety disorders. Can. J. Psychiatry Rev. Can. Psychiatry 62, 237–246 (2017).
Fan, F. et al. Development of the default-mode network during childhood and adolescence: A longitudinal resting-state fMRI study. Neuroimage 226, 117581 (2021).
Sherman, L. E. et al. Development of the default mode and central executive networks across early adolescence: A longitudinal study. Dev. Cogn. Neurosci. 10, 148–159 (2014).
Asselmann, E. & Beesdo-Baum, K. Predictors of the course of anxiety disorders in adolescents and young adults. Curr. Psychiatry Rep. 17, 7 (2015).
Spielberger, C. D., Gorsuch, R. L. & Lushene, R. E. The State-Trait Anxiety Inventory (Test Manual) (Consulting Psychologists Press, 1970).
Ercan, I. et al. Examining cut-off values for the state-trait anxiety inventory. Rev. Argent. Clin. Psicol. 24, 143–148 (2015).
Womble, M., Jennings, S., Schatz, P. & Elbin, R. J. A-173 clinical cutoffs on the state-trait anxiety inventory for concussion. Arch. Clin. Neuropsychol. 36, 1228 (2021).
Saviola, F. et al. Trait and state anxiety are mapped differently in the human brain. Sci. Rep. 10, 11112 (2020).
Tian, X. et al. Assessment of trait anxiety and prediction of changes in state anxiety using functional brain imaging: A test–retest study. NeuroImage 133, 408–416 (2016).
Modi, S., Kumar, M., Kumar, P. & Khushu, S. Aberrant functional connectivity of resting state networks associated with trait anxiety. Psychiatry Res. Neuroimaging 234, 25–34 (2015).
Sehlmeyer, C. et al. Neural correlates of trait anxiety in fear extinction. Psychol. Med. 41, 789–798 (2011).
Baur, V., Hänggi, J., Langer, N. & Jäncke, L. Resting-state functional and structural connectivity within an insula–amygdala route specifically index state and trait anxiety. Biol. Psychiatry 73, 85–92 (2013).
Morgenroth, E. et al. Using connectivity-based real-time fMRI neurofeedback to modulate attentional and resting state networks in people with high trait anxiety. NeuroImage Clin. 25, 102191 (2020).
Comte, M. et al. Effect of trait anxiety on prefrontal control mechanisms during emotional conflict. Hum. Brain Mapp. 36, 2207–2214 (2015).
Kim, M. J. & Whalen, P. J. The structural integrity of an amygdala–prefrontal pathway predicts trait anxiety. J. Neurosci. 29, 11614–11618 (2009).
Telzer, E. H. et al. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol. Psychol. 79, 216–222 (2008).
Britton, J. C. et al. Response to learned threat: An fMRI study in adolescent and adult anxiety. Am. J. Psychiatry 170, 1195–1204 (2013).
Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 15, 483–506 (2011).
Sylvester, C. M. et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 35, 527–535 (2012).
Khosla, M., Jamison, K., Ngo, G. H., Kuceyeski, A. & Sabuncu, M. R. Machine learning in resting-state fMRI analysis. Magn. Reson. Imaging 64, 101–121 (2019).
Baggio, T., Grecucci, A., Meconi, F. & Messina, I. Anxious brains: A combined data fusion machine learning approach to predict trait anxiety from morphometric features. Sensors 23, 610 (2023).
Liao, W. et al. Altered gray matter morphometry and resting-state functional and structural connectivity in social anxiety disorder. Brain Res. 1388, 167–177 (2011).
Menon, B. Towards a new model of understanding—The triple network, psychopathology and the structure of the mind. Med. Hypotheses 133, 109385 (2019).
Zhao, X.-H. et al. Altered default mode network activity in patient with anxiety disorders: An fMRI study. Eur. J. Radiol. 63, 373–378 (2007).
Dixon, M. L. et al. Emotion regulation in social anxiety disorder: Reappraisal and acceptance of negative self-beliefs. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 5, 119–129 (2020).
Xu, J. et al. Anxious brain networks: A coordinate-based activation likelihood estimation meta-analysis of resting-state functional connectivity studies in anxiety. Neurosci. Biobehav. Rev. 96, 21–30 (2019).
Coutinho, J. F. et al. Default mode network dissociation in depressive and anxiety states. Brain Imaging Behav. 10, 147–157 (2016).
Andreescu, C., Sheu, L. K., Tudorascu, D., Walker, S. & Aizenstein, H. The ages of anxiety—Differences across the lifespan in the default mode network functional connectivity in generalized anxiety disorder. Int. J. Geriatr. Psychiatry 29, 704–712 (2014).
Yuan, C. et al. Precuneus-related regional and network functional deficits in social anxiety disorder: A resting-state functional MRI study. Compr. Psychiatry 82, 22–29 (2018).
Lai, C.-H. The regional homogeneity of cingulate-precuneus regions: The putative biomarker for depression and anxiety. J. Affect. Disord. 229, 171–176 (2018).
Shin, Y.-W. et al. Increased resting-state functional connectivity between the anterior cingulate cortex and the precuneus in panic disorder: Resting-state connectivity in panic disorder. J. Affect. Disord. 150, 1091–1095 (2013).
Feurer, C. et al. Resting state functional connectivity correlates of rumination and worry in internalizing psychopathologies. Depress. Anxiety 38, 488–497 (2021).
Zhou, H.-X. et al. Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. NeuroImage 206, 116287 (2020).
Nolen-Hoeksema, S., Wisco, B. E. & Lyubomirsky, S. Rethinking rumination. Perspect. Psychol. Sci. 3, 400–424 (2008).
Hahn, A. et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage 56, 881–889 (2011).
Wright, P. et al. Dissociated responses in the amygdala and orbitofrontal cortex to bottom-up and top-down components of emotional evaluation. NeuroImage 39, 894–902 (2008).
Calhoun, V. D., Adali, T., Pearlson, G. D. & Pekar, J. J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 14, 140–151 (2001).
Salman, M. S. et al. Group ICA for identifying biomarkers in schizophrenia: ‘Adaptive’ networks via spatially constrained ICA show more sensitivity to group differences than spatio-temporal regression. NeuroImage Clin. 22, 101747 (2019).
Tsuchida, A. et al. The MRi-share database: Brain imaging in a cross-sectional cohort of 1870 university students. Brain Struct. Funct. 226, 2057–2085 (2021).
Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R. & Jacobs, G. A. Manual for the State-Trait Anxiety Inventory (Consulting Psychologists Press, 1983).
Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141 (2012).
Nieto-Castanon, A. Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN (Hilbert Press, 2020).
Andersson, J. L., Hutton, C., Ashburner, J., Turner, R. & Friston, K. Modeling geometric deformations in EPI time series. NeuroImage 13, 903–919 (2001).
Whitfield-Gabrieli, S., Nieto-Castanon, A. & Ghosh, S. Artifact Detection Tools (ART) (Cambridge, 2011).
Nieto-Castanon, A. Preparing fMRI Data for Statistical Analysis. Preprint at https://doi.org/10.48550/arXiv.2210.13564 (2022).
Calhoun, V. D. et al. The impact of T1 versus EPI spatial normalization templates for fMRI data analyses. Hum. Brain Mapp. 38, 5331–5342 (2017).
Alahmadi, A. A. S. Effects of different smoothing on global and regional resting functional connectivity. Neuroradiology 63, 99–109 (2021).
Hallquist, M. N., Hwang, K. & Luna, B. The nuisance of nuisance regression: Spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage 82, 208–225 (2013).
Bijsterbosch, J. et al. Introduction to Resting State fMRI Functional Connectivity (Oxford University Press, 2017).
Xia, M., Wang, J. & He, Y. BrainNet viewer: A network visualization tool for human brain connectomics. PLoS ONE 8, e68910 (2013).
Rorden, C. NITRC: MRIcroGL: Tool/Resource Info. https://www.nitrc.org/projects/mricrogl (2014).
Zidda, F. et al. Default mode network connectivity of fear- and anxiety-related cue and context conditioning. NeuroImage 165, 190–199 (2018).
Dixon, M. L. & Gross, J. J. Dynamic network organization of the self: Implications for affective experience. Curr. Opin. Behav. Sci. 39, 1–9 (2021).
Alvarez, R. P., Biggs, A., Chen, G., Pine, D. S. & Grillon, C. Contextual fear conditioning in humans: Cortical-hippocampal and amygdala contributions. J. Neurosci. 28, 6211–6219 (2008).
Andreatta, M. et al. Initial and sustained brain responses to contextual conditioned anxiety in humans. Cortex 63, 352–363 (2015).
Satpute, A. B., Mumford, J. A., Naliboff, B. D. & Poldrack, R. A. Human anterior and posterior hippocampus respond distinctly to state and trait anxiety. Emotion 12, 58–68 (2012).
Wang, J. et al. The critical mediating roles of the middle temporal gyrus and ventrolateral prefrontal cortex in the dynamic processing of interpersonal emotion regulation. NeuroImage 300, 120789 (2024).
Xie, X. et al. How do you make me feel better? Social cognitive emotion regulation and the default mode network. NeuroImage 134, 270–280 (2016).
Martins, B. & Mather, M. Default mode network and later-life emotion regulation: Linking functional connectivity patterns and emotional outcomes. In Emotion, Aging, and Health (eds Ong, A. D. & Löckenhoff, C. E.) 9–29 (American Psychological Association, 2016). https://doi.org/10.1037/14857-002.
Maddock, R. J., Garrett, A. S. & Buonocore, M. H. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum. Brain Mapp. 18, 30–41 (2002).
Lu, F. et al. Superior frontal gyrus and middle temporal gyrus connectivity mediates the relationship between neuroticism and thought suppression. Brain Imaging Behav. 16, 1400–1409 (2022).
Xu, J. et al. Delineating functional segregations of the human middle temporal gyrus with resting-state functional connectivity and coactivation patterns. Hum. Brain Mapp. 40, 5159–5171 (2019).
Yun, J.-Y. et al. The left middle temporal gyrus in the middle of an impaired social-affective communication network in social anxiety disorder. J. Affect. Disord. 214, 53–59 (2017).
Leech, R. & Sharp, D. J. The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32 (2014).
Gianaros, P. J. & Sheu, L. K. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: Emerging evidence for a brain-body pathway to coronary heart disease risk. NeuroImage 47, 922–936 (2009).
Brewer, J. A. & Garrison, K. A. The posterior cingulate cortex as a plausible mechanistic target of meditation: Findings from neuroimaging. Ann. N. Y. Acad. Sci. 1307, 19–27 (2014).
Busler, J. N., Yanes, J. A., Bird, R. T., Reid, M. A. & Robinson, J. L. Differential functional patterns of the human posterior cingulate cortex during activation and deactivation: A meta-analytic connectivity model. Exp. Brain Res. 237, 2367–2385 (2019).
Cowdrey, F. A., Filippini, N., Park, R. J., Smith, S. M. & McCabe, C. Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Hum. Brain Mapp. 35, 483–491 (2014).
Ciricugno, A. et al. Cerebellar neurostimulation for boosting social and affective functions: Implications for the rehabilitation of hereditary ataxia patients. Cerebellum Lond. Engl. 23, 1651–1677 (2024).
Chin, P. W. & Augustine, G. J. The cerebellum and anxiety. Front. Cell. Neurosci. 17, 1130505 (2023).
Elandaloussi, Y. et al. Understanding the relationship between cerebellar structure and social abilities. Mol. Autism 14, 18 (2023).
Li, K. et al. The spontaneous activity and functional network of the occipital cortex is correlated with state anxiety in healthy adults. Neurosci. Lett. 715, 134596 (2020).
Yang, X. et al. Network analysis reveals disrupted functional brain circuitry in drug-naive social anxiety disorder. NeuroImage 190, 213–223 (2019).
Liao, W. et al. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. NeuroImage 52, 1549–1558 (2010).
Cavanna, A. E. & Trimble, M. R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129, 564–583 (2006).
Yamaguchi, A. & Jitsuishi, T. Structural connectivity of the precuneus and its relation to resting-state networks. Neurosci. Res. https://doi.org/10.1016/j.neures.2023.12.004 (2023).
Geng, H. et al. Altered brain activation and connectivity during anticipation of uncertain threat in trait anxiety. Hum. Brain Mapp. 39, 3898–3914 (2018).
Knowles, K. A. & Olatunji, B. O. Specificity of trait anxiety in anxiety and depression: Meta-analysis of the state-trait anxiety inventory. Clin. Psychol. Rev. 82, 101928 (2020).
Gomez, R. & Francis, L. M. Generalised anxiety disorder: Relationships with Eysenck’s, Gray’s and Newman’s theories. Personal. Individ. Differ. 34, 3–17 (2003).
Weger, M. & Sandi, C. High anxiety trait: A vulnerable phenotype for stress-induced depression. Neurosci. Biobehav. Rev. 87, 27–37 (2018).
Funding
The preparation and initiation of the i-Share project was funded by the program ‘Invest for future’ (reference ANR-10-COHO-05). The i-Share Project had been supported by an unrestricted grant of the Nouvelle-Aquitaine Regional Council (Conseil Régional Nouvelle-Aquitaine) (Grant No. 4370420) and by the Bordeaux ‘Initiatives d’excellence’ (IdEx) program of the University of Bordeaux (ANR-10-IDEX-03-02). It has received grants from the Nouvelle-Aquitaine Regional Health Agency (Agence Régionale de Santé Nouvelle-Aquitaine, Grant No. 6066R-8), Public Health France (Santé Publique France, Grant No. 19DPPP023-0), and The National Institute against cancer INCa (Grant No. INCa_11502). The funding bodies were neither involved in the study design, or in the collection, analysis, or interpretation of the data.
Author information
Authors and Affiliations
Contributions
T.B.: conceptualization, methodology, formal analysis, writing—original draft. A.G.: conceptualization, methodology, writing—original draft, supervision. F.C.: data acquisition, writing—original draft, supervision. M.J.: data acquisition, writing—original draft, supervision. C.T.: data acquisition, supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
The study protocol was approved by the Comite de Protection des Personnes Sud-Ouest et Outre-Mer (local ethics committee CPP SOOMIII) with agreement nr 2015-A00850-49.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Baggio, T., Grecucci, A., Crivello, F. et al. Resting state connectivity patterns associated with trait anxiety in adolescence. Sci Rep 15, 9711 (2025). https://doi.org/10.1038/s41598-025-94790-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94790-9