Abstract
Objective: This study aimed to investigate the clinical efficacy and early outcomes of endoscopic microvascular decompression (MVD) for primary trigeminal neuralgia (TN) and provide clinical experience for the application of full endoscopic techniques in MVD surgery. Methods: This study retrospectively collected medical records of patients who underwent microvascular decompression (MVD) surgery at our institution between January 2020 and January 2023. According to predefined inclusion and exclusion criteria, a total of 137 patients were ultimately included in the study. To evaluate the severity of facial pain in these patients, we utilized the Barrow Neurological Institute (BNI) pain intensity rating system. Additionally, this study analyzed and compared the clinical outcomes of MVD procedures performed endoscopically versus those performed under microscopy. Results: There were no statistically significant differences between endoscopic and microscopic microvascular decompression (MVD) in terms of postoperative hospital stay, recurrence rate, complication incidence, and surgical duration (P > 0.05). However, regarding the efficacy of treatment, the effectiveness rate after endoscopic MVD was superior to that of microscopic MVD, with a statistically significant difference observed between the two groups (P < 0.05). Conclusion: Endoscopic microvascular decompression (MVD) for primary trigeminal neuralgia is a safe and effective treatment, with the critical success factor being the accurate localization of the vessel compressing the nerve. Compared to traditional microscopic MVD, endoscopic MVD shows superior postoperative outcomes, offering wide-angle and multi-angle views along with close-up inspection capabilities. However, it requires attention to overcoming limitations such as a lack of stereoscopic vision and potential blind spots.
Similar content being viewed by others
Introduction
Primary Trigeminal Neuralgia (PTN), also known as idiopathic trigeminal neuralgia, has unclear etiology and pathogenesis. Most scholars believe that the lesion occurs within the trigeminal ganglion and its sensory nerve root, possibly due to vascular compression, bony deformities in the petrous bone, and other factors leading to mechanical compression, traction, and metabolic disturbances of the nerve1,2. It can cause severe, debilitating facial pain, significantly affecting quality of life. Typically, it presents as “recurrent unilateral paroxysmal stabbing pain, with sudden onset and termination, along the distribution of the trigeminal nerve, usually lasting from a few seconds to two minutes, often occurring in the facial area supplied by the maxillary or mandibular branches.” Pain is often triggered by touching a specific sensitive area of the face, known as “trigger points.”3.
Treatment methods mainly include drug therapy, radiofrequency thermocoagulation, balloon compression of the trigeminal ganglion, glycerol injection, trigeminal nerve rhizotomy, stereotactic radiosurgery, and microvascular decompression (MVD)4,5,6. MVD has shown better efficacy compared to other treatment methods, providing longer pain relief duration, lower recurrence rates postoperatively, and preservation of nerve function, thus improving long-term quality of life for patients7,8. In recent years, the application value of endoscopy in cranial surgery has been demonstrated, such as in the treatment of lesions in the anterior skull base, saddle area, and slope9. Recently, endoscopy has been applied in lateral skull base surgery, especially in the surgical treatment of primary trigeminal neuralgia10. However, there are still few reports on the full endoscopic MVD for PTN11. This study aims to evaluate the clinical efficacy of full endoscopic MVD for PTN and share clinical experience.
Materials and methods
General information
This study retrospectively analyzed the clinical data of 137 patients who underwent microvascular decompression (MVD) for PTN at the Affiliated Hospital of Xuzhou Medical University from January 2020 to January 2023. The data collected included: gender, age, duration of follow-up, length of postoperative hospital stay, side of pain, distribution range of pain, duration of illness, degree of postoperative pain relief, recurrence, occurrence of complications, duration of surgery, identification of responsible vessels, and types of responsible vessels (Table 1). Preoperative imaging systems were used to assess the relationship between the affected side of the trigeminal nerve and adjacent blood vessels. Statistical analysis of the differences between the two groups was conducted using SPSS 26.0 software. This study was in accordance with the provisions of the Medical Ethics Committee of the Affiliated Hospital of Xuzhou Medical University. It has been reviewed by the Ethics Committee and exempted from the informed consent requirement, Ethics No. ( XYFY2023-KL441-01 ).
Inclusion and exclusion criteria
Inclusion criteria: Patients were included if they met the following criteria: (1) Diagnosis of PTN according to the 3rd edition of the International Classification of Headache Disorders; (2) Patients who experienced reduced efficacy or intolerance to standardized drug therapy; (3) Preoperative confirmation of the close relationship between the affected side of the trigeminal nerve and adjacent blood vessels through 3D FIESTA combined with 3D TOF sequence magnetic resonance imaging (Fig. 1); (4) In the treatment of patients with primary trigeminal neuralgia, a microvascular decompression surgery assisted by endoscopy or microscopy is employed throughout the procedure.
A: Magnetic resonance 3D TOF sequence scanning result; B: Magnetic resonance 3D FIESTA sequence scanning result; Preoperative magnetic resonance scan confirmed that there were anterior and posterior vessels in the upper part of the right trigeminal nerve, and it was in close contact with the right trigeminal nerve.
Exclusion criteria: Patients were excluded if they met any of the following criteria: (1) Secondary trigeminal neuralgia; (2) Patients with recurrent PTN after MVD treatment; (3) Patients with primary trigeminal neuralgia (PTN) who underwent other treatment modalities such as radiofrequency ablation, balloon compression, Gamma Knife radiosurgery, sensory root sectioning, etc.; (4) Patients who were unable to tolerate anesthesia and surgery or refused MVD treatment; (5) Patients with bilateral trigeminal neuralgia; (6) Patients lost to follow-up for various reasons during the follow-up period.
Surgical procedure
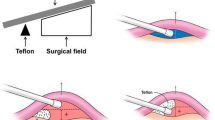
In the MVD surgery performed entirely under endoscopic guidance, 0° and 30° neuroendoscopes along with their corresponding surgical instruments were utilized (Fig. 2). Patients were placed in a lateral decubitus position with the back as close as possible to the edge of the operating table, and the head frame was secured to slightly tilt the head towards the incision side. A straight incision parallel and close to the midline was made, measuring 6–7 cm in length, and the skin, muscles, and periosteum were sequentially dissected. A bone window of approximately 2.5 cm × 2.5 cm was created using a bone drill, with the window positioned as close as possible to the transverse sinus and extending upward towards the lower edge of the transverse sinus. The dura mater was suspended along the periphery, and a “U” shaped incision was made in the dura mater, exposing the angle between the transverse and sigmoid sinuses. Under endoscopic guidance, the trigeminal nerve was dissected from the outer side to the inner side of the cerebellum, cerebrospinal fluid was slowly released, and the arachnoid membrane was thoroughly dissected to expose the trigeminal nerve. The 0°and 30°endoscopes were used to explore the entire length of the trigeminal nerve cisternal segment, identify the responsible vessels, and place Teflon pads for adequate decompression (Fig. 3). Before closing the skull, it was ensured that there was no active bleeding in the surgical field. Warm saline was gently injected under endoscopic observation to replace bloody cerebrospinal fluid and gas, while ensuring that the pads remained in place. The dura mater was meticulously sutured, the bone flap was replaced, and the skull was closed layer by layer.
In the MVD surgery conducted under microscopic guidance, patients were positioned in a lateral decubitus position and underwent retrosigmoid craniotomy via a postauricular straight incision under general anesthesia. Following this, cerebrospinal fluid was slowly released, and we patiently waited for cerebellar collapse to obtain sufficient operative space. The trigeminal nerve and its associated anatomical structures were fully exposed, and the root entry zone (REZ) of the trigeminal nerve was carefully examined under the microscope. To ensure a clear field of vision, the arachnoid membrane surrounding the trigeminal nerve was incised, thoroughly exposing the adjacent vessels. Once the culprit vessel was identified, it was adequately mobilized and separated using Teflon pads (Fig. 4). For cases where the culprit vessel could not be definitively identified, the procedure involved releasing the arachnoid bands around the trigeminal nerve and performing a neurolysis to improve the patient’s facial pain symptoms. Finally, the surgical area was thoroughly irrigated with warm saline, the bone flap was replaced, and the scalp was closed layer by layer.
Outcome measures
Follow-up of all enrolled patients was completed as of March 31, 2024. The follow-up period for patients postoperatively exceeded 12 months, with the longest follow-up time being 36 months. The postoperative efficacy, surgical duration, hospitalization time, recurrence rates, and occurrence of postoperative complications were statistically analyzed. Patients did not use analgesic or antiepileptic drugs (such as carbamazepine) postoperatively to observe and assess the efficacy, and pain relief was monitored through telephone and outpatient follow-ups.
Results
Postoperative efficacy was assessed using the Barrow Neurological Institute (BNI) pain intensity rating scale. Grade I: No pain; Grade II: Occasional pain, not requiring medication; Grade III: Intermittent pain, controlled with medication; Grade IV: Intermittent pain, not controlled with medication; Grade V: Severe pain/unrelieved pain. Grades I to III were defined as effective, with Grade I defined as complete cure and Grades II to III defined as partial relief.
From January 2020 to January 2023, this study included 137 patients with primary trigeminal neuralgia (PTN) who received microvascular decompression (MVD) surgery. Patients were allocated into two groups: the endoscopic group (n = 63), where 59 (93.7%) patients achieved complete postoperative cure and 4 (6.3%) patients had partial relief; and the microscopic group (n = 74), where 60 (81.1%) patients were completely cured, 8 (10.8%) patients had partial relief, and 6 (8.1%) patients showed no response to surgery. The statistical data revealed that the average operative time was 192.62 ± 7.38 min for the endoscopic group and 186.43 ± 5.39 min for the microscopic group. Average hospital stays were 7.73 ± 0.30 days for the endoscopic group and 8.08 ± 0.38 days for the microscopic group. Within the follow-up period of 12–36 months, recurrence was observed in 3 (4.8%) patients in the endoscopic group and 8 (10.8%) patients in the microscopic group. Additionally, 6 (9.5%) patients in the endoscopic group and 9 (12.2%) patients in the microscopic group reported postoperative complications (Table 2).
Discussion
Long-standing clinical practices in neurosurgery have demonstrated that nearly all typical cases of trigeminal neuralgia (PTN) originate from the compression of the trigeminal nerve root by responsible vessels in the cerebellopontine angle (CPA) zone12. Therefore, achieving a 100% cure rate has always been the eternal pursuit for neurosurgeons performing MVD surgery for PTN. As a highly refined form of minimally invasive neurosurgery, MVD surgery requires further dissemination of its operative techniques to minimize the occurrence of unacceptable severe complications in patients. Full endoscopic MVD treatment for PTN serves as a beneficial supplement and improvement to traditional microscopic MVD13, presenting an effective therapeutic approach. The application of endoscopy in neurosurgery dates back to the 1960s, and its scope has rapidly expanded to include lesions in the anterior skull base, saddle area, and slope14,15. Recently, endoscopy has been widely utilized in lateral skull base surgeries, particularly in the surgical treatment of trigeminal neuralgia16.
Surgical experience of full endoscopic MVD treatment for PTN
In trigeminal neuralgia surgery, the most common responsible vessel is the superior cerebellar artery17,18. However, the compression situation of vessels during surgery may differ from preoperative expectations. Therefore, we recommend comprehensive preoperative 3DFIESTA and 3DTOF examinations to improve the accuracy of responsible vessel identification19. Regarding patient positioning requirements, the use of the full endoscopic microvascular decompression (MVD) position does not require excessive forward tilting compared to traditional microscopic MVD. We adopt a complete lateral position to allow gravity-induced sagging of the cerebellum posteriorly. The surgical approach typically involves a retrosigmoid approach, operating below the surface or lateral aspect of the cerebellum to enter the subarachnoid space and release cerebrospinal fluid. During the cerebrospinal fluid release process, we recommend appropriately preserving the arachnoid membrane, as it can serve to protect the transverse sinus. Excessive dissection of the arachnoid membrane may lead to decreased protection of the transverse sinus during surgery, potentially resulting in serious consequences such as tearing of the transverse sinus or cerebellar injury13,20.
Once the cerebellum has sufficiently collapsed and an adequate operating space is obtained, the endoscope and related instruments are introduced, with the instruments kept at the forefront of the endoscope to enable the endoscope to follow the instruments into the surgical area. The endoscope is a telescopic instrument rather than a swinging instrument from side to side. Since the lateral side of the endoscope is out of view, changing the angle of view cannot rely solely on swinging the endoscope head. When it is necessary to change the endoscope angle, the endoscope should be removed first and then reinserted at the new angle. Any structure (such as the transverse sinus, perforating vessels, or nerves) between the two axes of rotation carries the risk of damage from the endoscope rod21. Therefore, regardless of how safe and broad the surgical field being operated on appears, it is essential to cultivate good habits to avoid injuring surrounding structures.
The key to the success of microvascular decompression surgery lies in the clear identification of vascular compression during surgery22,23. The majority of responsible vessels are located in the brainstem segment of the nerve, requiring limited and effective dissection of the nerve at its exit from the brainstem. When necessary, cerebellar-related vessels can be sacrificed, while vessels (veins or arteries) originating from the brainstem should be preserved as much as possible. After confirming the responsible vessel, a pad should be placed between the brainstem and the vessel to elevate the vessel, while simultaneously providing loose Teflon isolation between the vessel and the nerve for better outcomes.
Comparison of endoscopic and microscopic MVD procedures
The microscope projects a tubular beam of parallel light onto the surgical area and magnifies the field of view, necessitating that the light is directed accurately and directly onto the surgical target to observe anatomical structures clearly. To ensure that the light path reaches the surgical target in a straight line, it often requires retracting the cerebellum, which can increase the risk of postoperative complications if overdone during surgery24. In contrast, endoscopy allows for the comprehensive exposure of the three-dimensional structure of the cerebellopontine angle from multiple angles, providing full illumination and enabling close-up observation of the contact between nerves and vessels. This technique makes it possible for surgeons to more clearly identify and manage blind spots that are difficult to observe with traditional microscopic MVD, especially critical areas such as the root entry zone (REZ) of the trigeminal nerve and the Meckel’s cave. The application of endoscopy can fully expose the entire length of the trigeminal nerve within the subarachnoid pool, leading to more precise identification of the offending vessel. It not only facilitates the thorough separation of compressive vessels but also enhances the safety and accuracy of surgical operations, improving long-term patient outcomes.
In this study, we compared the efficacy and recurrence rates of microvascular decompression (MVD) procedures performed entirely endoscopically versus under microscopy. Results showed that patients who underwent full endoscopic MVD had a postoperative recurrence rate of 4.8%, while those who received microscopic MVD had a recurrence rate of 6.8%, the difference in recurrence rates between the two groups was not statistically significant (P > 0.05). Regarding treatment outcomes, the rate of complete pain relief after full endoscopic MVD reached 93.7%, whereas the rate for microscopic MVD was 81.1%, the difference in complete pain relief rates between the two groups was statistically significant (P < 0.05). Thus, although both approaches show similar recurrence rates, the full endoscopic MVD exhibits a notable advantage in enhancing the postoperative rate of complete pain relief. For patients seeking optimal pain relief, full endoscopic MVD may be a preferred treatment option.
Advantages and limitations of endoscopy
Advantages
(1) Wide-Angle View: Endoscopy can enter the cerebellopontine angle (CPA) area, providing a wide-angle view similar to a flask shape25. Compared to the magnified local structures through narrow channels by microscopes, endoscopy offers significant advantages in observing deep intracranial lesions, particularly suitable for monitoring from the Meckel’s cave to the root entry zone (REZ) of the trigeminal nerve during MVD surgery.
(2) Multi-Angle View: Endoscopy features multi-angle viewing capabilities that allow it to overcome obstructions and occlusions, directly observing anatomical blind spots inaccessible to microscopes, such as the Meckel’s cave, the ventromedial region of the trigeminal nerve, or structures within the internal auditory canal26. By using 30° or 45° endoscopes and rotating them, one can fully observe surrounding structures of the cisterns, even when obstructed by the petrous bone or veins27.
(3) Close-Up Observation: The close-up observation function of endoscopy combines optical and digital magnification effects to further enlarge the anatomical details of the surgical area. This feature aids in displaying subtle characteristics of vascular compression in the REZ of the trigeminal nerve, distinguishing between arterial and venous compressions, and accurately confirming the condition of neural compression28.
Limitations
(1) Visual Blind Spots: Despite offering wide-angle and multi-angle views, endoscopy’s close-up observation is limited to specific areas, with other extensive surgical pathways and areas behind or outside the scope potentially becoming blind spots29. This is a major disadvantage compared to microscopes.
(2) Two-Dimensional Images and Lack of Depth Perception: Endoscopic images are two-dimensional and exhibit a “fish-eye effect,” where the center is magnified while the periphery is compressed, lacking depth perception. This increases the difficulty of intraoperative manipulation, requiring surgeons to overcome visual distortion to identify true anatomical structures, presenting a particular challenge to beginners who may easily injure surrounding nerves and vessels30.
(3) Impact on Image Quality: Minor bleeding causing cerebrospinal fluid staining significantly degrades the quality of endoscopic images, making them difficult to observe, posing an additional challenge to the surgical process31.
Conclusion
This study demonstrates that performing microvascular decompression (MVD) using an endoscopic approach for the treatment of primary trigeminal neuralgia is a safe and effective method. During the procedure, accurately identifying and confirming the location of the vessel causing nerve compression is a crucial factor for surgical success. Compared with traditional microscopic MVD, endoscopic MVD exhibits superior postoperative efficacy. Endoscopic surgery is characterized by its wide-angle vision, multi-angle observation capabilities, and the capacity for close inspection. However, it also presents certain limitations, such as a lack of stereoscopic vision and potential blind spots behind the scope, which need to be recognized and addressed in practice.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information file.
References
Bendtsen, L. et al. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. 19, 784–796. https://doi.org/10.1016/S1474-4422(20)30233-7 (2020).
Bennetto, L., Patel, N. K. & Fuller, G. Trigeminal neuralgia and its management. BMJ 334, 201–205. https://doi.org/10.1136/bmj.39085.614792.BE (2007).
Khawaja, S. N. & Scrivani, S. J. Trigeminal Neuralgia. Dent. Clin. North. Am. 67, 99–115. https://doi.org/10.1016/j.cden.2022.07.008 (2023).
Allam, A. K., Sharma, H., Larkin, M. B. & Viswanathan, A. Trigeminal Neuralgia: Diagnosis and Treatment. Neurol. Clin. 41, 107–121. https://doi.org/10.1016/j.ncl.2022.09.001 (2023).
Tai, A. X. & Nayar, V. V. Update on Trigeminal Neuralgia. Curr. Treat. Options Neurol. 21, 42. https://doi.org/10.1007/s11940-019-0583-0 (2019).
European Academy of Neurology Guideline on Trigeminal Neuralgia - PubMed. (2024). https://pubmed.ncbi.nlm.nih.gov/30860637/. Accessed April 19.
Chen, L., Shang, Y., Zhang, Y. & Zhao, Y. Endoscopic microvascular decompression versus microscopic microvascular decompression for trigeminal neuralgia: A systematic review and meta-analysis. J. Clin. Neurosci. 117, 73–78. https://doi.org/10.1016/j.jocn.2023.09.009 (2023).
Wu, A. et al. Immediate and Long-Term Outcomes of Microvascular Decompression for Mixed Trigeminal Neuralgia. World Neurosurg. 117, e300–e307. https://doi.org/10.1016/j.wneu.2018.06.016 (2018).
Piazza, M. & Lee, J. Y. K. Endoscopic and Microscopic Microvascular Decompression. Neurosurg. Clin. N Am. 27, 305–313. https://doi.org/10.1016/j.nec.2016.02.008 (2016).
Sun, Z., Wang, Y., Cai, X., Xie, S. & Jiang, Z. Endoscopic Vascular Decompression for the Treatment of Trigeminal Neuralgia: Clinical Outcomes and Technical Note. J. Pain Res. 13, 2205–2211. https://doi.org/10.2147/JPR.S268441 (2020).
Di Stefano, G., La Cesa, S., Truini, A. & Cruccu, G. Natural history and outcome of 200 outpatients with classical trigeminal neuralgia treated with carbamazepine or oxcarbazepine in a tertiary centre for neuropathic pain. J. Headache Pain. 15, 34. https://doi.org/10.1186/1129-2377-15-34 (2014).
Jones, M. R. et al. A Comprehensive Review of Trigeminal Neuralgia. Curr. Pain Headache Rep. 23, 74. https://doi.org/10.1007/s11916-019-0810-0 (2019).
Tang, C-T., Baidya, N. B. & Ammirati, M. Endoscope-assisted neurovascular decompression of the trigeminal nerve: a cadaveric study. Neurosurg. Rev. 36, 403–410. https://doi.org/10.1007/s10143-012-0447-5 (2013).
Bove, I., Cheok, S., Ruzevick, J. J. & Zada, G. Endoscopic Endonasal and Keyhole Surgery for Skull Base Meningiomas. Neurosurg. Clin. N Am. 34, 393–402. https://doi.org/10.1016/j.nec.2023.02.003 (2023).
Lee, J. Y. K., Ramakrishnan, V. R., Chiu, A. G., Palmer, J. & Gausas, R. E. Endoscopic endonasal surgical resection of tumors of the medial orbital apex and wall. Clin. Neurol. Neurosurg. 114, 93–98. https://doi.org/10.1016/j.clineuro.2011.09.005 (2012).
Shimanskiĭ, V. N., Karnaukhov, V. V., Sergienko, T. A., Poshataev, V. K. & Semenov, M. S. [Endoscopic assistance in microvascular decompression of cranial nerves]. Zh Vopr Neirokhir Im N N Burdenko. 76, 3–10 (2012). discussion 10.
Yang, L. & Cheng, H. Surgical technique management of microvascular decompression for trigeminal neuralgia. Ideggyogy Sz. 75, 369–375. https://doi.org/10.18071/isz.75.0369 (2022).
So, R. J. et al. A racial analysis of pain outcomes following microvascular decompression for trigeminal neuralgia. J. Neurosurg. 139, 633–639. https://doi.org/10.3171/2022.12.JNS221884 (2023).
Wang, L., Zhang, X., Zhao, M. & Wang, Q. Assessment of epidermoid cyst with trigeminal neuralgia before neuroendoscopy: A high-resolution MR study based on 3D-FIESTA and MR angiography. Clin. Imaging. 91, 9–13. https://doi.org/10.1016/j.clinimag.2022.08.006 (2022).
Blue, R. et al. Complication Rates During Endoscopic Microvascular Decompression Surgery Are Low With or Without Petrosal Vein Sacrifice. World Neurosurg. 138, e420–e425. https://doi.org/10.1016/j.wneu.2020.02.142 (2020).
Zhu, G. T. et al. Application of complete endoscopic technique in microvascular decompression related tovertebrobasilar artery compression. Zhonghua Yi Xue Za Zhi. 99, 2597–2601. https://doi.org/10.3760/cma.j.issn.0376-2491.2019.33.008 (2019).
Soni, P. et al. Outcomes of microvascular decompression for trigeminal neuralgia with purely venous compression: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 198, 106230. https://doi.org/10.1016/j.clineuro.2020.106230 (2020).
Dumot, C. & Sindou, M. Veins of the Cerebellopontine Angle and Specific Complications of Sacrifice, with Special Emphasis on Microvascular Decompression Surgery. A Review. World Neurosurg. 117, 422–432. https://doi.org/10.1016/j.wneu.2018.06.160 (2018).
Li, Y. et al. A Meta-Analysis of Endoscopic Microvascular Decompression versus Microscopic Microvascular Decompression for the Treatment for Cranial Nerve Syndrome Caused by Vascular Compression. World Neurosurg. 126, 647–655e7. https://doi.org/10.1016/j.wneu.2019.01.220 (2019).
Di Stefano, G., Maarbjerg, S. & Truini, A. Trigeminal neuralgia secondary to multiple sclerosis: from the clinical picture to the treatment options. J. Headache Pain. 20, 20. https://doi.org/10.1186/s10194-019-0969-0 (2019).
Guan, H., Li, S. & Wang, X. Fully endoscopic microvascular decompression for trigeminal neuralgia: technical note and early outcomes. Neurosurg. Rev. 46, 292. https://doi.org/10.1007/s10143-023-02188-w (2023).
Bohman, L-E., Pierce, J., Stephen, J. H., Sandhu, S. & Lee, J. Y. K. Fully endoscopic microvascular decompression for trigeminal neuralgia: technique review and early outcomes. Neurosurg. Focus. 37, E18. https://doi.org/10.3171/2014.7.FOCUS14318 (2014).
Jarrahy, R., Eby, J. B., Cha, S. T. & Shahinian, H. K. Fully endoscopic vascular decompression of the trigeminal nerve. Minim. Invasive Neurosurg. 45, 32–35. https://doi.org/10.1055/s-2002-23586 (2002).
Xiang, H., Wu, G., Ouyang, J. & Liu, R. Prospective Study of Neuroendoscopy versus Microscopy: 213 Cases of Microvascular Decompression for Trigeminal Neuralgia Performed by One Neurosurgeon. World Neurosurg. 111, e335–e339. https://doi.org/10.1016/j.wneu.2017.12.051 (2018).
Wang, P., Li, Q., Wang, C. & Li, C. Complete neuroendoscopic versus microscopical trigeminal neuralgia microvascular decompression (MVD) in primary trigeminal neuralgia (PTN). Am. J. Transl Res. 13, 12905–12912 (2021).
Yun, G-Y. et al. Preliminary Report of Fully Endoscopic Microvascular Decompression. J. Korean Neurosurg. Soc: April. https://doi.org/10.3340/jkns.2024.0003 (2024).
Funding
This study is supported by the China Jiangsu High-level hospital construction project, GSPJS202407.
Author information
Authors and Affiliations
Contributions
Contributions of Authors: Bao Feng, Xin Chai, Yi Yu, Hao Xia, Junyi Wu, Yin Ren, PeiMin Yu, and Yufu Zhu contributed to this work. Bao Feng, Yi Yu, Hao Xia, Junyi Wu, Yin Ren, and PeiMin Yu participated in the collection and analysis of clinical data, performing the statistical analysis, and drafting portions of the manuscript. Xin Chai contributed to the critical revision of the manuscript for important intellectual content. Yufu Zhu, as the corresponding author, conceived and designed the study, participated in its coordination, helped to draft the manuscript, and ensured that all aspects of the work were appropriately investigated and resolved, including the accuracy and integrity of any data and figures. Each author has made significant contributions to different stages of the research and manuscript preparation, and all authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Feng, B., Chai, X., Yu, Y. et al. Endoscopic microvascular decompression for primary trigeminal neuralgia: surgical experience and early outcomes. Sci Rep 15, 10289 (2025). https://doi.org/10.1038/s41598-025-94797-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94797-2