Abstract
Endophytic fungi in medicinal plants aid in producing useful therapeutic compounds and enzymes. Among the most useful enzymes are cellulases. However, cellulase enzyme production in endophytic fungi of Azadirachta indica and Aloe secundiflora has not been comprehensively explored. The objective of this study was to; isolate and identify endophytic fungi from the leaves of young and mature plants of A. indica and A. secundiflora, determine colonization frequency of the endophytic fungi, and evaluate and optimize the cellulase production by the endophytic fungi on maize cob media. Eleven fungal endophytic isolates were obtained from the leaves of both A. secundiflora and A. indica, collected in Kitui and Kiambu Counties in total: Six from Kitui County and five from Kiambu County. Penicillium Sp. had highest colonization frequency in Kitui, while Candida sp. had highest in Kiambu. For enzyme optimization, isolates Candida boidinii, Galactomyces candidum, and Candida stellimalicola produced the highest amounts of Fpases and endoglucanases on third, sixth and ninth days. High exoglucanase producers were Colletotrichum gloeosporioides, Galactomyces candidum, and Candida stellimalicola. The endophytic communities within the leaves of A. indica and A. secundiflora are diverse. Maize cob agrowaste media can be used to cultivate the production of cellulases successfully in fungal endophytic isolates of A. indica and A. secundiflora. The study concluded that the endophytes of A. indica and A. secundiflora can be harnessed and optimized to secrete cellulase enzymes for commercial use, and especially isolates G. candidum and C. stellimalicola which yield significantly high amounts of total cellulases, endoglucanases and exoglucanases.
Similar content being viewed by others
Introduction
Endophytic fungi are microorganisms that live in plants and are diverse in nature1. They are abundant and present in the leaves, fruits, roots, flowers, and stems. Endophytic fungi can be found in all parts of the plant and especially the leaves. In Thai orchids, fifty-two endophytic isolates were obtained from five different species of orchids, with the leaves having the most abundant number of isolates2. Fifty-six percent of the isolates produced cellulases on solid media, which were the second most abundant enzymes produced2. Endophytic fungi form mutualistic, neutralistic, or antagonistic relationships with the host3. They have been isolated from tropical and temperate plants, desert plants, mangroves, Tundra in the Arctic, croplands, savannas, and grasslands4. Examples of endophytic fungi that have been isolated from plants include Fusarium sp., Colletotrichum sp., Alternaria sp., Aspergillus sp., Cladosporium sp., Penicillium sp., Trichoderma sp., and Xylaria sp., among others5. Species of endophytes isolated from A. indica include Cladosporium sp., Aspergillus sp., Colletotrichum sp., and Fusarium sp.6.
According to Khalil et al.4, medicinal plants are significantly unexplored when it comes to their functional associations with fungi. Medicinal plants contain endophytic fungi that secrete exoenzymes. The exoenzymes hydrolyse structural compounds of pathogens and destroy them, providing plants with protection against these organisms7. These enzymes break down lignocellulosic materials8. They include amylases, pectinases, cellulases, proteases, lipases, and laccases5. These enzymes can have a direct antagonistic effect on pathogens. The amount and type of enzymes secreted by endophytic fungi vary and may depend on the source plant. According to1, Colletotrichum gloeosporioides, Cladosporium cladosporioides, and Aspergillus versicolor are endophytic microbes with high amounts of cellulase activity.
According to9, naturally, these fungi significantly aid in plant biomass degradation by producing carbohydrate-active exoenzymes dedicated to the degeneration of plant polysaccharides. The activities of the enzymes have been observed over time but their applications in the commercial world are recent10. In addition to their degrading activities, these enzymes have become prominently significant and essential in industrial application due to their ease of production, relatively low costs, efficiency, consistency, small spaces needed for production, and the ability to modify and optimize the production process11. Cellulases from microbes are among these commercialized enzymes. Cellulases refer to a group of enzymes that comprise endoglucanases, exoglucanases, and β-glucosidases10. The endoglucanases hydrolyse cellulose chains producing cello-oligosaccharides and glucose10. Exoglucanases degrade cellulose, yielding the primary product cellobiose10. β-glucosidases act on cellobiose to yield glucose10. Anoop Kumar et al.10 state that cellulases have numerous applications in industries utilizing biotechnology and rank second in industrial use after proteases. In another study by12, cellulases were the second most prominent group of enzymes produced by endophytic fungi. The industries using cellulases include textile, animal feeds, paper and pulp, wine and brewery, laundry, and food 10. Industries showing very promising application of cellulases include the beverage, feed and food industries10.
According to Ejaz et al.13, the diverse uses of microbial-derived cellulases include clarifying fruit juices, tenderizing fruits, hydrolysing roasted coffee, and lessening roughage in dough, increasing taste and aroma in foods, and extracting essential oils and tea polyphenols from olives.13 report that cellulase enzymes are utilized in hydrolysis of biomass into sugars such as hexoses and pentoses that are fermented to yield biofuels. Using microbial cellulolytic processes reduced the cost of processing biomass by forty percent13. As per Sopalun and Iamtham14, there is an increase in industrial demand for novel sources of more potent enzymes. Reasons for these include the demand for more environmental-friendly bioprocesses and better thermostable enzymes13. According to Ejaz et al.13, there is an increase in the use of enzymes in commercial markets but the applications of the catalytic potential of cellulases are yet to be fully explored, especially in the food industry.
The main roadblock in enzyme application in industry is the high cost associated with producing the enzymes14. The increased demand for enzymes calls for more cost-effective alternatives in producing the enzymes, such as using low cost and effective substrates14. Sopalun and Iamtham14 report that agricultural countries such as Thailand produce large yields of agrowaste that pose challenges for disposal. These agrowastes include sugarcane bagasse, rice husk and corncob among others. The agrowastes are useful in producing biogas, biofuel and providing raw materials research work through solid state fermentation15. However, most of the agrowaste is underutilized and untreated, and usually destroyed through burning, landfilling or dumping15. Agrowaste media is beneficial because of its ease of availability, low cost, and eco-friendly utilization14. It is a great way to utilize raw material that would otherwise cause pollution14. This research study utilized maize (corn) cob as a substrate for the evaluation and optimization of cellulase enzymes. Maize is among the most commonly produced crops worldwide15. Such abundance means that it generates sufficient amounts of agrowaste that can be utilized for enzyme production at low costs. Moreover, El-Tayeb et al.16 determined that corn cob has the highest amount of cellulose based on dry weight, at 61.2% w/w, which makes it the most preferable substrate for producing cellulase enzymes. According to15, maize or corn provides commonly used substrate for solid state fermentation. The advantages of using solid state fermentation include low economic cost associated with the process, the ease of product recovery, reduced downstream processing, smaller size of the fermenter needed, and reduced energy needed for sterilizing and stirring15. The maize agrowaste medium and solid state fermentation are thus highly practical for research and industrial purposes.
The increase in demand for novel sources of thermostable and highly potent enzymes in industrial applications, the high demand for cellulases and the potential for cellulase application in various industries, the need for cheaper, available and eco-friendly processes, and the significant potential for research into medicinal plants and their endophytes are areas that need further exploration and research. Thus, the objective of this study was to isolate and identify fungal endophytes from medicinal plants A. indica and A. secundiflora by morphological and molecular characterization, and evaluate their potential for the production of cellulases under solid state fermentation using maize cobs as substrate.
Results
Morphological identification of all endophytic fungi
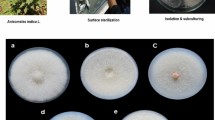
All colonies of the fungal isolates appeared smooth, except for isolate G1AL001. All isolates except M2L002 and G2AL001 appeared circular in Petri dish cultures. Isolates G1AL002 and G2L001 were non-filamentous, white, had aerial mycelia, and were septate. Isolate G2L001 was green and feathery in appearance. Isolates M2L001 (34 mm) and G1AL001 were fluffy, white, septate, with aerial mycelium (Table 1). Images of some of the fungi are shown below in Fig. 2.
The isolates were morphologically divided into three main clusters (A, B, and C). Clusters B and C were closer to each other than cluster A. Cluster A had two sub-clusters, I and II. Sub-cluster I was subdivided into two groups which showed close relationships between the isolates M2L001 and G1AL001 and M1L002 and M1L001. Sub-cluster II showed a close relationship between G2L001 and G1AL002. Cluster B had sub-clusters III and IV. Sub-cluster III had two sub-divisions, with isolates M2L002 and G2AL001. Sub-cluster IV had isolate M2L004. These isolates showed similar morphological traits. Cluster C had isolates M1L003 and G2AL002 (Fig. 1).
Genetic identification of the fungal endophytes
Based on nucleotide BLAST in the NCBI database, most isolates had a 100% sequence match with reference strains in the database (Table 2). Isolates G1AL001, 99.5%, G1AL002, 90.98%, and G2AL002 were grouped in the Genus Candida with similarity indices of 99.5%, 90.98%, and 100%, respectively. Isolates G2AL001 had 90.95% identity with isolates in the Genus Galactomyces, while isolates (Fig. 2); M1L001 and M1L002 had 100% similarity with reference sequences for the Genus Colletotrichum. Isolate M1L003 had 100% similarity with the reference sequence for Genus Fusarium. The isolate M2L001 matched with the reference sequence of Genus Trichoderma with a 100% match. Isolates M2L004 and M2L002 matched with reference sequences of Genus Penicillium, with 100% similarity, and isolate G2L001 had 99.78% similarity with the reference sequence of Genus Cladosporium (Table 2).
Phylogenetic analysis
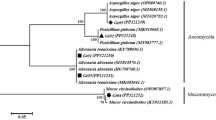
A phylogenetic tree based on the maximum likelihood method clustered the isolates into two main clusters (A and B). Cluster B had many isolates and two subclusters (i and ii). Subcluster I consisted of isolates related to the Candida genus and included C. boidinii and C. stellimalicola. Subcluster II was divided into two groups (1 and 2). Group 1 consisted of two main sub-groups; the first sub-group consisted of Clad. cladosprioides, and the second sub-group contained F. luffae. Group 2 consisted of isolates of the Colletotrichum Genus (Col. gloeosporioides and Col. fructicola), T. lixii, and Penicillium Genus (Penicillium sp. voucher AC11) (Fig. 3).
The optimal tree with the sum of branch length = 2.19115597 is shown (Fig. 3). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method17 and are in the units of the number of base substitutions per site. The analysis involved 21 nucleotide sequences. Codon positions included were 1st + 2nd + 3rd + Noncoding. All ambiguous positions were removed for each sequence pair. There were a total of 968 positions in the final dataset. Evolutionary analyses were conducted in MEGA X17;18.
Colonization frequency of endophytes in the sampled plant tissues
The colonization frequency of the different isolates was determined based on the formula in Sect. "Sampling". Twenty-six segments were used, divided into thirteen for each plant per County. These were further divided into seven segments for young plants and six mature plants. The colonization frequencies were presented in the form of percentages for each isolate. The isolates of leaves of mature A. secundiflora plants in Kitui County consisted of Penicillium sp. voucher AC11 (66.67%) and T. lixii (50%). F. luffae (42.86%) was isolated from young plant leaves of A. secundiflora. In A. indica, the mature plants were colonized by Penicillium sp. voucher AC11 (83.33%), while the young plants were colonized by Col. fructicola (14.29%) and Col. gloeosporioides (71.43%) (Fig. 4).
In Kiambu County, the leaves of mature plants of A. secundiflora hosted G. candidum (83.33%), while the young plant leaves contained C. boidinii (85.71%). The mature plant leaves of A. indica were colonized by C. stellimalicola (50%) and Clad. cladosporioides (16.67%). The young plants had leaves colonized by C. stellimalicola (42.86%) (Fig. 5).
Cellulase assays
Total cellulase enzyme production (FPase)
For FPase, most of the isolates showed decreased enzyme production from day 3 to day 6. Isolate G2AL001 (G. candidum) showed an increase in enzyme production from day 3 to day 9. Isolates G1AL001 (C. boidinii), M2L001 (T. lixii), MIL003 (F. luffae), M2L002 (Penicillium sp.), and M2L004 (Penicillium sp.) showed a decrease in enzyme production from day 3 to day 9. Isolates G1AL002 (C. stellimalicola), M1L002 (Col. fructicola), and M1L001 (Col. gloeosporioides) had a decrease in enzyme production from day 3 to day 6 and an increase in enzyme production from day 6 to day 9. Isolate G2L001 (Clad. cladosporioides) had an increase in enzyme production from day 3 to day 6 and a decrease in production from day 6 to day 9. Isolate G1AL002 produced the highest amount of total cellulase enzymes of 15.9 ± 0.28 IU/mL on day 3 which was not significantly higher than total cellulase enzymes produced by isolate M1L001 (14.9 ± 0.45 IU/mL). Isolate G2L001 produced the lowest total cellulase enzymes of 5.08 ± 0.02 IU/mL after three days of incubation. Isolate G2AL001 produced 18.9 ± 0.23 IU/mL on the 6th day of incubation which was significantly higher than FPase produced by all other fungal endophytes, while isolate M1L001 produced the lowest total cellulases on the 6th day of incubation at 5.29 ± 0.11 IU/mL. The highest FPase on the 9th day of incubation was 19.8 ± 0.31 IU/mL produced by isolate G1AL002 which was significantly higher than FPase recorded by all other fungal isolates (Table 3).
For total cellulase enzyme assay over time, there was a significant difference in the IU/mL (enzyme units) for days three, six, and nine (p < 0.05). Day 9 had the highest total cellulase enzyme production (11.82 ± 2.57 IU/mL), followed by day 3 (9.97 ± 0.64 IU/mL), and lastly day 6 (8.64 ± 0.81 IU/mL). G. candidum produced the highest amount over time (27.68 ± 5.94 IU/mL), while F. luffae had the lowest output over time (5.48 ± 0.52 IU/mL) at p < 0.05. The interaction between day of incubation and the fungal isolate was significant (p < 0.05).
Endoglucanase enzyme production
For endoglucanases, most of the isolates showed a decrease in enzyme production from day 3 to day 6. Isolate G1AL002 (C. stellimalicola) progressively produced more enzymes from day 3 to day 9. Isolates M1L001 (Col. gloeosporioides), M1L002 (Col. fructicola), M2L004 (Penicillium sp.), M1L003 (F. luffae), and M2L001 (T. lixii) had a decrease in enzyme production from day 3 to day 6, followed by an increase in production from day 6 to day 9. Isolates G1AL001 (C. boidinii), G2L001 (Clad. cladosporioides), and G2AL001 (G. candidum) peaked in enzyme production on day 6 and had the lowest enzyme production on day 3. Isolate M2L002 had a decrease in enzyme production from day 3 to day 9. Isolate G2AL001 produced 18.9 ± 0.34 IU/mL of endoglucanase enzyme which was only not significantly higher than endoglucanase enzyme produced by isolate G1AL002 on the 3rd day of incubation. On the 6th day of incubation, isolate G2AL001 produced 23.9 ± 0.43 IU/mL which was significantly higher than that produced by all other endophytic fungi. Isolate G1AL002 produced the highest endoglucanase enzymes of 23.7 ± 0.29 IU/mL on the 9th day of incubation, which was not significantly higher than that produced by isolate G2AL001 but was significantly higher than that produced by all other endophytic fungi (Table 4). Isolate G2L001 produced the lowest amount of endoglucanase enzyme on the 3rd day (7.38 ± 0.59 IU/mL), while isolate MIL003 produced the lowest amount of endoglucanase enzymes on the 6th day of incubation (5.25 ± 0.52 IU/mL) and on the 9th day of incubation (5.73 ± 0.16 IU/mL) (Table 4).
There was a significant difference (p < 0.05) in IU/mL for endoglucanase assays over time. The endoglucanase assay enzyme production was highest on day 3 (12.71 ± 0.74 IU/mL), while the enzyme production on days 6 (11.76 ± 1.03 IU/mL) and 9 (11.91 ± 1.07 IU/mL) showed no significant difference. G. candidum had the highest endoglucanase enzyme output over time (21.75 ± 0.75 IU/mL), while F. luffae had the lowest amounts of endoglucanases over time (6.55 ± 0.58 IU/mL). The interaction between day of incubation and the fungal isolate was significant (p < 0.05).
Exoglucanase enzyme production
All fungal isolates peaked in enzyme production for exoglucanase activity on day 3. Isolates G1AL001 (C. boidinii), G2L001 (Clad. cladosporioides), M1L001 (Col. gloeosporioides), M1L002 (Col. fructicola), M2L001 (T. lixii), M2L002 (Penicillium sp.), and M2L004 (Penicillium sp.) had a decline in enzyme production from day 3 to day 9. Isolates MIL003 (F. luffae), G2AL001 (G. candidum), and G1AL002 (C. stellimalicola) had a decrease in enzyme production from day 3 to day 6 and a slight increase in enzyme production from day 6 to day 9. Isolates G1AL002 and G2AL001 produced the highest amounts of exoglucanase enzymes that were not significantly different but were significantly higher than those produced by all the isolates on the 6th and 9th days of incubation. Isolates M1L001 (34.8 ± 0.36 IU/mL) and G1AL001 (32.8 ± 0.4 IU/mL) produced the highest amounts of exoglucanase enzymes that were not significantly different but were significantly higher than the other isolates on the 3rd day of incubation. The lowest amounts of exoglucanase enzymes were produced by the isolate G2L001 (12.7 ± 0.33 IU/mL) on the 3rd day of incubation, isolate MIL003 (6.62 ± 0.21 IU/mL) on the 6th day of incubation, and isolate M2L002 (4.82 ± 0.41 IU/mL) on the 9th day of incubation (Table 5).
For exoglucanases, there was a significant difference in IU/mL over time for days 3, 6, and 9 (p < 0.05). Day 3 had the highest amount of exoglucanase enzyme production (26.38 ± 1.13 IU/mL), day 6 had 13.57 ± 1.44 IU/mL, and day 9 had the lowest (10.35 ± 1.69 IU/mL). G. candidum (28.99 ± 0.20 IU/mL) and C. stellimalicola (28.83 ± 0.30 IU/mL) produced the highest amounts of exoglucanases over time, while Clad. cladosporioides produced the lowest amounts of exoglucanases over time (9.02 ± 0.98 IU/mL). The interaction between day of incubation and the fungal isolate was significant (p < 0.05).
Discussion
The choice of the leaves and maize cob as agrowaste medium
The leaves were chosen for endophytic isolation because they had fewer barriers to infection, lacking the outer bark, cuticle wax, and lignin layers compared to the other parts of the plant such as the stem and were thus prone to higher numbers of endophytic species, as observed by14. Bungtongdee et al.19 also obtained similar results showing that leaves have higher numbers of endophytic isolates. In comparing the abundance of endophytes in the leaves and stems of A. indica, Al-Daghari et al.20 found that the leaves had greater fungal taxa than the stems. The exposure of the leaves of medicinal plants A. Indica and A. secundiflora to external environment and infections meant that the endophytes within their leaves were greatly adapted to these external stressors. According to Chaudhary et al.21, endophytes have been shown to help plants cope with biotic stress. They also help in remediating abiotic stress22. Over time, the development of biotechnology has given rise to the use of enzymes for commercial reasons. The high cost of commercial enzymes has turned the focus of researchers to microbial enzymes23. Moreover, the process of harnessing the microbial enzymes is costly, but the cost can be cut by using innovative techniques such as employing agrowaste medium as raw material for the necessary fermentation processes15. In addition to maize cob being easily and ubiquitously available at low cost as agrowaste media, it is also has the highest amount of cellulose based on dry weight, at 61.2% w/w, as observed by16, which makes it the most preferable substrate for quantifying endophytic cellulase enzymes.
Fungal endophytic isolates of A. indica and A. secundiflora
The endophytes isolated are part of a wider endophytic community found in non-medicinal and medicinal plants. Different species of fungal isolates were isolated from A. secundiflora and A. indica. In concurrence, Amirita et al.24 report that a single plant or plant part can contain different endophytic isolates. Some factors determine the composition of communities of endophytes in plants. They include environmental factors, specificity of the organism towards the host, and composition of chemicals within the plants25,26. These factors could explain the occurrence of endophyte species in young or mature plants. As stated by27 diversity and colonization potential of endophytes in Coccoloba cerefeira depend on the amount of water in the leaves and the ratio of the polyphenol to the fresh or dry weight. These factors may contribute to the occurrence of one species in either young or mature plants or both27. The variation in the composition of microbial endophytes has been attributed to the level of chlorophyll present in the leaves27. The leaves of mature plants show wider variations in species of fungi compared to the leaves of young plants. Mature plants have an extended period to interact with fungi relative to young plants. The increased number of endophytes in mature plants is associated with their excellent nutrient potential due to higher rates of photosynthesis28.
Some species of fungi were observed in young plants but not in mature plants.28 reasoned that the changes that plants undergo as they grow can interfere with the innate environment of leaves and may favour different endophytic species over others as they mature. More species were isolated in samples from Kitui County compared to Kiambu County. There are few studies on the impact of the environment on the diversity of endophytic microbes. However, the relationship between the ecosystem and the plants significantly determines diversity29. The geographical area of study and ecology affect the endophytic communities in plants30. The interaction between the plants and the environment potentially leads to the variation of the endophytic community in Kitui and Kiambu Counties. Most endophytic organisms are transmitted horizontally; thus, the location and environment significantly determine the diversity of endophytic fungal species31.
Khalil et al.4 demonstrated that medicinal plants produce endophytes with the capability to produce cellulases, with the majority of the isolates being Penicillium sp. Similarly, the results of this study show that Penicillium sp. is highly abundant in the mature leaves of the A. indica (83.33%) and A. secundiflora (66.67%). Another study by Qureshi et al.32 showed that Penicillium sp. had the third highest colonization frequency (28.00%) out of eleven endophytic isolates from A. indica. Qureshi et al.32 isolated Clad. cladosporioides with a colonization frequency (CF) of 15.78% from the leaves of A. indica, which was similar to the 16.67% CF of Clad. cladosporioides in A. indica leaves. Qureshi et al.32 isolated Colletotrichum sp. from the leaves of A. indica (CF 13.33%), which concurred with the results obtained for Col. fructicola (CF 14.29%) in A. indica leaves. There are very limited studies on the endophytic communities of A. secundiflora, but the common endophytes from other Aloe sp. similar to those obtained in this study comprise of endophytes of Penicillium sp., Trichoderma sp., and Col. gloeosporioides33,34,35. T. lixii (CF 50.00%) is the second most dominant species in mature plant leaves of A. secundiflora in Kitui County. The results differ with those of Kiambu County, where G. candidum (CF 83.33%) is dominant in the mature plants of A. secundiflora. However, the mature leaves of A. secundiflora in Kitui County had more species that those in Kiambu County with only one species. F. luffae (CF 42.86%) is dominant in the young plants of A. secundiflora. C. boidinii (CF 85.71%) is the dominant species in the leaves of young A. secundiflora plants. In Kiambu County the dominant species of mature A. indica are C. stellimalicola (CF 50.00%) and Clad. cladosporioides. Wu et al.29 reported that the ecosystem significantly affects diversity in plant species. Furthermore, Chauhan et al.30 state that endophytic communities are affected significantly by ecology and geography. These variations in species may be the result of the ecological and geographical differences in Kitui and Kiambu counties.
Isolates of genera Candida, Trichoderma, Cladosporioides, and Colletotrichum belong to the phylum Ascomycota36,37,38,39. The isolates C. boidinii and T. lixii showed similarities in colony appearance, including filamentous, fluffy, white, aerial mycelium, circular and septate. Katoch et al.40 observed that isolates of T. lixii had septate hyphae, aerial mycelium, and white, filamentous colonies in concurrence with the obtained results. Isolates Col. gloeosporioides and Col. fructicola were smooth, circular, filamentous, aseptate, had aerial mycelia, and belonged to Genus Colletotrichum sp. The observations concur with morphological traits observed in Col. gloeosporioides and Col. fructicola38,41. C. stellimalicola is filamentous yeast, in agreement with Dupont et al.42. The morphological trait was shared by the isolates identified as C. stellimalicola and Clad. cladosporioides.36 observed varying degrees of septation in Genus Cladosporium depending on external factors. Fungal isolates identified as G. candidum and Penicillium sp. are filamentous fungi. According to Wang et al.43, the colony colour of Penicillium sp. varies depending on factors such as growth media. However, the colonies remain filamentous, with circular, entire margins.15 reported that the fungus G. candidum is filamentous, similar to the results obtained. The observations indicate that isolates of clusters B and C share morphological features. These include smooth margins, filamentous growth, and circular and septate colonies. F. luffae and C. stellimalicola (accession number ON077145) produced flat, spreading, and dense mycelia. Wang et al.44 studied Fusarium sp. complex and isolated F. luffae. The cultures were white, with dense mycelia, flat, spreading, and fast-growing. The results concur with the findings of the study.
Comparison of the amount of enzymes produced by the endophytic isolates
G. candidum and C. stellimalicola had the highest yields of endoglucanases over time. The production of exoglucanase enzymes by G. candidum and C. stellimalicola were also relatively high. The findings concur with studies showing G. candidum has high cellulase activity and produces exoglucanases45,46). However, Borisova et al.45 observed low endoglucanase production of G. candidum (1.86 IU/mL) in contrast to the high endoglucanase production of this study, with an overall production of 21.75 IU/mL. These differences may be linked to the differences in procedures for enzyme assay where Borisova et al.45 targeted 3–5% conversion rates, at 37 °C and pH 5.0, and reading the spectrophotometer at 404 nm. In this study, the pH is 4.8 and the reading is done at 540 nm. Subjecting the fungi to the same measurements of substrate, inoculum, pH and temperature, allows for an accurate measure of the capability of each endophytic fungi in producing cellulases. Even with the procedural differences, Borisova et al.45 acknowledge that G. candidum produced high amounts of cellulases and has huge potential in industrial application. Candida sp. evidently demonstrated their ability to produce highly active enzymes of degrading substrates such as cellulose and amylose47,48. According to Adelabu et al.47, Candida sp. possessed very cellulolytic activity with the highest day 3 cellulase production of 174.76 IU/mL by Candida tropicalis. Similarly, Candida stellimalicola had the highest production of total cellulases on day 3 at 15.9 IU/mL. The levels of cellulase activity vary among species even in the same Genus as observed in the study by Adelabu et al.47.
C. stellimalicola makes a good candidate for use in industries that employ cellulolytic enzymes46. Col. fructicola exhibited high exoglucanase production on day 3 and the most increased production of endoglucanases on the 9th day. Similarly, Colletotrichum sp. has been shown to secrete cellulases, among other enzymes1. Venkatesagowda et al.1 showed that Col. gloeosporioides had high endoglucanase production on a plate by exhibiting a zone of clearance of over 8.0 mm. The results of this study indicate that Col. gloeosporioides does produce relatively high amounts of endoglucanases, 12.9 IU/mL, on day 3, which is 6.0 IU/mL lower than the G. candidum. Venkatesagowda et al.1 also show that isolates Col. gloeosporioides and Clad. cladosporioides produce similar amounts of endoglucanases at 8.0 mm. In concurrence, the Table 4 of this study shows no significant difference in endoglucanase production of these isolates on days 6 and 9.
The exoglucanases yield of Clad. cladosporioides on day 3 is relatively higher than endoglucanase enzymes. Vázquez-Montoya et al.2 demonstrated that Clad. cladosporioides did produce exoglucanases in avicel-rich media (205 IU/L) and endoglucanases in CMC media (606 IU/L). Even though the Clad. cladosporioides in this study had the lowest amount of exoglucanases over time among all the isolates (9.02 IU/mL), it was higher than the amount obtained by Vázquez-Montoya et al.2, who used Moringa oleifera straw as agrowaste media. It indicates that the maize cob substrate may be better at harnessing enzymes from microbes. Clad. cladosporioides showed relatively higher endoglucanases over time from day 3 to day 9 and decreased exoglucanases over time. Similarly,49 reported significantly less cellulase activity when the substrates used were avicel or filter paper compared to CMC.
C. boidinii has higher activity in avicel relative to CMC and total cellulase activity. The activity of the enzymes in all three assays is higher on the third day and decreases over time. This yeast has been shown to degrade methanol and is a potential source of enzymes in the biotechnology industry50. The cellulase enzyme activity of isolate T. lixii was highest on the third day and decreased over time. T. lixii has been isolated as an endophyte40. Similar to the results, Li et al.51 reported that Trichoderma sp. produced enzymes that degraded cellulose with the maximum secretion of enzymes detected within the first two days of the experiment and decreased over time. T. lixii has the potential to be used for industrial purposes, just like Trichoderma harzianum has been applied for biotechnological purposes for its ability to produce enzymes that degrade lignin and cellulose substrates52,53. M’barek et al.54 showed that Trichoderma sp. possessed high cellulose-degrading activity, with Trichoderma/Hypocrea lixii exhibiting the highest cellulolytic activity with a blue halo zone of 172 mm2. This study showed that T. lixii produces cellulase enzymes, although at a significantly lower amount than most of the isolates. In the study by54.
Trichoderma sp. has higher cellulolytic activity than Penicillium sp. (94 mm2). The results contrast to this study, where there are slight significant differences in cellulase production, with Penicillium sp. producing more cellulases. It can be a factor of using different media, differing methodologies, test conditions, and also different strains of the same Genus. According to Chaverri et al.55, T. lixii is part of the species belonging to the T. harzianum complex. Most studies have been directed toward the potential of Trichoderma sp. as a biocontrol organism for phytopathogenic microbes56,57. Li et al.58 detected significant activity of exoglucanases and endoglucanase in T. harzianum. Isolates M2L004 and M2L002 (Penicillium sp.) had optimal yields on day 3 for all assays and the lowest yields on day 9. In a study, endophytic strains of Penicillium sp. exhibited secretion of significant amounts of cellulases compared to other endophytic organisms59. M’barek et al.54 showed that Penicillium sp. have high cellulolytic activity. However the strains used in those studies, the raw materials and methodologies differ from those used in this study. Penicillium sp. in this study produce significantly higher amounts of cellulases than a few isolates; they still produce lower amounts of cellulases than most isolates.
These differences are visible in different studies as well. The amount of FPase produced in this study was higher than that produced in the study by Syed et al.8, at 1.2 IU/mL, but the amount of endoglucanases in the study by Syed et al.8 (19 IU/mL) is higher than that obtained in this study. Nonetheless, the Penicillium sp. isolated from medicinal plants produces significant amounts of cellulases that can be harnessed. Production of endoglucanases and exoglucanases was higher relative to the FPase in concurrence with the study by Syed et al.8, with FPase and endoglucanases. Panagiotou et al.38 demonstrated the ability of Fusarium sp. strains to produce exoglucanases and endoglucanases. Isolate F. luffae had a higher exoglucanase activity than the other assays. The lowest activity of the isolate was observed in FPase. The results concur with those of60, who report that Fusarium sp. secreted significantly higher quantities of endoglucanases (128 IU/g) than FPase (95.2 IU/g). Fusarium sp. is the prominent organism in producing cellulases in rice straw61.
Fungal endophytic isolates of A. secundiflora has more enzyme activity for total cellulase, endoglucanases, and exoglucanases. It could result from the fact that the fungal endophytes isolated from A. secundiflora comprise Candida species, which, according to studies by47,48, have been shown to produce high quantities of cellulases. Galactomyces candidum isolated from A. secundiflora has been shown to possess high cellulase activity45,46. Fungal endophytic isolates of A. indica had relatively lower enzyme activity. It could be attributed to substrate specificity in isolates such as F. luffae, which could produce more cellulases in substrates other than maize cobs. According to38, Fusarium sp. had better exoglucanase and endoglucanase activity than FPase activity. Isolate Clad. cladosporioides performed better for endoglucanases and exoglucanases, although relatively lower than other isolates. Vázquez-Montoya et al.2 demonstrated the selectivity of Clad. cladosporioides by showing a higher exoglucanase activity than endoglucanase activity.
There was a significant difference in enzyme units of cellulases assays over time. The enzyme outputs significantly differed on days 3, 6, and 9. It can be attributed to the growth rate of the endophyte and ability to utilize the substrate. Li et al.51 shows that some species of fungi peak in enzyme production on the first two days of growth and then decline in production over extended periods of time. An example is Fusarium sp. that produces significant cellulase amounts for CMC and FPase assays60. Similar activity was observed for Penicillium sp.8. According to Sunitha et al.62 internal factors of the host, including age and external factors, such as climate and geographical location or habitat determine the requirements of the host and endophyte that facilitate enzyme production.
Conclusions
The present study confirms that the endophytic communities of A. secundiflora and A. indica vary from one region to another, for example, the mature leaves of A. indica in Kitui County are dominated by Penicillium sp., while those in Kiambu County are dominated by Candida stellimalicola. The communities within the leaves of these medicinal plants are diverse. The present study found maize cob agrowaste media can be used to cultivate the production of cellulases successfully in fungal endophytic isolates of A. indica and A. secundiflora. The study concluded that the endophytes of A. indica and A. secundiflora can be harnessed and optimized to produce cellulase enzymes in large scale for commercial use, and especially isolates G. candidum and C. stellimalicola which yield significantly high amounts of total cellulases, endoglucanases and exoglucanases. In addition, enzyme production peaks occur at different time intervals for different isolates. While some of the isolated organisms may belong to potentially pathogenic genera, the fungal isolates selected, under controlled conditions, are typically not pathogenic. For large-scale production, we recommend using of GRAS (genetically recognised as safe) strains or genetically modified non-pathogenic strains. The solid-state fermentation process coupled with genetic improvements of strains and adherence to strict safety protocols are essential in large-scale production. These strategies would ensure that the production of cellulase can be safely and efficiently scaled for industrial applications. More research should be directed towards identifying novel endophytic sources of enzymes in medicinal plants and optimizing the production of biofuels in these endophytes. Further research is needed to evaluate and optimize the endophytes as sources for other groups of enzymes. It is possible to combine different agrowaste raw materials to develop the optimal media for cellulase production.
Materials and methods
Sampling
Samples were collected from Kanyonyoo in Kitui County (1°11′ 3ʺ S, 37° 49′11ʺ E) and Gachie in Kiambu County (1° 13′ 0ʺ S, 36° 47′ 0ʺ E). The samples were collected in August 2021 during the dry season. The permission for collection of the samples was granted by the owners of the sampling locations. The samples were identified and deposited in the Kenyatta University Herbarium with the help of staff in the Plant Sciences Department. The voucher specimen numbers provided for A. Secundiflora and A. indica and are PKM 001 and PKM 002 respectively. The mature A. secundiflora leaves sampled were over 30 cm in length, with a base leaf width of 10 cm and above. The young leaves were less than 30 cm long, with a leaf base width of less than 8 cm. The young leaves of A. indica sampled were purplish or reddish. The mature leaves were green, with dented edges, and between 4 and 8 cm in length. The leaves of A. secundiflora and A. indica were transported to Kenyatta University Microbiology Laboratories in labelled plastic bags and processed separately for each county within 24 h.
The leaves were washed with running tap water and allowed to air dry before being sterilized by immersing them in 70% ethanol for two minutes, followed by a minute in 3.5% sodium hypochlorite and rinsing five times using double distilled water. They were then dried using sterile blotting paper in a laminar flow cabinet. The leaves were cut aseptically using a sterile blade into 1 cm by 1 cm segments4. The segments prepared were inoculated on potato dextrose agar (PDA) containing streptomycin to inhibit bacterial contamination. They were incubated for five days at room temperature. The cut surfaces were placed in contact with the culture media to promote the growth of endophytes on the plate. The double-distilled water from the final rinsing was incubated on a control Petri dish on PDA to confirm that the surfaces of the leaves were sterile63;5. The emerging fungal hyphae were sub-cultured on fresh PDA and incubated for five days at room temperature. The isolates were differentiated based on morphology, colour, and characteristics of the culture64.
The colonization frequency by an endophyte was calculated using Eq. 1 below, as described by Vyawahare et al.5.
Macroscopic and microscopic morphological identification of fungal isolates
The fungal endophytic isolates were studied macroscopically by observing features of the colony, including shape, colour, size, and mycelia. Small portions of the mycelia of each fungus were then loaded onto slides and stained using Lacto phenol cotton blue and observed using a compound microscope for spores, as described by65.
DNA isolation
The mycelia of the fungal isolates were transferred to sterile microcentrifuge tubes containing 500 μL of sterile normal saline. The tubes were centrifuged to pellet the hyphae, and the normal saline was decanted. This process was repeated thrice to get rid of extracellular polymeric substances66. This was followed by cell lysis. Cell lysis buffer pH 8.0 containing (2% w/v CTAB, 1.4 M NaCl, 100 mM Tris–HCl pH 8.0, 20 mM EDTA pH 8.0, and 0.3% Mercaptoethanol) was added to the tubes 66. The samples were vortexed for 1 min and then incubated at 65 °C for one hour. They were then centrifuged for 5 min at 13,000 rpm. The liquid phase was transferred to sterile microcentrifuge tubes, and an equal volume of chloroform:isoamyl alcohol (24:1) was added 66. The mixture was gently mixed by inversion and incubated at − 20 °C for 30 min 66. Centrifuging was done at 13,000 rpm for 4 min, and the supernatant was transferred to a fresh tube. DNA was precipitated by adding 500 μL of chilled absolute ethanol to the supernatant, gently inverted, and left to for 1 h at room temperature 7. This mixture was centrifuged at 12,000 rpm for 4 min to pellet the DNA. The supernatant was discarded, and the pellet was washed with 110 μL of chilled 70% ethanol and centrifuged at 12,000 rpm for 2 min 66. After decanting the 70% ethanol, the pellet was air dried by inversion on sterile paper towels, followed by elution of the DNA using 50 mL TE buffer pH 8.066.
DNA amplification
The extracted DNA was amplified using ITS 1 (5’ – TCCGTAGGTGAACCTGCGG – 3’) and ITS 4 (5’ – TCCTCCGCTTATTGATATGC – 3’), using a 25 µL One Taq Quick-load 2X master mix with a standard buffer from New England BioLabs. The reaction mixture contained 1.25 µL of each primer (10 µM), 12.5 µL, 2X master mix with buffer, 9.0 µL PCR water, and 1.0 µL of isolate DNA. The PCR was set for amplification for 35 cycles66. Initial denaturation was set at 94 °C (2 min), followed by 35 cycles of denaturation at 94 °C (45 s), annealing at 52 °C (45 s), and extension at 72 °C (2 min)66. The final extension was set at 72 °C (5 min). The amplification was a modification of the amplification perimeters used by66.
DNA gel electrophoresis, sequencing, and identification of endophytic fungal isolates
After DNA amplification, the PCR products were subjected to gel electrophoresis. DNA loading buffer (3 µL) containing SYBR GREEN dye was mixed with 3 µL aliquots of the PCR product and pipetted into the 1% agarose gel wells in 0.5X Tris borate-EDTA (TBE) buffer and run at 80 V for 30 min and visualized with a UV trans-illuminator67, to determine the quality and quantity of the PCR products. The amplified fragments were sent to MACROGEN in the Netherlands, Europe for standard DNA sequencing using the sanger dideoxy method and identified with the aid of the National Centre for Biotechnology Information genome sequence database.
Solid state fermentation (SSF) for cellulase enzyme production
Preparation of the substrate
Cellulase enzymes were produced under SSF using maize cobs as substrates. To remove moisture, the maize cobs were dried for three days at room temperature. The maize cobs were then ground and sieved using a 2 mm sieve. The maize cob powder was used as a carbon source in the solid-state fermentation (SSF) process. According to Kanengoni et al.68, the carbon source composition of maize cob is primarily made up of cellulose (45–55%), hemicellulose (25–35%), and lignin (20–30%). These polysaccharides are the key substrates for cellulase enzyme production by the endophytic fungi. Furthermore, maize cob contains small quantities of lipids, proteins, and essential minerals that support growth and enzyme secretion in fungi. Five grams of the substrate were dispersed into 350 mL bottles, and 10 mL of the Bushnell-Haas solution (g/L) with constituents as described by69, (0.1 g of MgSO4.7H2O, 0.5 g of Na2HPO4, 0.5 g of KH2PO4, 0.5 g of NH4NO3, 0.05 g FeCl3.6H2O, and 0.02 g CaCl2) was dispersed into each bottle. The bottles were plugged with cotton wool and sterilized at 121 °C, 15 psi, for 20 min. The bottles were cooled to room temperature and inoculated with 2.5 mL of inoculum for each fungus69.
Preparation of the inoculum and fermentation
The endophytic fungal isolates were inoculated in PDA for 5 days. The inoculum was adjusted to 1 × 106 CFU, and 2.5 mL were dispersed into 350 mL bottles with maize cobs prepared as in Sect. "Preparation of the substrate".23 Filamentous fungi cultured on PDA for 5 days were scrapped off the media and blended in 5% sucrose solution for 30 s, and 2.5 mL of the blended inoculum was used to inoculate 350 mL bottles prepared as in Sect. "Preparation of the substrate"23. The inoculum was thoroughly mixed with the substrate, and the bottles were incubated at room temperature. Samples were taken in triplicates at 3, 6, and 9 days after inoculation for enzyme extraction for each fungus23.
Enzyme extraction
The cellulase enzymes were extracted by adding 50 mL of 50 mM citrate buffer, pH 4.8, to each bottle and left to stand for 2 h at room temperature. The contents of the bottles were filtered and strained using clean cheesecloth. The filtrate was collected and centrifuged at 10,000× g for 10 min23. The supernatant containing the enzymes was collected and stored at − 20 °C awaiting bioassays23.
Enzyme assays
The crude enzyme extracts were assayed for the total cellulase (FPase), endoglucanase, and exoglucanase activities.
Total cellulase activity (FPase)
Filter paper assay was adopted to determine the total cellulase enzyme activity according to70. Strips of Whatman Filter Paper No. 1 measuring 6 cm by 1 cm were cut, folded, and placed into 2.0 mL labelled microcentrifuge tubes. Five hundred microliters of 50 mM citrate buffer pH 4.8 were added to the tubes, and then five hundred microliters of the harvested enzymes (the supernatant obtained in Sect. "Enzyme extraction") were added. The control tubes had five hundred microliters of citrate buffer added instead of the enzyme extract. For the standards, one gram of glucose was dissolved in citrate buffer pH 4.8 in a 100 mL volumetric flask. Different concentrations of glucose were prepared by serial dilution in thirteen microcentrifuge tubes. A modified version of the method by70 was used to determine glucose concentration range. The range of glucose concentration was from 0 to 2.4 mg/mL (0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8, 2.0, 2.2, and 2.4). The tubes containing the glucose standards and crude enzymes were incubated at 50 °C in a water bath for 1 h, and the amount of reducing sugars produced was determined using the DNS reagent (10 g of 3,5-dinitrosalycyclic acid, 0.5 g sodium sulfite, and 10 g NaOH) as described by70. Seven hundred microliters of DNS were added to all the enzyme bioassay activity tubes and standard tubes to stop the reaction14. The tubes were then boiled for 5 min and cooled to room temperature. Three hundred microliters of 400 g/L potassium sodium tartrate (PTS) were added to the tubes containing enzymes, glucose standards, and negative control. The absorbance of the reducing sugars produced was determined at 540 nm using a JENWAY 6300 spectrophotometer, quantified using a glucose standard curve, and converted to enzyme units using the Eq. 2 below, as described by Rahnama et al.23:
where f refers to final absorbance, c refers to the intercept, df stands for dilution factor, m refers to the slope from the standard curve, Sv stands for sample volume, and T is time expressed in minutes23.
Endoglucanases activity
Endoglucanase activity was determined using 1% Carboxymethylcellulose (CMC) prepared in 50 mM citrate buffer pH 4.8 substrate as described by Shuangqi et al.71. Five hundred microliters of the CMC solution were transferred into 2.0 mL microcentrifuge tubes, and five hundred microliters of the extracted enzyme were added, while 500 µL of buffer were added to the control tubes71. The glucose standards were prepared as described in Sect. "Total cellulase activity (FPase)". The tubes were incubated in a 50 °C water bath for 30 min. The amount of reducing sugars produced was quantified as described for FPase71.
Exoglucanases activity
Exoglucanase activity was determined using 1.25% microcrystalline cellulose (avicel) prepared in 100 mM sodium acetate buffer, pH 4.8. Five hundred (500) µL of avicel solution and 500 µL of the enzyme extract were added to 2 mL microcentrifuge tubes71. The control tubes contained 500 µL of the buffer. The tubes were incubated in a 50 °C water bath for 2 h71. The reducing sugars produced were quantified as described for FPase.
Data analysis
Consensus for sequences generated using ITS1 and ITS4 primers were created using BioEdit Software 7.1 after base calling using Chromas Lite 2.0 Software. A BLAST (Basic Local Alignment Search Tool) search of the consensus sequence on the National Centre for Biotechnology Information database was used for molecular identification of the isolates. The isolate consensus and identity sequences were aligned using the MUSCLE algorithm in MEGA X software72. The phylogenetic tree was developed using the neighbour-joining method, and evolutionary distances were determined using the maximum composite likelihood of the MEGA X statistical tool. The R statistical tool was used to analyse enzyme bioassay data. Two-way ANOVA was used to determine any significant difference in enzyme production on the third, sixth, and ninth days of the study using Tukey’s HSD. Differences were considered significant if the p-value ≤ 0.0567.
Data availability
Sequence data that support the findings of this study have been deposited in the DNA Data Bank of Japan (DDBJ) with the link https://ddbj.nig.ac.jp/arsa/search?lang=en&cond=quick_search&sortTarget=score&sortOrder=desc&displayFields=PrimaryAccessionNumber%2CDefinition%2CSequenceLength%2CMolecularType%2COrganism&query=mwendwa&operator=AND&filterQuery=Division%3APLN.
References
Venkatesagowda, B., Ponugupaty, E., Barbosa, A. M. & Dekker, R. F. Diversity of plant oil seed-associated fungi isolated from seven oil-bearing seeds and their potential for the production of lipolytic enzymes. World J. Microbiol. Biotech. 28(1), 71–80 (2012).
Vázquez-Montoya, E. L., Castro-Ochoa, L. D., Maldonado-Mendoza, I. E., Luna-Suárez, S. & Castro-Martínez, C. Moringa straw as cellulase production inducer and cellulolytic fungi source. Revista Argentina de Microbiologia. 52(1), 4–12. https://doi.org/10.1016/j.ram.2019.02.005 (2020).
Jia, M. et al. A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front. Microbiol. https://doi.org/10.3389/fmicb.2016.00906 (2016).
Khalil, A. M. A. et al. Isolation and characterization of fungal endophytes isolated from medicinal plant ephedra pachyclada as plant growth-promoting. Biomolecules 11, 140 (2021).
Vyawahare, M. A., Jagtap, P. R., Gangurde, A. B., Kukreja, G. P. & Mane, R. S. Isolation of endophytes from roots of Aloe vera. Int. J. Sci. Adv. Res. Technol. 5(1), 273–276 (2019).
Patil, M., Pagare, J., Patil, S. & Sidhu, A. Extracellular enzymatic activities of endophytic fungi isolated from various medicinal plants. Int. J. Curr. Microbiol. Appl. Sci. 4(3), 1035–1042 (2015).
Zewdinesh, D. Z., Bizuayehu, T. A. & Tesfaye, D. B. Optimizing DNA isolation protocol for rosemary (Rosemarinus officinalis L) accessions. Afr. J. Biotechnol. 18, 895–900 (2019).
Syed, S., Riyaz-Ul-Hassan, S. & Johri, S. A novel cellulase from an endophyte, Penicillium sp. NFCCI 2862. Am. J. Microbiol. Res. 1(4), 84–91 (2013).
Sunitha HV, Hedge SC. Evaluation and optimization of pectinase production by endophytic fungi Talaromyces sp. isolated from Calophyllum inophyllum. International Journal of Engineering Technology Science and Research. 2017. p. 610. http://ijetsr.com/images/short_pdf/1511590722_610-620-ieteb331_ijetsr.pdf
Kumar, A., Kurup, C., Snishamol, C. & Prabhu, N. Role of cellulases in food, feed, and beverage industries. Energy, Environ, Sustainabil. https://doi.org/10.1007/978-981-13-3263-0_17 (2018).
Liu, X. & Chandrakant, K. Microbial enzymes of use in industry 405–444 (Elsevier eBooks, 2023). https://doi.org/10.1016/b978-0-443-19059-9.00021-9.
Sopalun, K., Laosripaiboon, W., Wachirachaikarn, A. & Iamtham, S. Biological potential and chemical composition of bioactive compounds from endophytic fungi associated with Thai mangrove plants. South Afr. J. Botany. 141, 66–76. https://doi.org/10.1016/j.sajb.2021.04.031 (2021).
Ejaz, U., Sohail, M. & Ghanemi, A. Cellulases: from bioactivity to a variety of industrial applications. Biomimetics. 6(3), 44. https://doi.org/10.3390/biomimetics6030044 (2021).
Sopalun, K. & Iamtham, S. Isolation and screening of extracellular enzymatic activity of endophytic fungi isolated from Thai orchids. South Afr. J. Botany https://doi.org/10.1016/j.sajb.2020.02.005 (2020).
Song, S. et al. First report of a new potato disease caused by Galactomyces candidum F12 in China. J. Integr. Agricult. 19(10), 2470–2476. https://doi.org/10.1016/S2095-3119(20)63257-9 (2020).
El-Tayeb, T. S., Abdelhafez, A. A., Ali, S. H. & Ramadan, E. M. Effect of acid hydrolysis and fungal biotreatment on agro-industrial wastes for obtainment of free sugars for bioethanol production. Brazil. J. Microbiol. 43(4), 1523–1535. https://doi.org/10.1590/s1517-83822012000400037 (2012).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35(6), 1547–1549. https://doi.org/10.1093/molbev/msy096 (2018).
Fernández-Escobar, M., Millán, J., Chirife, A. D., Ortega-Mora, L. M. & Calero-Bernal, R. Molecular survey for cyst-forming coccidia (Toxoplasma gondii, Neospora caninum, Sarcocystis spp.) in Mediterranean periurban micromammals. Parasitol. Res. 119(8), 2679–2686. https://doi.org/10.1007/s00436-020-06777-2 (2020).
Bungtongdee, N., Sopalun, K., Laosripaiboon, W. & Iamtham, S. The chemical composition, antifungal, antioxidant and antimutagenicity properties of bioactive compounds from fungal endophytes associated with Thai orchids. J. Phytopathol. 167(1), 56–64. https://doi.org/10.1111/jph.12773 (2018).
Al-Daghari N, Maharachchikumbura S, Al-Moqbali D, Al-Saady N, Al-Sadi AM. Fungal diversity in leaves and stems of neem (Azadirachta indica). International Journal of Scientific and Technology Research. 2020;9:793–8. https://aarinena.org/wp-content/uploads/2021/03/Fungal-Diversity-In-Leaves-And-Stems-Of-Neem-azadirachta-Indica.pdf
Chaudhary, P., Agri, U., Chaudhary, A., Kumar, A. & Kumar, G. Endophytes and their potential in biotic stress management and crop production. Front. Microbiol. https://doi.org/10.3389/fmicb.2022.933017 (2022).
Khare, E., Mishra, J. & Arora, N. K. Multifaceted interactions between endophytes and plant: developments and prospects. Front. Microbiol. https://doi.org/10.3389/fmicb.2018.02732 (2018).
Gad, A. M., Suleiman, W. B., El-Sheikh, H. H., El-mezayen, H. A. & Beltagy, E. A. Characterization of cellulase from geotrichum candidum strain gad1 approaching bioethanol production. Arab. J. Sci. Eng. 47, 6837–6850 (2022).
Amirita, A., Sindhu, P., Swetha, J., Vasanthi, N. S. & Kannan, K. P. Enumeration of endophytic fungi from medicinal plants and screening of extracellular enzymes. World J. Sci. Technol. 2(2), 13–19 (2012).
Harrison, J. G. & Griffin, E. A. The diversity and distribution of endophytes across biomes, plant phylogeny and host tissues: how far have we come and where do we go from here?. Environ. Microbiol. 22, 2107–2123 (2020).
Bertonha, L. C. et al. Screening of Fusarium sp. for xylan and cellulose hydrolyzing enzymes and perspectives for the saccharification of delignified sugarcane bagasse. Biocatal Agricult Biotechnol 16, 385–389 (2018).
Sanchez-Azofeifa, A., Oki, Y., Fernandes, G. W., Ball, R. A. & Gamon, J. Relationships between endophyte diversity and leaf optical properties. Trees 26(2), 291–299 (2012).
Fan, Y., Gao, L., Chang, P. & Li, Z. Endophytic fungal community in grape is correlated to foliar age and domestication. Ann. Microbiol. https://doi.org/10.1186/s13213-020-01574-9 (2020).
Wu, W. et al. Beneficial relationships between endophytic bacteria and medicinal plants. Front. Plant Sci. https://doi.org/10.3389/fpls.2021.646146 (2021).
Chauhan, N. M., Gutama, A. D. & Aysa, A. Endophytic fungal diversity isolated from different agro-ecosystem of Enset (Ensete ventericosum) in Gedeo zone, SNNPRS Ethiopia. BioMed Central Microbiol. 19(1), 1–10 (2019).
Christian, N., Sullivan, C., Visser, N. D. & Clay, K. Plant host and geographic location drive endophyte community composition in the face of perturbation. Microbial Ecol. 72(3), 621–632 (2016).
Rahnama, N., Foo, H. L., Rahman, A., Ariff, A. & Shah, M. Saccharification of rice straw by cellulase from a local Trichoderma harzianum SNRS3 for biobutanol production. BioMed Central Biotechnol. https://doi.org/10.1186/s12896-014-0103-y (2014).
Al-Rashdi, F. K. H. et al. Endophytic fungi from the medicinal plant Aloe dhufarensis Lavranos exhibit antagonistic potential against phytopathogenic fungi. S. Afr. J. Bot. 147, 1078–1085. https://doi.org/10.1016/j.sajb.2020.05.022 (2022).
Chowdhary, K. & Sharma, S. Plant growth promotion and biocontrol potential of fungal endophytes in the inflorescence of Aloe vera L. Proceed. Nat. Acad. Sci., India Sect. B: Biol. Sci. 90(5), 1045–1055. https://doi.org/10.1007/s40011-020-01173-3 (2020).
Taher, M. A. et al. Antimicrobial activities of endophytic fungi residing in Aloe vera against diabetic wound pathogens. Malays. J. Microbiol. https://doi.org/10.21161/mjm.200824 (2020).
Bensch, K., Braun, U., Groenewald, J. Z. & Crous, P. W. The genus Cladosporium. Stud. Mycol. 72(1), 1–401. https://doi.org/10.3114/sim0003 (2012).
Koundal S, Cojandaraj L. Candida species - morphology, medical aspects and pathogenic spectrum. European Journal of Molecular and Clinical Medicine. 2020;07(07):4015–21. https://ejmcm.com/article_5257_6e1e040ad44e2d9a96c9258312c643a2.pdf
Papade V, Potdukhe S, Navsupe D, Taral A. Morphological characters of Colletotrichum gloeosporioides from various hosts. International Journal of Chemical Studies. 2019;7(4):75–8. https://www.chemijournal.com/archives/2019/vol7issue4/PartB/7-3-731-234.pdf
Venice, F. et al. Genome sequence of trichoderma lixii MUT3171, a promising strain for mycoremediation of PAH-contaminated sites. Microorganisms 8, 1258 (2020).
Katoch, M., Singh, D., Kapoor, K. K. & Vishwakarma, R. A. Trichoderma lixii (IIIM-B4), an endophyte of Bacopa monnieri L producing peptaibols. BioMed Central Microbiol. https://doi.org/10.1186/s12866-019-1477-8 (2019).
Yang, Z. et al. First report of colletotrichum fructicola causing anthracnose on pouteria campechiana in China. Plant Dis. 105, 708–708 (2021).
Dupont, D. et al. Donor derived candida stellimalicola in a clinical specimen: Preservation fluid contamination during pancreas procurement. Mycopathologia 183, 573–577 (2017).
Wang, X. C., Chen, K., Zeng, Z. Q. & Zhuang, W. Y. Phylogeny and morphological analyses of Penicillium section Sclerotiora (Fungi) lead to the discovery of five new species. Sci. Rep. https://doi.org/10.1038/s41598-017-08697-1 (2017).
Wang, M. M., Chen, Q., Diao, Y. Z., Duan, W. J. & Cai, L. Fusarium incarnatum-equiseti complex from China. Persoonia – Mol. Phylogeny Evol. Fungi 43(1), 70–89. https://doi.org/10.3767/persoonia.2019.43.03 (2019).
Borisova, A. S. et al. Sequencing, biochemical characterization, crystal structure and molecular dynamics of cellobiohydrolase Cel7A from Geotrichum candidum 3C. Federat Eur. Biochem. Soci. J. 282(23), 4515–4537 (2015).
Ladevèze, S. et al. The yeast Geotrichum candidum encodes functional lytic polysaccharide monooxygenases. Biotechnol. Biofuels https://doi.org/10.1186/s13068-017-0903-0 (2017).
Adelabu, B. A., Kareem, S. O., Adeogun, A. I. & Ademolu, K. O. Direct bioconversion of sorghum straw to ethanol in a single-step process by Candida species. Jordan J. Biol. Sci. 11, 57–63 (2018).
Adelabu, B., Kareem, S., Adeogun, A. & Wakil, S. M. Optimization of cellulase enzyme from sorghum straw by yeasts isolated from plant feeding-termite Zonocerus variegatus: https://www.semanticscholar.org/paper/Optimization-of-cellulase-enzyme-from-sorghum-straw-Adelabu-Kareem/4a9ec02eaf4fbba7bfd9f4d7a0cb4d83e7d4bd2f (2019).
Mushimiyimana, I. A statistical strategy for the production of cellulase, xylanase and α-amylase by cladosporium cladosporioides. Fungal Territory 2, 16 (2019).
Oda, S., Yurimoto, H., Nitta, N., Sasano, Y. & Sakai, Y. Molecular characterization of hap complex components responsible for methanol-inducible gene expression in the methylotrophic yeast Candida boidinii. Eukaryot. cell. 14(3), 278–285 (2015).
Li, J. X. et al. Rapid production of lignocellulolytic enzymes by Trichoderma harzianum LZ117 isolated from Tibet for biomass degradation. Biores. Technol. 292, 122063 (2019).
Benoliel, B., Torres, F. A. G. & Moraes, L. M. P. A novel promising Trichoderma harzianum strain for the production of a cellulolytic complex using sugarcane bagasse in natura. SpringerPlus. 2(1), 1–7 (2013).
Filho, F., Horta, M. A. C., Beloti, L. L., Santos, D. & Souza, A. P. Carbohydrate-active enzymes in Trichoderma harzianum: A bioinformatic analysis bioprospecting for key enzymes for the biofuels industry. BioMed Central Gen. 18(1), 1–12. https://doi.org/10.1186/s12864-017-4181-9 (2017).
M’barek, H. N. et al. Isolation, screening and identification of ligno-cellulolytic fungi from northern central Morocco. BASE https://doi.org/10.25518/1780-4507.18182 (2019).
Chaverri, P. et al. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 107(3), 558–590. https://doi.org/10.3852/14-147 (2015).
El-Debaiky, S. A. Antagonistic studies and hyphal interactions of the new antagonist Aspergillus piperis against some phytopathogenic fungi in vitro in comparison with Trichoderma harzianum. Microbial. Pathogen. 113, 135–143 (2017).
Ghazanfar, M. U., Raza, M., Raza, W. & Qamar, M. I. 2018 Trichoderma as potential biocontrol agent, its exploitation in agriculture: A review. Plant Protect. 2
Li, J.-X. et al. 2020 Diversity of Cellulase-Producing Filamentous Fungi From Tibet and Transcriptomic Analysis of a Superior Cellulase Producer Trichoderma harzianum LZ117. Front. Microbiol, 11
Baldrian, P. et al. Production of extracellular enzymes and degradation of biopolymers by saprotrophic microfungi from the upper layers of forest soil. Plant Soil. 338(1), 111–125 (2011).
Qureshi, A. H., Ali, S. & Pandey, P. A. K. Isolation and identification of endophytic fungi inhabiting Azadirachta indica A juss from different regions of Jabalpur (MP) India. Res. J. Life Sci., Bioinform., Pharmaceut. Chem. Sci. https://doi.org/10.26479/2019.0503.22 (2019).
Indira, D., Sharmila, D., Balasubramanian, P., Thirugnanam, A. & Jayabalan, R. Utilization of sea water based media for the production and characterization of cellulase by Fusarium subglutinans MTCC 11891. Biocatal. Agricult. Biotechnol. 7, 187–192 (2016).
Sunitha, V. H., Devi, D. N. & Srinivas, C. Extracellular enzymatic activity of endophytic fungal strains isolated from medicinal plants. World J. Agricult. Sci. 9(1), 01–09 (2013).
Sharaf, M. H., Abdelaziz, A. M., Kalaba, M. H., Radwan, A. A. & Hashem, A. H. Antimicrobial, antioxidant, cytotoxic activities and phytochemical analysis of fungal endophytes isolated from ocimum basilicum. Appl. Biochem. Biotechnol. 194, 1271–1289 (2021).
Tibpromma, S. et al. Identification of endophytic fungi from leaves of Pandanaceae based on their morphotypes and DNA sequence data from southern Thailand. MycoKeys 33, 25–67 (2018).
Gaddeyya G, Niharika P, Bharathi P, Kumar R. Isolation and identification of soil mycoflora in different crop fields at Salur Mandal. Advances in Applied Science Research. 2012;3(4):2020–6. https://www.primescholars.com/articles/isolation-and-identification-of-soil-mycoflora-in-different-crop-fieldsat-salur-mandal.pdf
Kouakou, J.-L., Gonedelé-Bi, S., Assamoi, J.-B. & Assanvo N’Guetta, S.-P. Optimization of the Cetyltrimethylammonium bromide (CTAB) DNA extraction protocol using forest elephant dung samples. MethodsX 9, 101867 (2022).
Muthini M. Diversity, symbiotic efficiency and effect of water hyacinth compost on population of rhizobia nodulating Phaseolus vulgaris in Lake Victoria basin [PhD/MSc Thesis]. https://ir-library.ku.ac.ke/bitstream/handle/123456789/14992/Diversity,%20symbiotic%20efficiency%20and%20effect.pdf?sequence=1. 2016.
Kanengoni, A. T., Chimonyo, M., Ndimba, B. K. & Dzama, K. Potential of using maize cobs in pig diets — a review. Asian-Australas. J. Animal Sci. 28(12), 1669–1679. https://doi.org/10.5713/ajas.15.0053 (2015).
Hamza UD, Mohammed IA, Sale A. Potentials of bacterial isolates in bioremediation of petroleum refinery wastewater. Journal of Applied Phytotechnology in Environmental Sanitation. 2012;1(3):131–8. https://www.researchgate.net/publication/266348646_Potentials_of_bacterial_isolates_in_bioremediation_of_petroleum_refinery_wastewater
Farinas, C. S., Damaso, T. & Couri, S. Cellulase Activity Assays: A Practical Guide 69–98 (Bentham Science Publishers eBooks, 2013). https://doi.org/10.2174/9781608053001113010008.
Grata, K. Determining cellulolytic activity of microorganisms. Chem-Didactics-Ecol-Metrol 25, 133–143 (2020).
Keklik, G. Understanding evolutionary relationships and analysis methods through mega software. Int. J. New Horizons Sci. 1, 83–90 (2023).
Acknowledgements
We thank Lawrence Alaro, Morris Muthini, Mutai Mourine, the Biochemistry, Microbiology and Biotechnology staff members, and the staff in the Plant Sciences department at Kenyatta University for the exceptional technical assistance.
Funding
This work was self-sponsored and did not receive any grants.
Author information
Authors and Affiliations
Contributions
PKM: drafted the proposal, collected data, carried out the experiments, analysis, interpretation, and prepared the manuscript draft. AK and JMM: reviewed the proposal, analysis and interpretation, preparation and review of the manuscript draft. GO: enzyme assays and data analysis. All authors reviewed and approved the final draft version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mwendwa, P.K., Omwenga, G.I., Maingi, J.M. et al. Evaluation of cellulase production by endophytic fungi isolated from young and mature leaves of medicinal plants using maize cob substrate. Sci Rep 15, 17842 (2025). https://doi.org/10.1038/s41598-025-94864-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94864-8