Abstract
This study retrospectively explored the characteristics of 5838 children with enterovirus infection in our hospital from 2018 to 2023. In addition, children with enterovirus (EV: EV typing was performed using RT-PCR) infection exhibiting clinical manifestations of viral encephalitis were investigated. Pharyngeal swabs or fecal samples from outpatients and inpatients from our hospital were collected from 2018 to 2023 and were subjected to EV nucleic acid detection using real-time fluorescence quantitative PCR. Furthermore, cerebrospinal fluid EV nucleic acid detection was performed for children with clinical manifestations of viral encephalitis. Descriptive epidemiological methods were used to analyze the age, sex, and etiology of EV infection cases. Statistical analyses were performed with SPSS 20.0. The statistical data were expressed as percentages, and the χ2 test was used for statistical analysis. A total of 9676 children were included in this study, and 5838 (60.33%) showed positive EV nucleic acid test results. These included 1909 cases of Coxsackievirus group A type 6 (CV-A6) (32.70%), 259 cases of Coxsackievirus group A type 16 (CV-A16) (4.44%), 252 cases of Coxsackievirus group A type 10 (CV-A10) (4.32%), and 34 cases of enterovirus type 71 (EV-A71) (0.58%). A total of 3384 other uncategorized EVs (57.97%) were found. The detection rates of EV-A71 and CV-A16 decreased year by year, while the detection rates of other EV nucleic acids increased year by year. Cerebrospinal fluid (CSF) EV nucleic acid detection was performed on 1520 children with positive EV nucleic acid throat swabs or stool samples showing clinical manifestations of viral encephalitis; a total of 140 positive cases (9.21%) were detected, including CV-A16 2.14% (3/140), CV-A10 1.43% (2/140), CV-A6 9.29% (13/140), EV-A71 0%(0/140), and other uncategorized EVs 87.14% (122/140). Among the 140 cerebrospinal fluid EV-positive children, 32 had typical hand-foot-mouth disease or herpetic angina, and 108 had only fever and upper respiratory tract infection. Real-time fluorescence quantitative PCR detection and virus typing can greatly improve the diagnosis rate of EV. Multi-sample EV nucleic acid detection and virus typing in children with viral encephalitis effectively improve the etiological diagnosis rate. Nonetheless, the development of multivalent vaccines remains the most economical and effective measure to prevent and control EV infection.
Similar content being viewed by others
EV is a general term indicating a class of pathogens belonging to the microribonucleic acid (RNA) virus family (Picornaviridae), which displays highly similar virus structure and pathogenic mechanism. Currently, 124 serotypes have been reported, with at least 66 EV types associated with human diseases. Depending on their tissue tropism, EVs can cause various clinical manifestations, and diseases resulting from EV infections are collectively termed enterovirus-associated diseases1,2. Seven species of human rhinovirus are known to infect humans, including EV-A, EV-B, EV-C, EV-D, and human rhinovirus (HRV)-A, HRV-B, and HRV-C. Among them, the EV-A/ EV-B/ EV-C representative EV-A71, CV-A/B, and poliovirus (PV) mainly cause hand-foot-mouth disease(HFMD), myocarditis, and poliovirus, respectively. In addition, EV-D68 and HRV typically cause respiratory influenza-like symptoms3,4,5,6,7. EV is mainly transmitted through the fecal-oral route, and the virus primarily replicates in the human intestine. Most infections are recessive, but infants and immunocompromised adults may have obvious symptoms. EVs of different serotypes may lead to different clinical manifestations. Young children with EV infection may present with symptoms indicating viral encephalitis, and the pathogen diagnosis of viral encephalitis remains a global challenge. The clinical diagnosis of viral encephalitis mainly relies on medical history, clinical symptoms, cerebrospinal fluid examination, and imaging examination, among which cerebrospinal fluid examination results are particularly important. About 50-60% of viral encephalitis patients cannot be identified by the existing conventional diagnostic methods(cerebrospinal Fluid Cytological and Biochemical Profiling8,9,10,11. Furthermore, hundreds of pathogens may cause viral encephalitis in children, with EV being one of the important ones in China12. The current study employed real-time fluorescence quantitative polymerase chain reaction (qPCR) to detect EV nucleic acid from throat swabs or fecal samples in children treated in our hospital. Moreover, cerebrospinal fluid EV nucleic acid detection was performed in children with clinical manifestations of viral encephalitis, combined with imaging examination to improve the diagnosis rate of viral encephalitis children. EV nucleic acid detection and virus typing of stool, throat swabs, cerebrospinal fluid, and other samples can improve the etiological diagnosis rate. Positive etiological test results further confirm the clinical manifestations and imaging manifestations of viral encephalitis children, providing a rapid and accurate basis for clinical diagnosis. This study aims to elucidate the epidemiological characteristics of EV in Hangzhou from 2018 to 2023 and to analyze the distribution of different EV serotypes in pediatric infections, thereby improving the diagnostic rate of EV-associated viral encephalitis.
Materials and methods
Study population
A total of 9676 children treated in Hangzhou Children’s Hospital from January 2018 to December 2023 were selected as the study objects. Pharyngeal swabs or stool samples were collected on the day of admission or the second day.In a cohort of 1520 children who tested positive for EV and exhibited clinical features consistent with viral encephalitis, CSF specimens were collected. These specimens were subsequently subjected to EV nucleic acid testing. Additionally, imaging studies were performed when clinically indicated. Informed consent was obtained from all parents and/or their legal guardian(s).
Methods
-
1.
Diagnostic Criteria for Encephalitis: Encephalitis is an inflammatory process of the brain parenchyma, which usually presents with fever, headache, and mental status changes. The diagnostic criteria of diseases were based on the relevant clinical diagnostic criteria described in Nelson Pediatrics(page389-391)13.
-
2.
Sample processing: All samples collected were sent to the PCR laboratory of the hospital under cold chain conditions for viral nucleic acid detection.
-
3.
Instruments and materials: Viral nucleic acid extraction reagent QIAamp Viral RNA Mini kit (Qiagen Company, Germany), one-step real-time test kit (Guangzhou Sun Yat-sen University Daan Gene Co., LTD.), ABI 7500 real-time fluorescent quantitative PCR gene amplification instrument (ABI Corporation, USA).
-
4.
Sample detection: A one-step real-time fluorescence quantitative RT-PCR method was used to detect EV-A71, CV-A16, CV-A6, CV-A10, and EV universal RNA viruses.
-
5.
Viral RNA extraction: 140ul of the supernatant of the specimen(Pharyngeal swabs or stool samples) was collected, and the viral RNA was extracted in strict accordance with the operation instructions of the extraction kit. Finally, 50ul of the RNA extraction solution was eluted and stored at -20°C for later use.
-
6.
RT-PCR detection: The extracted RNA was dissolved in 50 µL of TE buffer. The reaction volume was 25 µL, containing 2.5 mM dNTPs, 200 nM of each primer, 15 mM magnesium chloride, 200 U of M-MLV reverse transcriptase, 2.5 U of Taq DNA polymerase, 5 µL of total RNA template, and 1× PCR buffer. Reverse transcription and PCR were performed consecutively for each sample using an ABI 7500 Real-Time PCR System (Applied Biosystems, USA). Reverse transcription parameters: 40°C for 25 min. PCR parameters: initial denaturation at 94°C for 3 min; followed by 40 cycles of denaturation at 93°C for 15 s, annealing and extension at 55°C for 45 s. Single-point fluorescence detection was set at 55°C, with the fluorescence channel configured for FAM. The “start value” was set between 3 and 15, and the “end value” was set between 5 and 20. The amplification curve of the negative control was flat or below the threshold line.
-
7.
Ethical statement: All methods were conducted in accordance with relevant guidelines and regulations. Informed consent was obtained from the parents of all child patients for the use of human tissue samples, and the study was approved by the Medical Ethics Committee of Hangzhou Children’s Hospital, with the ethics reference number (2020)Annual Review (Research) No. (03).
Statistical analysis
SPSS 20.0 software was used for statistical analysis, and the statistical data were expressed as percentages, and the χ2 test was used for comparison between groups. P < 0.05 indicated statistically significant differences.
Results
Sample submission and pathogen detection
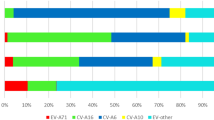
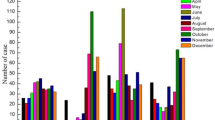
From January 2018 to December 2023, a total of 9676 clinical samples were subjected to EV viral nucleic acid detection, and 5838 (60.33%) positive EV samples were detected, with a male-to-female ratio of 1.67:1, and ages ranging from 4 days to 15 years (2.2 ± 2 years). The disease distribution of the 5838 EV-positive children included 2863 cases of hand-foot-mouth disease, 1520 cases of viral encephalitis (including 1 case of CV-A6-associated acute necrotic encephalopathy), 1176 cases of herpetic angina, 174 cases of upper respiratory tract infection, 60 cases of lower respiratory tract infection, and 45 cases of digestive tract infection. The enterovirus test results indicated 1909 cases of CV-A6 (32.70%), 259 cases of CV-A16(4.44%), 252 cases of CV-A10 (4.32%), and 34 cases of EV-A71 (0.58%). In addition, 3384 other EVs were uncategorized (57.97%). The results revealed that EV-A71 and CV-A16 decreased year by year from 2018 to 2023, and the detection rate of these two types of enterovirus was nearly 0% in the past two years. However, CV-A6 still accounted for a significant proportion of EV infection cases. The details of the positive detection of EV nucleic acid during 2018–2023 are shown in Fig. 1. After 2019, the number of EV nucleic acid samples decreased (P < 0.01), as shown in Table 1. Although the number of samples decreased compared with 2018 and 2019, the positive detection rate of EV nucleic acid still showed an increasing trend, showing a statistically significant difference between the positive detection rates of EV nucleic acid across different years (χ2 = 441.62, P < 0.01), as shown in Table 1.
Cerebrospinal fluid test results of EV encephalitis patients
A total of 1520 children with clinical manifestations of viral encephalitis were subjected to CSF EV nucleic acid detection, including 945 males and 575 females aged from 4 days to 15 years. Among them, 140 cases (9.21%) were positive for CSF EV nucleic acid. The positive cases included CV-A16 2.14% (3/140), CV-A10 1.43% (2/140), CV-A6 9.29% (13/140), EV-A71 0% (0/140), and other uncategorized EV 87.14% (122/140). The detection rate of cerebrospinal fluid EV-A71 in children with viral encephalitis from 2018 to 2023 was 0%. Among the 140 cerebrospinal fluid EV nucleic acid-positive children, 32 cases exhibited typical herpetic angina or hand-foot-mouth disease clinical manifestations in addition to fever (herpes angina or accompanied by herpes on the hands and feet); 108 cases only had fever or upper respiratory tract infection symptoms, without any obvious herpes angina or herpes on the hands, feet, and buttocks.
Discussion
Based on the analysis of 9676 samples, this study reveals the epidemiological trends of EV infections in children in Hangzhou and identifies the predominant serotypes. Furthermore, it confirms that enteroviruses are one of the common etiological agents of viral encephalitis in children. EV related diseases are one of the more common infectious diseases in humans, which can cause a variety of acute infectious diseases and often lead to sudden public health events14. From 2008 to 2012, most of the HFMD cases in China were caused by EV-A71 or CV-A16 infection, and more than 90% of the death cases were caused by the co-infection of both15. From 2008 to 2015, about 570,000 confirmed cases of HFMD were reported in China, and the positive rates of EV-A71, CV-A16, and other EVs were 44%, 25%, and 31%, respectively. EV-A71 infection was the primary infection in severe cases and deaths16.
The monovalent EV-A71 vaccine was approved in China in 2016, following which the number of severe cases and deaths of HFMD caused by EV-A71 showed a decreasing trend. The test results of the samples in this study showed that the detection rate of EV-A71 and CV-A16 decreased year by year from 2018 to 2023, and the detection rate of the above two types of enterovirus reached 0% in the past two years. However, due to the absence of cross-protection between EV serotypes, the monovalent EV-A71 vaccine does not protect against infection from other EV serotypes, as evidenced by the similar number of EV infection cases after the monovalent EV-A71 vaccine was launched. At the end of 2019, due to the normalization of prevention and control measures after the outbreak of novel coronavirus and the enhancement of public awareness of infectious disease prevention, the number of EV nucleic acid cases decreased significantly. Although the number of samples has decreased compared with 2018 and 2019, the detection rate of EV has not decreased but has shown an increasing trend. In terms of virus type, CV-A6 and other EV types have surpassed EV-A71 and CV-A16 to become the main pathogens of EV-related infectious diseases, which is consistent with the epidemiological survey data reported in other regions of China17,18,19,20,21,22.
Fever symptoms are almost always present in the acute stage of EV infection. Comprehensive analysis is conducted based on the presence of other accompanying symptoms, such as rashes (considering the time, place, shape, etc.), respiratory system, central nervous system, or circulatory system-related symptoms. However, considering the large number of EV serotypes, different serotypes of EVs can cause a variety of clinical symptoms. Hence, making a correct diagnosis based solely on clinical manifestations is challenging23. The results of this study found 2863 cases of hand-foot-mouth disease, 1520 cases of viral encephalitis, 1176 cases of herpetic angina, 174 cases of upper respiratory tract infection, 60 cases of lower respiratory tract infection, and 45 cases of digestive tract infection among 5838 EV-positive children. The results revealed that EV nucleic acid detection of different specimens in children with suspected EV infection, especially in children with viral encephalitis, can improve the detection rate of EV-related disease pathogens. Due to the large number of EV serotypes, further analyses are required to determine the EV serotypes.
Considering the lack of effective antiviral drugs for EV infection, vaccines remain the most economical and effective measure for the prevention and control of infection. In 2016, the monovalent inactivated EV-A71 vaccine developed by three domestic manufacturers was approved in China, showing protection rates against HFMD caused by EV-A71 of over 90% for all three monovalent EV-A71 inactivated vaccines24,25. The vaccines effectively reduced the number of deaths of HFMD caused by EV-A71. The EV-A71 vaccination rate among children in Zhejiang Province was 24.1%, playing a critical role in preventing severe HFMD26. With the increase in EV-A71 vaccination, the cases of EV-A71 infection decreased significantly, but the cases of other EV infections demonstrated no significant decrease but showed an increasing trend. Due to the continuous changes in the pathogen spectrum of EV infection, developing multivalent vaccines is particularly important27.
In conclusion, based on the findings of this study, we have observed that pediatric enterovirus infections can manifest with a diverse array of clinical presentations. Relying solely on clinical symptoms for diagnosis can easily lead to misdiagnosis, which in turn may adversely affect treatment outcomes. Furthermore, there are no significant distinguishing features in the clinical manifestations, cerebrospinal fluid cytology, or biochemical markers among cases of viral encephalitis caused by different viruses. Given that the severity of encephalitis can vary depending on the causative pathogen, performing nucleic acid testing on multiple specimens, particularly EV nucleic acid testing and virus typing, is of paramount importance for children with suspected encephalitis.
Nevertheless, the limitations of this study should be acknowledged. First, only limited EV serotypes such as CV-A16, CV-A10, CV-A6, and EV-A71 were tested. Many EVs in the test results were unclassified, and accurate etiological diagnosis of EV-related diseases could not be made. In response to the above problems, more effective detection methods should be developed, such as the application of metagenomic sequencing technology for pathogenic nucleic acid detection, which can effectively identify unknown viruses. The different types of CV and related imaging findings can be further explored.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Nikolay, B. Cauchemez S.Enterovirus outbreak dynamics.science. 361(6404): 755–756 (2018).
Lefkowitz, E. J. et al. Virus taxonomy:the database of the international committee on taxonomy of viruses (ICTV). Nucleic Acids Res. 46 (D1), D708–D717 (2018).
Gao, L. et al. Spectrum of enterovirus serotypes causing uncomplicated hand,foot,and mouth disease and enteroviral diagnostic yield of different clinical samples. Clin. Infect. Dis. 67 (11), 1729–1735 (2018).
Mirand, A. & Peigue-Lafeuille, H. Acute flaccid myelitis and enteroviruses: an ongoing story. Lancet 385 (9978), 1601–1602 (2015).
Yea, C. Bitnun A,Branson HM,et al.association of outcomes in acute flaccid myelitis with identification of enterovirus at presentation:a Canadian,nationwide,longitudinal study.lancet child. Adolesc. Health. 4 (11), 828–836 (2020).
Nkosi, N. et al. Molecular characterisation and epidemiology of enterovirus-associated aseptic meningitis in the Western and Eastern cape Provinces,South Africa 2018–2019. J. Clin. Virol. 139, 104845 (2021).
Bonnin, A. et al. Case of a healthy infant born following antenatal enterovirus myocarditis and hydrops. J. Clin. Virol. 61 (3), 459–462 (2014).
Glaser, C. A. et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin. Infect. Dis. 43 (12), 1565–1577 (2006).
Granerod, J. et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect. Dis., 10(12):835–844 (2010).
Glaser C.A. et al. In search of encephalitis etiologies: diagnostic challenges in the California encephalitis Project,1998–2000. Clin. Infect. Dis. 36 (6), 731–742 (2003).
Tyler, K. L. A. Viral Encephalitis N Engl. J. Med. 379(6):557–566. (2018).
Rudolph, H., Schroten, H. & Tenenbaum, T. Enterovirus infections of the central nervous system in children: an update. Pediatr. Infect. Dis. J. 35 (5), 567–569 (2016).
Robert, M. & Kliegman, J. W. St. Geme III.Nelson Textbook of Pediatrics 21st Edition.Elsevier.2019.
Christian, K. A. et al. What we are watching–five top global infectious disease threats, 2012: a perspective from CDC’s global disease detection operations center. Emerg. Health Threats J. 6, 20632 (2013).
Huang, D. Q. et al. Characteristic analysis of hand foot mouth disease death cases in 2008–2021 in Hubei Province. J. Math. Med., 2:104–109 (2023).
Huang, J. et al. Epidemiology of recurrent hand, foot and mouth disease, China, 2008–2015. Emerg. Infect. Dis. 24 (3), 432–442 (2018).
Kang, D. J. et al. Epidemiological characteristics of hand-foot-mouth disease in Sichuan Province, 2015–2020.Practical Preventive Medicine29(8):920–923 (2022).
Zhang, X. et al. Hand-Foot-and-Mouth Disease-Associated enterovirus and the development of multivalent HFMD vaccines. Int. J. Mol. Sci. 24 (1), 169 (2022).
Yang, Q. et al. Molecular epidemiology and clinical characteristics of enteroviruses associated HFMD in Chengdu, China, 2013–2022. Virol. J. 20 (1), 202 (2023).
Wang, J. & Zhang, S. Epidemiological characteristics and trends of hand-foot-mouth disease in Shanghai, China from 2011 to 2021. Front. Public. Health. 11, 1162209 (2023).
Chen, S. et al. Emerging concerns of atypical hand foot and mouth disease caused by Recombinant coxsackievirus A6 variants in Henan, China. J. Med. Virol. 95 (12), e29316 (2023).
Wu, Y. et al. Spatiotemporal cluster patterns of hand, foot, and mouth disease at the Province level in Mainland China, 2011–2018. PLoS One. 17 (8), e0270061 (2022).
Zhou, H. T. et al. Changes in enterovirus serotype constituent ratios altered the clinical features of infected children in Guangdong Province, China, from 2010 to 2013. BMC Infect. Dis., 16:399 (2016).
Zhu, F. C. et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 381 (9882), 2024–2032 (2013).
Zhu, F. et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China.N. Engl. J. Med. 370 (9), 818–828 (2014).
Ye, L. et al. Vaccination coverage estimates and utilization patterns of inactivated enterovirus 71 vaccine post vaccine introduction in Ningbo, China. BMC Public. Health. 21 (1), 1118 (2021).
Liu, Q., Tong, X. & Huang, Z. Towards broadly protective polyvalent vaccines against hand, foot and mouth disease. Microbes Infect. 17 (2), 155–162 (2015).
Acknowledgements
This research is supported by the grants to Hangzhou Health Science and Technology Plan (Key) project(No. 2016Z082).
Author information
Authors and Affiliations
Contributions
Q.L. and X.Y.L. wrote the main manuscript text. S.T. and Z.H.Q., X.F.Z., and S.Y.Z. contributed to the data analysis and interpretation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lao, Q., Lin, X., Teng, S. et al. Epidemiological characteristics of 5838 cases of enterovirus infection in children in Hangzhou from 2018 to 2023. Sci Rep 15, 10167 (2025). https://doi.org/10.1038/s41598-025-94883-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94883-5