Abstract
Gauzes are used in various ways, including wiping blood, compressing organs, and as markers during surgery. However, losing gauze during surgery can lead to pieces being left inside the body, causing time-consuming searches and unnecessary radiation exposure. To address this, we developed a fluorescent gauze using indocyanine green (ICG), which fluoresces under near-infrared (NIR) light. This study aimed to evaluate the fluorescence intensity (FI) of the gauze and confirm its fluorescence in a porcine abdominal cavity. We dissolved 25 mg of ICG in 10 ml of glycerol, ethanol, distilled water, and 5% bovine serum albumin, then diluted each solution 5- to 106-fold with distilled water. The gauze was dyed using these solutions. A rigid laparoscope observed the fluorescent gauze in a dark room under NIR light, and FI was measured. The gauze treated with a 100-fold dilution of each ICG dyestuff showed the strongest fluorescence. This gauze was then placed in a pig’s abdominal cavity and observed under NIR light, demonstrating that the fluorescence could penetrate two or three layers of the mesentery. Our fluorescent gauze, confirmed both ex vivo and in vivo, should be manufactured for clinical use and further validated for its utility.
Similar content being viewed by others
Introduction
Gauze is an essential medical material with various applications, and surgical gauze is indispensable to surgeons. It is used not only to wipe blood from surgical fields but also to compress organs and as a marker for surgical manipulation. Surgical gauze is occasionally lost during surgery. Gauze remnants can cause health problems and are occasionally found as retained surgical sponges (Gossypiboma)1. Gawande et al. reported that the incidence of retained sponges and instruments varied from 1/8801 to 1/18,760 during inpatient operations in non-specialty acute care hospitals2. Retained surgical gauze is a rare and preventable problem that persists despite the use of gauze with radiopaque markers and standardized protocols for gauze counting. The search for lost gauze is time-consuming, especially in laparoscopic surgery, and it involves unnecessary radiation exposure to patients and medical staff.
Indocyanine green (ICG) fluorescence-guided surgery is widely performed, and during this procedure camera devices equipped with near-infrared (NIR) light are available in many countries. We considered that a fluorescent gauze dyed with ICG that fluoresces under NIR light would be useful for identifying lost gauze in the body during surgery. During this study, we produced a fluorescent gauze dyed with ICG and evaluated the fluorescence intensity (FI) of the gauze ex vivo and in vivo.
Materials and methods
This study was conducted at the Medical Device Innovation Platform, following the Foundation for Kobe International Medical Alliance Animal Experimentation Regulations, and it was approved by the Institutional Animal Care and Use Committee (permission number: K-23-036). All procedures in the animal study were performed in accordance with the institutional ethical standards in compliance with the ARRIVE guidelines (https://arriveguidelines.org) and all relevant guidelines and regulations. A pig for this study was provided by the IVTEC Corporation Animal Experimentation Regulations (permission number IVTeC No.23-067). This experimental study was designed in compliance with the 3Rs principles (Replacement, Refinement, and Reduction) and developed in congruence with the best animal welfare conditions. Accordingly, it was initiated ex vivo and performed in a pig model to replicate human characteristics, thereby facilitating its reproducibility and potential transfer. In this study, a 38 kg female pig was used and housed in a group and acclimatized for 1 week in an enriched environment with circadian cycles of light darkness, constant humidity, and temperature conditions. The subject fasted for 24 h before surgery, had ad libitum access to water, and was sedated (xylazine 2 mg/kg + ketamine 10 mg/kg IM) before the procedure to decrease stress. Anesthesia induction was achieved through isoflurane inhalation before intubation and maintained with rocuronium 0.6 mg/kg along with inhaled sevoflurane 2–5%. Finally, the animals were euthanized using a lethal intravenous dose of potassium chloride before being discarded.
Preparation of ICG diluted solutions
Indocyanine green was used as the fluorescent agent. Glycerol (NACALAI TESQUE, INC., Japan), ethanol (99.5, FUJIFILM Wako Pure Chemical Co., Japan), distilled water, and 5% bovine serum albumin were used as solvents. ICG (Diagnogreen for injection 25 mg, Daiichi-Sankyo Co., Japan) was dissolved in 10 ml of solvent solution. Each ICG solution was diluted 10- to 104-fold with distilled water to prepare the ICG-diluted solutions.
Preparation of fluorescent gauze
Each ICG solution was diluted with distilled water from 5- to 106-fold to prepare each dyestuff. Each sterilized gauze (Stellaze 5 × 5 cm; Hakujuji, Japan) was soaked in the dyestuff for several minutes and then dried using a hair dryer for 15 min in a dark room, and subsequently stored under light-shielded conditions for 2 days before use.
Near-Infrared imaging
A laparoscopic camera system (VISERA ELITE II; Olympus, Tokyo, Japan) was used for NIR imaging. The diluted ICG solutions and fluorescent gauze were observed under white and NIR light (magenta and monochrome modes). The camera and NIR light settings were as follows: contrast set, normal; IR sensitivity, set high; IR exposure time, 1 field.
Ex vivo setting
The laparoscope was placed 20 cm from the ICG-diluted solutions on the tube holder and 25 cm from the fluorescent gauze on the table. Undyed gauze was used as a negative control and an ICG reference card (Diagnostic Green GmbH, Germany) was used as a positive control. The objects were observed in a dark room.
In vivo setting
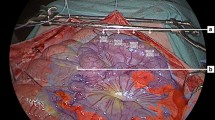
The laparoscope was placed approximately 5 cm from the fluorescent gauze. The strongest fluorescent gauze dyed with each dyestuff was placed in the abdominal cavity of the pig through a laparoscopic trocar. The gauze was covered with mesenteries to check tissue penetration of the fluorescence. The thickness of the mesentery was approximately 2–3 mm per layer. In addition, each fluorescent gauze was placed under bleeding conditions.
Measurement of the fluorescence intensity
Using monochrome mode images, the FIs of the ICG-diluted solutions and fluorescent gauze were measured using the Java-based image processing program, ImageJ. In this study, fluorescence intensity (FI) was defined as the brightness value per pixel, measured using monochrome images (256 intensity levels; 0 = darkest, 255 = brightest). Under bleeding conditions, magenta images were separated into three monochrome 8-bit images: Red, Green, and Blue. The green images were used. Six regions of interest (ROIs) of random sizes were selected within each sample, and the mean FI across these ROIs was calculated. The total area of the ROIs was set to at least 100 pixels for the ICG-diluted solutions and at least 1000 pixels for the fluorescent gauze samples.
Statistical analysis
The mean FI data of each subject were compared using the Tukey–Kramer method. Statistical significance was set at p < 0.05. All calculations were performed using JMP software version Pro 17 (SAS Institute, Cary, NC, USA).
Results
Fluorescence intensity of the ICG diluted solution in ex vivo (Figs. 1 and 2)
A photograph of a series of ICG diluted solutions. Twenty-five mg of ICG was dissolved in 10 ml of each solvent (glycerol, ethanol, distilled water, and 5% bovine serum albumin). Each ICG solution was diluted with distilled water (from left to right: undiluted, x10, x102, x103, and x104 diluted) under white light and NIR light for monochrome mode. A, Glycerol. B, Ethanol. C, Distilled water, D, 5% bovine serum albumin. * means p value < 0.05.
The mean FI values of the ICG-diluted solutions in the glycerol group were 25.4, 51.5, 59.2, 69.1, and 54.3 for the undiluted solution, 10-, 102-, 103-, and 104-fold diluted solutions, respectively. Similarly, the values were 46.8, 76.0, 80.7, 88.6, and 75.6 in the ethanol group; 3.1, 3.3, 8.3, 13.6, and 7.7 in the water group; and 3.3, 14.2, 28.6, 34.5, and 22.6 in the albumin group. Each 1000-fold diluted ICG solution had the highest FI value. Among the 1000-fold ICG-diluted solutions, the FI was significantly stronger in the ethanol, glycerol, albumin, and water groups.
Fluorescence intensity of the fluorescent gauze in ex vivo (Figs. 3 and 4)
A series of fluorescent gauze dyed with each ICG diluted solution. Twenty-five mg of ICG was dissolved with 10 ml of each solvent. Each ICG solution was diluted with distilled water to produce each dyestuff. (from top to bottom: glycerol, ethanol, distilled water, and 5% bovine serum albumin; from left to right: x5, x10, x102, x103, x104, x105, and x106 diluted). A, white light. B, NIR light for magenta mode. C, NIR light for monochrome mode.
The mean FI values were 15.6, 27.0, 49.3, 24.0, 7.6, 5.6, and 5.2 in the gauze dyed with 5-, 10-, 102-, 103-, 104-, 105-, and 106-fold dyestuffs of glycerol, respectively. Similarly, the values were 7.4, 14.3, 51.4, 50.3, 9.4, 6.6, and 4.8 in the ethanol group, 6.3, 14.1, 41.0, 22.9, 6.2, 5.1, and 3.8 in the water group, 4.8, 13.9, 30.3, 17.6, 4.9, 4.8, and 4.2 in the albumin group. Each fluorescent gauze dyed with 100-fold dyestuff exhibited the strongest FI. Among the fluorescent gauzes dyed with the 100-fold dyestuff, the FIs of those in the ethanol and glycerol groups were significantly stronger than those in the water group, and the FI of those in the water group was significantly stronger than those in the albumin group.
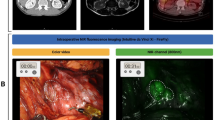
The fluorescent gauze in vivo
After the fluorescent gauze dyed with 100-fold diluted dyestuff was inserted into the porcine abdominal cavity, each gauze exhibited strong fluorescence under NIR imaging (Supplementary Video 1). The fluorescence of the gauze dyed with the 100-fold diluted dyestuff in glycerol penetrated up to three layers of the mesentery, whereas that of ethanol, water, and albumin penetrated up to two layers of the mesentery (Supplementary Video 1). Photographs of the gauze dyed with the 100-fold diluted dyestuff of pure ICG solution are shown in Fig. 5. Under bleeding conditions, the fluorescence intensity of the fluorescent gauze slightly decreased after blood absorption, as shown in Supplementary Video 2). In the in vivo setting, the mean FI values were 253.3, 253.2, 251.8, and 253.3 for the gauze dyed with 10²-fold diluted solutions of glycerol, ethanol, water, and albumin, respectively. In contrast, the mean FI values for the fluorescent gauze after blood absorption were 226, 228.2, 178.2, and 216, respectively. There was a significant difference in fluorescence intensity between the fluorescent gauze before and after blood absorption for each group.
Discussion
We developed a gauze with strong fluorescence emission under NIR imaging, ex vivo and in vivo. This is the first experimental report showing that gauze dyed with ICG dyestuff emits fluorescence and that the FI varies with the concentration of ICG dyestuff, with the gauze fluorescence penetrating several layers of the mesentery.
In this study, ICG dissolved in four solvents was used to confirm its fluorescence as a dyestuff before preparing the fluorescence gauze. While a pure ICG diluted solution exhibits an unstable and weak FI due to ICG aggregation, dimethyl sulfoxide, methanol, and ethanol are known to enhance ICG fluorescence, as well as blood plasma3. In this study, the addition of glycerol to ICG enhanced the ICG FI. These four solvents were chosen to ensure uniform and stable dyeing of the gauze. The fluorescence intensity of ICG does not always correspond to its concentration because of the quenching effect4. This study also demonstrated that cellulose alone, a component of the gauze, enhanced ICG fluorescence. Therefore, it is important to note that no additional enhancers were required to produce the fluorescent gauze. In addition, a high concentration of the ICG-diluted solution did not result in a high FI in the gauze.
Although each ICG-diluted solution exhibited the strongest fluorescence intensity at a dilution of 10³-fold, the fluorescent gauze samples showed maximum fluorescence intensity at a dilution of 10²-fold. We speculate that this difference is related to interactions between ICG molecules and cellulose fibers in the gauze. Binding of ICG to hydroxyl groups in cellulose fibers might inhibit aggregation of ICG molecules, thereby reducing quenching effects and resulting in more stable fluorescence intensity. This mechanism potentially explains why fluorescence intensity differs between liquid ICG solutions and dyed gauze samples.
ICG fluorescence-guided surgery is widely used during various surgeries4. Recently, NIR fluorescent solid materials have been developed5, and several fluorescent medical devices have been used in image-guided surgery6,7. The developed NIR-coating material can be used in various medical applications8. In a previous study, a fluorescent gauze coated with a Cy-C18 TPB-based coating material soaked in blood was identified using an NIR laparoscopic system. However, no fluorescent gauze is commercially available. To the best of our knowledge, there has been only one case report on the use of a fluorescent gauze with ICG. Kumata et al. reported that ICG-containing gauze was useful in identifying the dissection layer under NIR imaging during laparoscopic intersphincteric resection9. The authors used fluorescent gauze soaked in a liquid prepared by dissolving 25 mg of ICG in 10 ml of water containing sodium arginine. Although this gauze can be easily prepared in a clinical setting, the authors did not consider the concentrations of ICG and arginine required for viscosity enhancement. Moreover, the use of gauze must be approved by an ethics committee because it is off-label for ICG. Thus, fluorescent gauze must be manufactured under the Pharmaceutical Affairs Law. Based on our results, a pure ICG-diluted solution seems to be an optimal dyestuff because glycerol and ethanol are chemical compounds and albumin is a biologically derived product. More importantly, ICG is safe for injection. Ethanol and glycerol may potentially affect biological tissues upon contact; thus, further investigations are required to evaluate their safety. It is essential to conduct additional in vivo safety assessments before clinical application.
Fluorescent gauze is useful in various operations, especially in minimally invasive procedures. First, it is expected to reduce the incidence of gauze loss during surgery. When a lost gauze is recognized after gauze counting, fluorescence can be used to locate any material that is behind organs and tissues using NIR imaging before transferring the C-arm fluoroscope for radiation. In addition, fluorescent gauze can be used for ICG fluorescence-guided surgery. Therefore, it may serve as an effective marker for surgical manipulation. For example, surgeons often include gauze in the gap between the mesentery and retroperitoneum as a marker of peritoneal dissection during colorectal surgery. Tissue penetration of fluorescence is an important factor for fluorescence markers used during surgical procedures. In this study, fluorescence from the gauze penetrated multiple mesentery layers. The observed tissue penetration corresponds approximately to a depth of 4–9 mm (2–3 layers). The available literature currently reports a penetration depth ranging from approximately 5–10 mm4. The results are consistent with previous reports regarding near-infrared fluorescence penetration through biological tissues.
This study has several limitations. First, our gauze is a prototype of a fluorescent gauze. Although the strongest fluorescence was observed in the gauze dyed with a 100-fold dilution of each ICG solution, the optimal concentration of the dye solution remains unknown. In the measurement of the FI, selection bias in the determination of ROIs was unavoidable due to the random selection process used for ROI placement. Additionally, uneven dyeing may occur on the fluorescent gauze, although a manufacturing system can be used to produce a uniformly dyed gauze. Fluorescent residues may potentially transfer to tissues upon contact with the gauze, especially in a bleeding setting, because the gauze was only dyed with the ICG solution and dried. Thus, dyeing, refining, bleaching, drying, and sterilization methods should also be considered. Conversely, we should know that FI may be attenuated during each process. As shown in Fig. 6, the fluorescence intensity of the prepared gauze has the potential to be maintained for a long period. Further studies are required to determine optimal storage methods and the maximum duration for maintaining fluorescence intensity. Another key factor is the strength of the NIR light of the device, which is strongly related to the FI.
A fluorescent gauze dyed with a 100-fold diluted pure ICG solution was stored under light-shielded conditions and maintained stable fluorescence intensity for over three years. Left: newly prepared gauze. Right: gauze prepared three and a half years ago. A, white light. B, NIR light in magenta mode. C, NIR light in monochrome mode.
Our study evaluated a limited number of fluorescent gauzes using a single laparoscopic NIR imaging device. Therefore, further studies are required. Secondly, the binding of ICG to cellulose may emit fluorescence under NIR light. This process needs to be elucidated by molecular structural analysis. Finally, fluorescent gauze for clinical use should be manufactured according to medical gauze standards, and its safety and usefulness should be validated in various operations.
Conclusions
We developed a fluorescent gauze using ICG, and strong fluorescence emission was confirmed using laparoscopic NIR imaging, ex vivo and in vivo. This gauze should be manufactured for clinical use and its usefulness should be validated.
Data availability
All the data obtained and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Abbreviations
- ICG:
-
Indocyanine green
- FI:
-
Fluorescence intensity
- NIR:
-
Near-infrared
References
Bani-Hani, K. E., Gharaibeh, K. A. & Yaghan, R. J. Retained surgical sponges (gossypiboma). Asian J. Surg. 28, 109–115 (2005).
Gawande, A. A., Studdert, D. M., Orav, E. J., Brennan, T. A. & Zinner, M. J. Risk factors for retained instruments and sponges after surgery. New. Eng. J. Med. 348, 229–235 (2003).
Benson, R. C. & Kues, H. A. Fluorescence properties of indocyanine green as related to angiography. Phys. Med. Biol. 23, 159–163 (1978).
Kusano, M., Kokudo, N., Toi, M. & Kaibori, M. ICG Fluorescence Imaging and Navigation Surgery (Springer, 2016).
Anayama, T. et al. Near-infrared fluorescent solid material for visualizing indwelling devices implanted for medical use. Surg. Endosc. 34, 4206–4213 (2020).
Namikawa, T. et al. Novel endoscopic marking clip equipped with resin-conjugated fluorescent indocyanine green during laparoscopic surgery for Gastrointestinal cancer. Langenbecks Arch. Surg. 405, 503–508 (2020).
Ryu, S. et al. Laparoscopic fluorescence navigation for left-sided colon and rectal cancer: Blood flow evaluation, vessel and ureteral navigation, clip marking and trans-anal tube insertion. Surg. Oncol. 35, 434–440 (2020).
Ashoka, A. H. et al. Near-infrared fluorescent coatings of medical devices for image-guided surgery. Biomaterials 261, 120306 (2020).
Kumata, H. et al. Efficacy of intraoperative fluorescence imaging using indocyanine green-containing gauze in identifying the appropriate dissection layer in laparoscopic intersphincteric resection: A case report. Clin. Case Rep. 10, e6356 (2022).
Funding
The authors have no source of funding to declare.
Author information
Authors and Affiliations
Contributions
Study conception and design: Urade, TsuchidaAcquisition of data: Urade, Tsuchida, Oji, Fujiwara, Fukuoka, YasudaAnalysis and interpretation of data: Urade, Tsuchida, Oji Drafting of manuscript: Urade, OjiCritical revision: FukumotoAll authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests. S. T. and T.U. have submitted a patent application (PCT International Application) protecting the methodology described in this article. A preprinted version of this manuscript has been published on Research Square (https://doi.org/10.21203/rs.3.rs-4519399/v1).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary Material 2
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Urade, T., Tsuchida, S., Oji, K. et al. Development of a novel fluorescent gauze using indocyanine green. Sci Rep 15, 10189 (2025). https://doi.org/10.1038/s41598-025-94944-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94944-9