Abstract
Salt stress is one of the main challenges for plant development and production. To address this issue, new strategies with organic compounds in soils affected by salinity are being investigated. This study investigated how onion (Allium cepa) responds to salt stress induced by NaCl (150 mM), in conjunction with Cordia verbenacea essential oil. Chemical analyses of the soil and essential oil were performed to identify their main chemical constituents. Seeds of A. cepa were planted and irrigated daily with test solutions. After 21 days, germination rates were evaluated, and leaf length and leaf area were measured. After 42 days, the root length was analyzed. In addition, molecular analysis of the main constituents of the essential oil was conducted using molecular docking with a protein from Allium cepa. The results showed that the concentration of 500 μg/mL of Cordia verbenacea essential oil + 150 mM NaCl significantly increased the length of the leaves, mitigating the adverse effects of salt stress. The treatment also resulted in an increase in the number of roots, suggesting a potential protective effect. The in-silico analysis revealed that ( ±)-α-Pinene, (-)-β-caryophyllene and Alloaromadendrene predominantly formed alkyl interactions, suggesting that such interactions may contribute to the protective effects observed on leaf and root growth. These findings indicate that Cordia verbenacea essential oil may be a promising tool to minimize the impacts of salt stress on plants, promoting the growth and sustainable productivity of A. cepa under saline conditions.
Similar content being viewed by others
Introduction
The term stress refers to any type of external pressure, whether abiotic (such as salinity, heat, water scarcity, etc.) or biotic (such as herbivore action), that limits the rate of photosynthesis and reduces the plants’ ability to convert energy into biomass1,2.
The adverse impact of excess minerals such as sodium (Na+) and/or chloride (Cl-) in plants is known as salt stress3,4,5,6. Salt stress represents one of the most significant factors limiting crop growth and production. There are several sustainable approaches to mitigate this effect, both preventive and proactive, including soil recovery, that can be implemented separately or in combination to enhance plant resistance to salt and improve crop nutrition under conditions affected by this issue7,8.
New research strategies have been exploring the benefits of different organic amendments for plant growth in saline or sodic soils. These strategies report reduction in oxidative and osmotic stress, improvement in conductance and stomatal density, increased seed germination rate, and stimulation of microbial activities7,8, among other benefits.
The application of organic materials has shown promising results, improving the saline soil biome and enriching it with compounds, green manure, poultry manure, and sugarcane residues (pressed lime)9. Cordia verbenacea is a flowering shrub commonly known in Brazil as “Erva baleeira”, belonging to the family of Boraginaceae10,11. It is mainly found throughout the Brazilian Atlantic Forest, as well as in coastal regions and low-lying areas of the Amazon12.
Substantial evidence from the literature indicates that Cordia verbenacea aerial parts are often used in Brazilian folk medicine due to its remarkable antirheumatic, analgesic, and healing properties. The aerial parts are commonly used in the form of herbal extracts, decoctions, and infusions13,14,15.
Allium cepa Linn., a widely cultivated edible bulb from the family Alliaceae, is among the oldest known crops16. The A. cepa assay, a rapid toxicity indicator, evaluates nuclear abnormalities, chromosomal aberrations, mitotic index alterations, and root growth inhibition17,18. In Bangladesh, onion cultivation is widespread but hampered by salinity stress in coastal regions, as it is a glycophytic crop. Salinity affects 1.06 million hectares (32% of coastal land), significantly reducing agricultural productivity19.
Treating saline stress with plant-based organic materials is a promising and relevant approach, considering the increasing impact of soil salinization on agriculture and global food security7,20.Saline soils compromise crop productivity, leading to environmental management and biodiversity loss21.The use of plant organic compounds not only improves soil physical and chemical properties, but also promotes beneficial microbial activity, essential for soil health22,23. Furthermore, many of these materials have bioactive properties that can mitigate the effects of saline stress on plants, favoring physiological and biochemical adaptations that increase crop resilience24. Therefore, this study was undertaken to investigate the effects of Cordia verbenacea essential oil on the emergence of Allium cepa L. Seeds exposed to saline stress. In addition, we investigated the major compounds present in essential oil through molecular analyses, to provide a deeper understanding of its effects.
Material and methods
Reagents used and sowing location
The essential oil of Cordia verbenacea was obtained from a standard supplier. The seeds of A. cepa used for germination were purchased from a local agricultural supply store. Other chemicals, such as acetic acid, distilled water, and NaCl, were obtained from suppliers recognized for their standard quality.
Seed procurement and compliance
The seeds used in the experiment were purchased from a store specialized in agronomy products, which eradicated the need for additional documentation, offering convenience and security in the acquisition process. Baia Periforme onion seeds (071—Allium cepa) from the brand A Super Semente (ISLA), lot number 163245–009 S2, were utilized. These seeds demonstrated a germination rate of 94% and a purity of 100%, ensuring their quality and reliability for the experiment. Harvested in 2022/2023 and valid until May 2025, these seeds guarantee viability throughout the experimental period. In tests involving onions, compliance with the IUNC (International Union For Nature Conservation) policy declaration on research involving endangered species or the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) is not required because onions (Allium cepa) are not classified as endangered species. They are widely cultivated agricultural crops and are not subject to the regulations and protections that apply to endangered or threatened species. Therefore, research on onions does not necessitate adherence to these specific international conservation policies.
GC–MS analysis
Oil analysis was performed using a Shimadzu GC MS – QP2010 series (GC/MS system): Rtx-5MS capillary column (30 m × 0.25 mm, 0.25 μm film thickness); helium carrier gas at 1.5 mL/min; injector temperature 250 ºC; detector temperature 290º C; column temperature 60º C – 180º C at 5º C/min, then 180º – 280º C at 10º C/min (10 min). Scanning speed was 0.5 scan/sec from m/z 40 to 350. Split ratio (1:200). Injected volume: 1 µL of [25 µL (essential oil) / 5 mL CHCl3] (1: 200). Solvent cut time = 2.5 min. The mass spectrometer was operated using 70 eV of ionization energy. Identification of individual components was based on their mass spectral fragmentation based on Mass spectral library NIST 08, retention indices, and comparison with published data.
Physicochemical analysis of the soil
The soil samples from the study area were collected at depths of 20 and 40 cm. They were then sent to the laboratory, where the concentrations of pH, phosphorus, potassium, sodium, calcium, magnesium, aluminum, hydrogen, aluminum, and cation exchange capacity (CEC) were measured following the methodology proposed by Teixeira et al. (2017)25.
Cultivation of Allium cepa plants and experimental treatments
The cultivation of Allium cepa was conducted in a greenhouse with shade netting, following the protocol outlined by Fiskesjo (1985)26 with minor revisions. The seeds (120) of Allium cepa were planted at a depth of 2 cm in the soil and were watered daily as follow: Control (distilled water at pH 7), NaCl (150 mM) diluted in distilled water, and NaCl (150 mM) in combination with different concentrations of the essential oil of Cordia verbenacea (100, 300, and 500 µg/mL). Readings were taken on the 21st day after each treatment was applied. The mean effective concentrations (IC50 and IC20) were determined using linear regression. Additionally, the number of germinated seeds was counted, and the relative inhibition of seed growth was calculated and expressed as a percentage.
Phytotoxicity index analysis
Following a modified protocol by Li et al. (2022)27, Allium cepa seedlings were assessed on the 21th day post-sowing, using a phytotoxicity classification system. The data were then statistically analyzed, and the phytotoxicity damage index was calculated with the following formula:
where, PI (%) represents the phytotoxicity index (%), PG is the phytotoxicity score, HL denotes the highest level of observed phytotoxicity, and NP is the number of plants evaluated.
Leaf area estimation
To estimate leaf area, we used methods based on linear measurements, specifically leaf length and width. This procedure was adapted from previous studies on vineyards and fruit trees28, peppers 29 and tomatoes30, which provided a foundation for calculating the leaf area of Allium cepa (onion). We applied the ellipse formula, which allows for greater accuracy by considering the elliptical shape of onion leaves. The formula used is:
where, L represents the leaf length, C is the maximum leaf width, and π is a constant approximately equal to 3.14159. The division by 4 is necessary because the area formula for an ellipse is based on the semi-axes, whereas L and C represent the full axes. By dividing by 4, we adjust the formula to reflect the semi-axes, making it especially suitable for elongated leaves, such as those of Allium cepa.
Root length measurements and sampling
For a detailed assessment of root growth rates, a precisely calibrated digital caliper was used to measure root length. Twenty plants were randomly selected for each treatment. Measurements were taken 42 days after germination began to ensure reliable and representative data collection. This process was repeated three times to ensure the consistency and robustness of the results obtained.
Recipient treatment
The target of interest chosen from the literature review was subjected to molecular docking. The target protein (PDB id: 5GTE) with its respective ligand was obtained from the Protein Data Bank (PDB). The PDB is a repository of protein data and their three-dimensional structures. Various types of information are associated with each PDB file entry, including atomic coordinates in three-dimensional space, polymer sequence, and metadata (Berman, 2000)31. The removal of the protein inhibitor and water molecules from the receptor structure was performed using the Discovery Studio 2021 Client software.
Ligand treatment
The compounds ( ±)-α-Pinene, (-)-β-caryophyllene and alloaromadendrene were chosen for in silico evaluation via molecular docking. The ligand selected for this investigation was crafted in 3D using ACD/ChemSketch software, while the 2D model was obtained from ChemSpider ( ±)-α-Pinene ID: 6402, (-)-β-caryophyllene ID: 4,444,848, and alloaromadendrene ID: 10,478,311). Employing the Autodock VINA system within the PyRx software32, the compounds underwent ‘rigid protein-flexible ligand’ docking. Following the docking process, ligands adopting the most stable conformations were scrutinized using Discovery software.
Grid calculation and docking
The grid calculation involved processing 100 conformations using the Autodock VINA system within the PyRx software. For the ligand–protein docking procedure, the grid dimensions were set at 40 × 40x40 Å along the X, Y, and Z axes, with a grid spacing of 0.375 Å. Center coordinates for the grid were specified as 16.819, 71.967, and 31.192 Å for the X, Y, and Z axes respectively. These coordinates were determined based on the binding site location derived from known ligands previously co-crystallized with the protein and archived in the Protein Data Bank (Fig. 1). Following this, the interaction energy between the ligands and the amino acids of the 5GTE protein was calculated using the Discovery Studio software. This software enables the computation of free binding energy by analyzing its underlying energetic components, such as van der Waals forces, electrostatic bonds, and hydrogen bonds33.
Statistical analysis
Analysis of variance (ANOVA) was performed among different treatments. Significant differences between treatments were assessed by ANOVA, followed by Tukey’s post hoc test (p < 0.05), and the data are expressed as the mean values ± SEM of 3 triplicate.
Results
Chemical composition of Cordia verbenacea essential oil
Table 1 shows the chemical composition of the essential of Cordia verbenacea. The most abundant compounds were α-pinene, caryophyllene and tricyclo 2.2.1 (2,6) heptane representing respectively 33.81%, 15.61% and 14.55%. Similarly, the minor components were α-thujene (1.65%), nerolidol (2.38%) and tetradecane (3.19%) (Table 1). This result suggest that Cordia verbenacea essential oil is mostly composed of compounds from the class of terpene (α-pinene, caryophyllene).
Characteristics of the soil used
When characterizing the soil, we analyzed the presence of parameters at two distinct depths, 0–10 cm and 20–40 cm, and did not identify significant differences between the levels of coarse sand, fine sand, silt, clay, and natural clay. However, upon examining the chemical compounds, we observed slightly differences, but not significant. For instance, in the assortment complex, the Ca2+ content was 14.00 cmol/kg at the depth of 0–10 cm and 13.10 cmol/kg at the depth of 20–40 cm. K+ had a concentration of 3.35 cmol/kg at the surface (0–10 cm) and 2.80 cmol/kg at the depth of 20–40 cm, revealing a difference, although not significant (Table 2). The concentration of Na+ was 0.90 cmol/kg at the surface, showing a higher rate compared to the analysis conducted at 20–40 cm depth (0.75 cmol/kg). However, the organic matter (OM) content showed a considerable difference, being 50.08 g/kg in the 0–10 cm layer and 40.51 g/kg in the 20–40 cm layer, indicating a significant variation.
Effect of Cordia verbenacea essential oil (EOCv) on seed germination
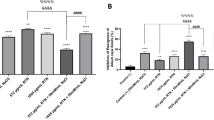
As depicted in Fig. 2A, the treatment of seeds with NaCl (150 mM) caused a significant reduction of about 38% in the emergence of Allium cepa compared to the control group, with an effective concentration of 37 µg/mL (EC50). However, co-treatment of NaCl (150 mM) with different concentrations of EOCv (100–500 µg/mL) did not show any effect compared to NaCl (150 mM) alone (p < 0.05). Similar result was obtained in the inhibition of emergence of Allium cepa seeds (Fig. 2B) and in the number of rootlets (Table 3). This result demonstrates some protection against the adverse effects of NaCl on the number of roots, and suggest a potential benefit of EOCv in promoting root development, even under conditions of NaCl-induced salt stress.
Effects of Cordia verbenacea essential oil (EOCv) on seed germination in the presence of NaCl (A) and inhibition of seed germination in the presence of NaCl (B). Seed germination rates were recorded 21 days after planting under various treatments: control; treatment with NaCl 150 mM, 100, 300, and 500 µg/mL. Data are expressed as mean ± SEM for 3 replicates. * denotes significant difference compared to the control.
Phytotoxicity
In the phytotoxicity analysis, NaCl (150 mM) caused a significant increase in the phytotoxicity index (~ 27%) compared to control (p < 0.05). Treatment with EOCv (100–500 µg/mL) did not show any significant effect compared to NaCl (150 mM) alone (p > 0.05) (Fig. 3), indicating that EOCv did not provide protection against saline stress damage (Fig. 3).
Effects of Cordia verbenacea essential oil (EOCv) on seed phytotoxicity in the presence of NaCl. Phytotoxicity rates were recorded on day 21 after planting under the treatments: control; NaCl treatment 150 mM, 100, 300 and 500 µg/mL. Data are expressed as mean ± SEM for 3 replicates. * denotes significant difference compared to control.
Leaf length and leaf area estimation
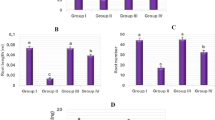
It was observed that the effect of different concentrations of NaCl combined with Cordia Verbenacea essential oil (EOCv) on the leaf length of Allium cepa resulted in a significant reduction compared to the control group (p < 0.05). However, concentrations of 300 µg/mL and 500 µg/mL showed a significant increase both compared to NaCl 150 mM and between each other (Fig. 4A). This effect was observed in leaf area (cm2), where NaCl resulted in a reduced leaf area compared to the control. However, EOCv treatment caused an increase in the leaf area in a concentration dependent manner, with 300 and 500 µg/mL exhibiting significant improvements compared to NaCl (Fig. 4B and 5). These results suggest a possible positive influence of higher concentrations of EOCv on leaf length, partially counteracting the negative effects of NaCl.
Effects of different concentrations of NaCl + EOCv on leaf length in Allium cepa (A) and effect of different concentrations of NaCl + EOCv on leaf area of Allium cepa (B). Vertical bars denote SEM, n = 8. * indicates significant difference compared to control; # indicates significant difference compared to NaCl; & indicates significant difference compared to NaCl + EOCv (100 µg/mL) (p < 0.05).
Root length
Figure 6 shows roots length of Allium cepa after 42 days of treatment with NaCl (150 mM) alone or in combination with EOCv (100–500 µg/mL). NaCl 150 mM caused a significant reduction of roots length compared to the control group (p < 0.05). However, concentrations of 300 µg/mL and 500 µg/mL of EOCv in combination with 150 mM NaCl showed a significant increase in the roots length when compared to NaCl (150 mM, Fig. 6). These results suggest that higher concentrations of EOCv may have a positive effect on root length, counteracting the negative effects induced by NaCl. (Fig. 6. (A).
Effects of different concentrations of NaCl + EOCv on root length in Allium cepa, with 42 days after emergence. Vertical bars denote SEM, n = 8. * denotes significant difference compared to control; # indicates significant difference compared to NaCl (p < 0.05). Root length calculation was obtained from measurements of 20 seedlings.
Molecular analysis
Trehalose showed an affinity of −6.9 kcal/mol, with binding at sites GLN B:161, LEU B:28, THR B:157, GLN B:153, and ALA B:27, where sites GLN B:161, THR B:157, and GLN B:153 were of the conventional hydrogen bond type. LEU B:28 was a carbon hydrogen bond, and ALA B:27 was of the alkyl type (Fig. 7G). The docking results highlighted several key interactions and molecular properties. Hydrogen bond donors were observed in interactions at the LEU B:28 site (Fig. 7A), while the solvent-accessible surface (SAS) predominantly ranged between 22.5 and 25 Å2 (Fig. 7B). Aromatic interactions were characterized as weak, with a lever-to-edge geometry (Fig. 7C). The interpolated charge was close to 0, indicating minimal polarization of the ligand (Fig. 7D). Conventional hydrogen bonds exhibited hydrophobicity values between −1 and −2 (Fig. 7E), and the ionizability remained stable with minimal variation (Fig. 7F).
In silico molecular docking analysis with the target protein, crystal structure of the onion lachrymatory factor synthase (5GTE) and the Trehalose ligand, including analysis of hydrogen bonds (H-Bonds) (A), solvent-accessible surface area (SAS) (B), aromatics (C), interpolated charges (D), hydrophobicity (E), ionizability (F), and 2D binding analysis with the target protein (G).
( ±)-α-Pinene showed an affinity of −5.0 kcal/mol, with alkyl bonds at sites LEU B:28, LEU D: 28 and ALA B:27 (Fig. 8G). The docking analysis revealed neutral hydrogen bond interactions in the vicinity of the ligands (Fig. 8A). The solvent-accessible surface (SAS) ranged from 22.5 to 25 Å2 at binding sites interacting with the ligand (Fig. 8B). Aromatic interactions were slightly oriented toward an edge geometry (Fig. 8C), and no interpolated charge was detected (Fig. 8D). Hydrophobicity values near the ligand ranged from 1 to 2 (Fig. 8E), while ionizability remained neutral, indicating no significant variations (Fig. 8F).
In silico molecular docking analysis with the target protein, crystal structure of the onion lachrymatory factor synthase (5GTE) and the ( ±)-α-Pinene ligand, including analysis of hydrogen bonds (H-Bonds) (A), solvent-accessible surface area (SAS) (B), aromatics (C), interpolated charges (D), hydrophobicity (E), ionizability (F), and 2D binding analysis with the target protein (G).
Alloaromadendrene showed an affinity of −6.6 kcal/mol and alkyl bond at sites PRO B:30 and ALA B:27 (Fig. 9G). The docking analysis showed no donor or acceptor reactions in hydrogen bonds (Fig. 9A). The solvent-accessible surface (SAS) was close to 12.5 to 10 Å2 at the ALA B:27 site (Fig. 9B). Aromatic interactions displayed a slight edge orientation (Fig. 9C), and no interpolated charge was detected (Fig. 9D). Hydrophobicity at the ALA B:27 site ranged from 1 to 2, while for other ligands, it varied between −2 and −3 (Fig. 9E). Ionizability near the PRO B:30 site was slightly acidic, suggesting a localized pH effect at these binding sites (Fig. 9F).
In silico molecular docking analysis with the target protein, crystal structure of the onion lachrymatory factor synthase (5GTE) and the Alloaromadendrene ligand, including analysis of hydrogen bonds (H-Bonds) (A), solvent-accessible surface area (SAS) (B), aromatics (C), interpolated charges (D), hydrophobicity (E), ionizability (F), and 2D binding analysis with the target protein (G).
Caryophyllene showed an affinity of −6.5 kcal/mol, with alkyl bonds at sites ALA D:27 and LEU B:28 (Fig. 10G). The docking results showed no reactions involving hydrogen bonds (Fig. 10A). The solvent-accessible surface area (SASA) was predominantly between 22.5 and 25 Å2 at the binding sites (Fig. 10B). Aromatic interactions were slightly oriented toward an edge geometry (Fig. 10C), and the interpolated charge remained close to 0, indicating minimal electronic polarization (Fig. 10D). Hydrophobicity values ranged from 1 to 2 at the binding sites, while ionizability remained neutral in proximity to the ligand (Fig. 10F).
In silico molecular docking analysis with the target protein, crystal structure of the onion lachrymatory factor synthase (5GTE) and the (-)-β-caryophyllene ligand, including analysis of hydrogen bonds (H-Bonds) (A), solvent-accessible surface area (SAS) (B), aromatics (C), interpolated charges (D), hydrophobicity (E), ionizability (F), and 2D binding analysis with the target protein (G).
Discussion
Salt-induced stress in plants is known in agriculture and plant sciences to play a major role in soil by negatively impacting ion levels, which results in oxidative damage. Essential oils from plants origin containing a variety of compounds including terpenes and phenols can potentially be helpful in managing saline stress. In the current study, essential oil of Cordia verbenacea (EOCv) exhibited a variety of chemical components including α-pinene as the major component, representing 33.81% of the composition. Other components like Caryophyllene with a proportion of 15.61%, and Tricyclo 2.2.1 (2,6) heptane, with a proportion of 14.55% were also represented. According to Xanthis et al. (2021)34, α-pinene demonstrated moderate activity in the scavenging of DPPH and ABTS radicals, with 47.9 ± 2.78% and 49.28 ± 3.55% inhibition, respectively. Also, β-Caryophyllene was defined as an antioxidant by Ames-Sibin et al. (2018)35. Several studies have highlighted the beneficial role of the cell wall in response of the plant to salt stress36,37,38.
The study by Ugras et al. (2024)39 demonstrated the protective effects of essential oil (EO) components from Origanum onites on Allium cepa root cells subjected to lead nitrate-induced toxicity. Among the evaluated EO constituents, α-pinene exhibited the strongest protective activity. Similarly, EO from Campomanesia xanthocarpa, with β-caryophyllene as its major constituent (8.87%), displayed genotoxic and antiproliferative properties when tested in infusions using the in vivo root tip assay of Allium cepa40.
It is important to note that the study of essential oils in plant protection under saline conditions is still an emerging field, with much of the existing research focusing on how salinity affects essential oil production rather than their potential as biostimulants or stress protectants. In a study by Ben Saad et al. (2024)41, the authors demonstrated that Rosmarinus officinalis essential oil enhanced germination and biomass accumulation under salinity, likely due to its α-pinene content (10.1 ± 0.02%). In comparison, in the current study, Cordia verbenacea exhibited a higher α-pinene concentration (33.81%), suggesting potential advantages in stress tolerance. Additionally, research has shown that essential oils from Baccharis dracunculifolia, Schinus terebinthifolius, and Porophyllum ruderale effectively control phytopathogenic fungi, supporting their protective roles in plant health42.
Although limited studies have specifically examined C. verbenacea under saline conditions, its essential oil composition is known to vary based on environmental factors such as seasonal changes, which could influence its bioactivity in salt-stressed environments43,44. For instance, Day (2016) reported that essential oils extracted from different tissues of safflower (Carthamus tinctorius) exhibited differential effects on the germination of wheat, barley, sunflower, and chickpea. Similarly, growing evidence supports the biostimulant role of phenolic compounds and plant extracts in seed germination, rooting, and seedling growth, whether applied as seed treatments, foliar sprays, or soil amendments41,45. Given these findings, further comparative studies on the efficacy of various essential oils under saline conditions are necessary to better assess their potential applications in plant stress management.
The protective characteristics of Cordia verbenacea essential oil (CVE) were observed in leaf and root development, but were not evident during germination, where the treatments showed similar effects. When Allium cepa seeds were exposed to a concentration of 150 mM NaCl, there was a significant change compared to the control group, evidencing the negative impact of NaCl on seed development. This salt stress resulted in reduced root growth, reflecting lower elongation and cell division. Salt stress, such as that caused by high NaCl concentrations, interferes with osmosis and water uptake, leading to suppression of cell expansion and, consequently, impaired plant growth6,46,47.
Particularly, increased salt-concentration, can cause water absorption issues due to the osmotic stress that it creates, thereby restricting root elongation and inhibiting cell division. This results in shorter roots and reduced root biomass, impairing the plant’s ability to absorb water and nutrients. Additionally, salt stress leads to the accumulation of reactive oxygen species (ROS), which damage cellular structures and disrupt metabolic processes48,49,50,51. In this context, antioxidants compounds like terpenes found in Cordia verbenacea essential oil may help to maintain the cellular integrity of A. cepa and consequently prevent damage to vital structures of the plant.
The cell wall plays a vital role in determining the shape and function of the cell, serving as the first line of defense against salt stress. Furthermore, salt stress induces water deficiency in plant cells, causing changes in cell turgor pressure. The cell wall provides mechanical resistance to cope with these changes in turgor pressure25. The cell wall acts as a vital support system for plants, helping them endure changes in their environment and cope with osmotic stress37,52.Alterations in key physiological and metabolic mechanisms have adverse repercussions on seed germination and seedling growth, resulting in delayed development of both shoot and root systems, as well as reductions in fresh and dry weight, chlorophyll content, and its synthesis38 .However, our results demonstrated that EOCv did not protect against delayed seed development, with an inhibition similar to that of NaCl, but with a significant difference in relation to the control. This effect was also noted in seed germination.
Salinity poses a significant challenge to crop productivity, as it has a negative impact on both seed germination and seedling growth. This phenomenon affects plant development by disrupting osmosis, imbalancing nutrient channels, and causing ionic toxicity38. This toxicity was evidenced in the phytotoxicity analyses, where all treatments showed significant differences in relation to the control. However, the phytotoxicity rate tends to reduce with increasing EOCv concentration.
The plant transporters existing in the roots play an important role in the absorption of minerals from the soil or hydroponic medium. The uptake of minerals and water is closely related, as both involve these transporters. Among them, aquaporins are of particular importance as they regulate the absorption and transport of water and minerals across cell membranes53. Our results showed that NaCl treatment caused a significant reduction in root length compared to the control, but EOCv in combination with NaCl resulted in increased root length at concentrations of 300 and 500 µg/mL to the control root length.
It was observed that treatment with the essential oil of Cordia Verbenacea (EOCv) resulted in a proportional increase in leaf length as the concentration of EOCv increased. This suggests that EOCv attenuated the impact of NaCl on leaf development, with the concentration of 500 µg/mL showing a significant effect compared to NaCl alone. This trend was also observed when analyzing root length, where the highest concentration of EOCv resulted in greater root development. These results indicate that EOCv possibly provides protection against salt stress caused by NaCl. Our results corroborate with that Chatterjee & Majumder (2010)54 that demonstrated that the exposure of Allium cepa root tips to varying concentrations of NaCl significantly decreased root growth rates, especially at higher concentrations (0.24 and 0.48 M NaCl), which led to notable growth inhibition over 72 h and reduced the mitotic index, indicating an impact on cell division. Additionally, another study revealed that NaCl stress caused chromosomal abnormalities, particularly at elevated levels, along with observable DNA fragmentation and cell death in root meristem cells55,56,57,58.
Trehalose, evidenced at sites GLN B:161, THR B:157, and GLN B:153, established conventional hydrogen bonds. This molecule exhibits remarkable hydrophilicity due to its inability to form internal hydrogen bonds59. This characteristic makes it a valuable molecular, membranous, and proteinaceous preservative46. In situations of dehydration or freezing, trehalose interacts through hydrogen bonds with adjacent macromolecules and membranes, replacing water molecules60. After extreme dehydration, it crystallizes, assuming a vitreous appearance, a distinctive characteristic61,62.
In response to saline stress, plants employ an adaptive strategy to regulate cellular osmotic potential, producing osmolytes or compatible osmoprotectants, such as sugars, among which trehalose stands out63,64. In the current study, the compound α-Pinene established a specific interaction with site LEU B:28, with this interaction classified as an alkyl bond. However, upon examining trehalose, we noticed that, although it also showed bonding with the same site, this interaction was identified as a carbon-hydrogen bond, indicating a different nature of molecular interaction. The presence of this distinction suggests potential implications in the properties and biological functions of these compounds56,65,66,67. Additionally, when investigating Alloaromadendrene, we found that it also established a similar alkyl-type bond with site ALA B:27 as trehalose68,69,70 This similarity in the nature of bonding suggests a possible correlation between the structural properties and molecular interactions of these compounds71,72,73.
The formation of alkyl-alkyl bonds is essential in the construction of molecules, and the stereochemistry of the carbons involved plays a crucial role in determining their structures and properties. Therefore, the development of techniques that allow the formation of these bonds with enantioselective control is of great importance in organic synthesis74,75.
Conclusion
The study highlights the significant potential of Cordia verbenacea essential oil (EOCv) to enhance salt tolerance in onion crops by promoting leaf and root growth and mitigating the adverse effects of salt stress on onion growth. In silico analysis identified alkyl interactions between EOCv compounds such as ( ±)-α-pinene, (-)-β-caryophyllene and alloaromadendrene that may suggest a molecular protective mechanism. However, further biochemical and histochemical investigations are required to elucidate its precise biochemical mechanisms, potential interactions with stress-related metabolic pathways, and long-term implications for plant adaptation in saline environments. Such studies will be essential for developing targeted strategies to enhance plant resilience and promote sustainable agricultural practices in salt-affected regions.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Grime, J. P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 111(982), 1169–1194 (1977).
de Medeiros, R. L. S., de Paula, R. C., de Souza, J. V. O. & Fernandes, J. P. P. Abiotic stress on seed germination and plant growth of Zeyheria tuberculosa. J. For. Res. 34(5), 1511–1522. https://doi.org/10.1007/s11676-023-01608-3 (2023).
Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 167(3), 645–663. https://doi.org/10.1111/j.1469-8137.2005.01487.x (2005).
Okon, O. G. Effect of salinity on physiological processes in plants. In Microorganisms in saline environments: Strategies and functions (eds Giri, B. & Varma, A.) 237–262 (Springer International Publishing, 2019). https://doi.org/10.1007/978-3-030-18975-4_10.
Roy, S. & Chowdhury, N. Salt Stress in Plants and Amelioration Strategies: A Critical. In Abiotic stress in plants Vol. 391 (eds Fahad, S. et al.) (IntechOpen, 2021).
Balasubramaniam, T., Shen, G., Esmaeili, N. & Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants (Basel, Switzerland) 12(12), 2253. https://doi.org/10.3390/plants12122253 (2023).
Ondrasek, G. et al. Salt Stress in Plants and Mitigation Approaches. Plants (Basel, Switzerland) 11(6), 717. https://doi.org/10.3390/plants11060717 (2022).
Ahmed, A., Kurian, J. & Raghavan, V. Biochar influences on agricultural soils, crop production, and the environment: A review. Environ. Rev. 24(4), 495–502. https://doi.org/10.1139/er-2016-0008 (2016).
Liu, B., Soundararajan, P. & Manivannan, A. Mechanisms of Silicon-Mediated Amelioration of Salt Stress in Plants. Plants (Basel, Switzerland) 8(9), 307. https://doi.org/10.3390/plants8090307 (2019).
Liu, D. et al. Biochar and compost enhance soil quality and growth of roselle (Hibiscus sabdariffa L.) under saline conditions. Sci. Rep. 11(1), 8739. https://doi.org/10.1038/s41598-021-88293-6 (2021).
Matias, E. F. et al. Seasonal variation, chemical composition and biological activity of the essential oil of Cordia verbenacea DC (Boraginaceae) and the sabinene. Ind. Crops Prod. 87, 45–53. https://doi.org/10.1016/j.indcrop.2016.04.028 (2016).
Matias, E. F. F. et al. The genus Cordia: Botanists, ethno, chemical and pharmacological aspects. Rev. Bras 25, 542–552. https://doi.org/10.1016/j.bjp.2015.05.012 (2015).
Sertié, J. A., Woisky, R. G., Wiezel, G. & Rodrigues, M. Pharmacological assay of Cordia verbenacea V: Oral and topical anti-inflammatory activity, analgesic effect and fetus toxicity of a crude leaf extract. Phytomed.: Int. J. Phytother. Phytopharmacol. 12(5), 338–344. https://doi.org/10.1016/j.phymed.2003.09.013 (2005).
Martim, J. K. P., Maranho, L. T. & Costa-Casagrande, T. A. Review: Role of the chemical compounds present in the essential oil and in the extract of Cordia verbenacea DC as an anti-inflammatory, antimicrobial and healing product. J. Ethnopharmacol. 265, 113300. https://doi.org/10.1016/j.jep.2020.113300 (2021).
Vettorello, I. et al. Analgesic Efficacy of Cordia Verbenacea-based Gel in the Reduction of Pain Associated with Use of Separator Elastics. Braz. J. Dev. 7(6), 63855–63868. https://doi.org/10.34117/bjdv7n6-661 (2021).
Suri, S. et al. The beneficial effect of Allium Cepa bulb extract on reproduction of rats; A two-generation study on fecundity and sex hormones. PLoS ONE 19(3), e0294999. https://doi.org/10.1371/journal.pone.0294999 (2024).
Mota, T. F. M., Sampaio, A. R., Vasconcelos, M. W. & de Ghisi, N. C. Allium cepa test vs. insecticides: A scientometric and meta-analytical review. Environ. Sci. Poll. Res. Int. 29(28), 42678–42691. https://doi.org/10.1007/s11356-021-15953-5 (2022).
Haq, I., Kumar, S., Raj, A., Lohani, M. & Satyanarayana, G. N. Genotoxicity assessment of pulp and paper mill effluent before and after bacterial degradation using Allium cepa test. Chemosphere 169, 642–650. https://doi.org/10.1016/j.chemosphere.2016.11.101 (2017).
Alam, M. A. et al. Performance valuation of onion (Allium cepa L.) genotypes under different levels of salinity for the development of cultivars suitable for saline regions. Front. Plant Sci. 14, 1154051. https://doi.org/10.3389/fpls.2023.1154051 (2023).
El-Ramady, H. et al. Review of crop response to soil salinity stress: Possible approaches from leaching to nano-management. Soil Syst. 8(1), 11. https://doi.org/10.3390/soilsystems8010011 (2024).
Cuevas, J., Daliakopoulos, I. N., del Moral, F., Hueso, J. J. & Tsanis, I. K. A review of soil-improving cropping systems for soil salinization. Agronomy 9(6), 295. https://doi.org/10.3390/agronomy9060295 (2019).
Usharani, K. V., Roopashree, K. M. & Naik, D. Role of soil physical, chemical and biological properties for soil health improvement and sustainable agriculture. J. Pharmacogn. Phytochem. 8(5), 1256–1267 (2019).
Wei, X. et al. Enhancing soil health and plant growth through microbial fertilizers: Mechanisms, benefits, and sustainable agricultural practices. Agronomy 14(3), 609. https://doi.org/10.3390/agronomy14030609 (2024).
Acosta-Motos, J. R. et al. Towards a sustainable agriculture: Strategies involving phytoprotectants against salt stress. Agronomy 10(2), 194. https://doi.org/10.3390/agronomy10020194 (2020).
Teixeira, P. C., Donagemma, G. K., Fontana, A., & Teixeira, W. G. Manual de métodos de análise de solo. Available at: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1085209 (2017).
Fiskesjö, G. The Allium test as a standard in environmental monitoring. Hereditas 102(1), 99–112. https://doi.org/10.1111/j.1601-5223.1985.tb00471.x (1985).
Li, X., Riaz, M., Song, B. & Liu, H. Phytotoxicity response of sugar beet (Beta vulgaris L.) seedlings to herbicide fomesafen in soil. Ecotoxicol. Environ. Saf. 239, 113628. https://doi.org/10.1016/j.ecoenv.2022.113628 (2022).
Ackley, W. B., Crandall, P. C., & Russell, T. C. The use of linear measurements in estimating leaf areas. (1958).
Ray, R. C. & Singh, R. P. Leaf area estimation in capsicum (Capsicum annuum L.). Sci. Hortic. 39(3), 181–188 (1989).
Astegiano, E. D., Favaro, J. C. & Bouzo, C. A. Estimation of leaf area for several tomatoes varieties using foliar linear measurements. Investig. Agrar.: Prod. y Prot. Veg. 16, 249e256 (2001).
Berman, H. M. et al. The Protein Data Bank. Nucl. Acids Res. 28(1), 235–242. https://doi.org/10.1093/nar/28.1.235 (2000).
Trott, O. & Olson, A. J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31(2), 455–461. https://doi.org/10.1002/jcc.21334 (2010).
Dos Santos, C. A. L. et al. Mentha arvensis oil exhibits repellent acute toxic and antioxidant activities in Nauphoeta cinerea. Sci. Rep. 14(1), 21599. https://doi.org/10.1038/s41598-024-72722-3 (2024).
Xanthis, V. et al. Antioxidant and Cytoprotective Potential of the Essential Oil Pistacia lentiscus var. chia and Its Major Components Myrcene and α-Pinene. Antioxidants (Basel, Switzerland) 10(1), 127. https://doi.org/10.3390/antiox10010127 (2021).
Ames-Sibin, A. P. et al. β-Caryophyllene, the major constituent of copaiba oil, reduces systemic inflammation and oxidative stress in arthritic rats. J. Cell. Biochem. 119(12), 10262–10277. https://doi.org/10.1002/jcb.27369 (2018).
Endler, A. et al. A Mechanism for Sustained Cellulose Synthesis during Salt Stress. Cell 162(6), 1353–1364. https://doi.org/10.1016/j.cell.2015.08.028 (2015).
Houston, K., Tucker, M. R., Chowdhury, J., Shirley, N. & Little, A. The Plant Cell Wall: A Complex and Dynamic Structure As Revealed by the Responses of Genes under Stress Conditions. Front. Plant Sci. 7, 984. https://doi.org/10.3389/fpls.2016.00984 (2016).
Byrt, C. S., Munns, R., Burton, R. A., Gilliham, M. & Wege, S. Root cell wall solutions for crop plants in saline soils. Plant Sci.: Int. J. Exp. Plant Biol. 269, 47–55. https://doi.org/10.1016/j.plantsci.2017.12.012 (2018).
Ugras, S., Rasgele, P. G., Temizce, S., Emire, Z. & Dirmenci, T. Protective Effects of Origanum onites and Its Components on Lead-Nitrate Induced Genotoxicity in Root Cells of Allium cepa L. Rec. Nat. Prod. 18(1), 143–154. https://doi.org/10.25135/rnp.440.2311.2990 (2024).
Pastori, T. et al. Genotoxic effects of Campomanesia xanthocarpa extracts on Allium cepa vegetal system. Pharm. Biol. 51(10), 1249–1255. https://doi.org/10.3109/13880209.2013.786097 (2013).
Ben Saad, R. et al. Rosmarinus officinalis L. essential oil enhances salt stress tolerance of durum wheat seedlings through ROS detoxification and stimulation of antioxidant defense. Protoplasma 261(6), 1207–1220. https://doi.org/10.1007/s00709-024-01965-8 (2024).
Fonseca, M. C. M. et al. Potencial de óleos essenciais de plantas medicinais no controle de fitopatógenos. Rev. Bras. Plant. Med. 17, 45–50 (2015).
Queiroz, T. B., Mendes, A. D. R., Silva, J. C. R. L., Fonseca, F. D. & Martins, E. R. Teor e composição química do óleo essencial de erva-baleeira (Varronia curassavica Jaqc.) em função dos horários de coleta. Rev. Bras. Plant. Med. 18(1 suppl 1), 356–362 (2016).
Day, S. Impact of essential oils obtained from safflower stem and roots on germination and seedling growth of wheat, barley, sunflower and chickpea. Turk. J. Agric. Food Sci. Technol. 4(8), 706–711 (2016).
Kisiriko, M. et al. Phenolics from Medicinal and Aromatic Plants: Characterisation and Potential as Biostimulants and Bioprotectants. Molecules (Basel, Switzerland) 26(21), 6343. https://doi.org/10.3390/molecules26216343 (2021).
Munns, R. & Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59(1), 651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911 (2008).
Safdar, H. et al. A review: Impact of salinity on plant growth. Nat. Sci 17(1), 34–40. https://doi.org/10.7537/marsnsj170119.06 (2019).
Arif, Y., Singh, P., Siddiqui, H., Bajguz, A. & Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem.: PPB 156, 64–77. https://doi.org/10.1016/j.plaphy.2020.08.042 (2020).
Flowers, T. J. & Colmer, T. D. Plant salt tolerance: Adaptations in halophytes. Ann. Botany 115(3), 327–331. https://doi.org/10.1093/aob/mcu267 (2015).
Hossain, M. A. et al. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 6, 420. https://doi.org/10.3389/fpls.2015.00420 (2015).
Acosta-Motos, J. R. et al. Plant responses to salt stress: adaptive mechanisms. Agronomy 7(1), 18. https://doi.org/10.3390/agronomy7010018 (2017).
Devi, E. L. et al. Adaptation strategies and defence mechanisms of plants during environmental stress. Med. Plants Environ. Chall. https://doi.org/10.1007/978-3-319-68717-9_20 (2017).
López-Gómez, M. & Lluch, C. Trehalose and abiotic stress tolerance. In Abiotic stress responses in plants: metabolism, productivity and sustainability (eds Ahmad, P. & Prasad, M. N. V.) 253–265 (Springer New York, 2012). https://doi.org/10.1007/978-1-4614-0634-1_14.
Chatterjee, J. & Majumder, A. L. Salt-induced abnormalities on root tip mitotic cells of Allium cepa: prevention by inositol pretreatment. Protoplasma 245(1–4), 165–172. https://doi.org/10.1007/s00709-010-0170-4 (2010).
Smail, H. O. Impact of different concentration of sodium chloride on the root growth, cell division and chromosomal abnormalities in the root tips of Allium cepa. J. Adv. Lab. Res. Biol. 11(1), 1–6 (2020).
Yang, X. et al. OsTTG1, a WD40 repeat gene, regulates anthocyanin biosynthesis in rice. Plant J. 107(1), 198–214. https://doi.org/10.1111/tpj.15285 (2021).
Chen, F. et al. Effects of Litsea cubeba essential oil on growth performance, blood antioxidation, immune function, apparent digestibility of nutrients, and fecal microflora of pigs. Front. Pharmacol. 14, 1166022. https://doi.org/10.3389/fphar.2023.1166022 (2023).
Bi, Y. et al. Effects of Bacillus subtilis on cotton physiology and growth under water and salt stress. Agric. Water Manag. 303, 109038. https://doi.org/10.1016/j.agwat.2024.109038 (2024).
Paul, S. & Paul, S. Trehalose induced modifications in the solvation pattern of N-methylacetamide. J. Phys. chem. B 118(4), 1052–1063. https://doi.org/10.1021/jp407782x (2014).
Crowe, J. H. Trehalose as a “chemical chaperone”: fact and fantasy. Adv. Exp. Med. Biol. 594, 143–158. https://doi.org/10.1007/978-0-387-39975-1_13 (2007).
Cesaro, A., De Giacomo, O. & Sussich, F. Water interplay in trehalose polymorphism. Food Chem. 106(4), 1318–1328. https://doi.org/10.1016/j.foodchem.2007.01.082 (2008).
Zhang, L. et al. Enhancing Aphid Resistance in Horticultural Crops: A Breeding Prospective. Hortic. Res. https://doi.org/10.1093/hr/uhae275 (2024).
Shahbaz, M., Abid, A., Masood, A. & Waraich, E. A. Foliar-applied trehalose modulates growth, mineral nutrition, photosynthetic ability, and oxidative defense system of rice (Oryza sativa L.) under saline stress. J. Plant Nutr. 40(4), 584–599. https://doi.org/10.1080/01904167.2016.1263319 (2017).
Nawaz, M. et al. Trehalose: A promising osmo-protectant against salinity stress-physiological and molecular mechanisms and future prospective. Mol. Biol. Rep. 49(12), 11255–11271. https://doi.org/10.1007/s11033-022-07681-x (2022).
Wang, N., Zhang, Z., Zhang, Y., Xu, X. & Guan, Q. Fe-Mn oxide activating persufate for the in-situ chemical remediation of organic contaminated groundwater. Sep. Purif. Technol. 355, 129566. https://doi.org/10.1016/j.seppur.2024.129566 (2025).
Zhuang, Q. et al. Catalysis Enhancement of Co3O4 through the Epitaxial Growth of Inert ZnO in Peroxymonosulfate Activation: The Catalytic Mechanism of Surface Hydroxyls in Singlet Oxygen Generation. Cryst. Growth & Des. https://doi.org/10.1021/acs.cgd.4c01357 (2024).
Wang, M., Zhang, S., Li, R. & Zhao, Q. Unraveling the specialized metabolic pathways in medicinal plant genomes: A review. Front. Plant Sci 15, 1459533. https://doi.org/10.3389/fpls.2024.1459533 (2024).
Han, Y. et al. Improving Aerobic Digestion of Food Waste by Adding a Personalized Microbial Inoculum. Curr. Microbiol. 81(9), 277. https://doi.org/10.1007/s00284-024-03796-5 (2024).
Chen, S. et al. TMV-CP based rational design and discovery of α-Amide phosphate derivatives as anti plant viral agents. Bioorganic Chem. 147, 107415. https://doi.org/10.1016/j.bioorg.2024.107415 (2024).
Liu, Y. et al. Beneficial microorganisms: Regulating growth and defense for plant welfare. Plant Biotechnol. J. https://doi.org/10.1111/pbi.14554 (2024).
Zhao, Y. et al. Dimensionality and scales of preferential flow in soils of Shale Hills hillslope simulated using HYDRUS. Vadose Zone J. 23(4), e20367. https://doi.org/10.1002/vzj2.20367 (2024).
Wu, X. & Zhao, Y. A Novel Heat Pulse Method in Determining “Effective” Thermal Properties in Frozen Soil. Water Resour. Res. https://doi.org/10.1029/2024WR037537 (2024).
Zhu, Y., Dai, H. & Yuan, S. The competition between heterotrophic denitrification and DNRA pathways in hyporheic zone and its impact on the fate of nitrate. J. Hydrol. 626, 130175. https://doi.org/10.1016/j.jhydrol.2023.130175 (2023).
He, L. et al. Prediction of bedload transport inside vegetation canopies with natural morphology. J. Hydrodyn. 36(3), 556–569. https://doi.org/10.1007/s42241-024-0033-7 (2024).
Peng, C. et al. Alginate oligosaccharides trigger multiple defence responses in tobacco and induce resistance to Phytophthora infestans. Front.Plant Sci. 16, 1506873. https://doi.org/10.3389/fpls.2025.1506873 (2025).
Acknowledgements
The authors extend their appreciation to King Saud University for funding this work through Researchers Supporting Project number (RSP2025R133), King Saud University, Riyadh, Saudi Arabia.This research was also supported by Ceará Research Support Foundation (FUNCAP), concession BP3-0139-00018.02.00/18.
Author information
Authors and Affiliations
Contributions
Each author participated sufficiently in taking public responsibility for appropriate portions of the content. Study conception and design: CALS and USHD, conceived the idea, designed the experiments and wrote the manuscript, LMB, and AED analyzed the data performed the experiments, MI, AMES, AA, MRA, SRL, and JPK analyzed the data and revised the manuscript. All authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
dos Santos, C.A.L., Damasceno, U.S.H., Barros, L.M. et al. Molecular analysis of the emergence of Allium cepa L. Seeds response to saline stress and treatment with essential oil of Cordia verbenacea. Sci Rep 15, 24389 (2025). https://doi.org/10.1038/s41598-025-94973-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94973-4