Abstract
To use the FDA Adverse Event Reporting System (FAERS) database to identify drugs associated with orthostatic hypotension. Adverse event reports of orthostatic hypotension from Q1 2004 to Q3 2024 were obtained from the FAERS and JADER databases. We employed algorithms such as the reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network (BCPNN), and the multi-item gamma Poisson shrinker (MGPS) for signal detection. JADER database was used to validate the findings from FAERS analysis. We identified 15,737 adverse events associated with orthostatic hypotension, involving 15,480 patients for analysis. The patient demographic included 6,745 males (43.5%) and 7,248 females (46.8%), with the largest group comprising adults over 65 years (7,654 cases, 49.4%). The three drugs with the highest ROR risk signals were terazosin [ROR (95% CI): 153.96 (124.57-190.28)], rasagiline [ROR (95% CI): 37.46 (29.99–46.78)], and doxazosin [ROR (95% CI): 37.06 (31.32–43.86)]. Apomorphine, abalopatine and levodopa were associated with the shortest onset time of orthostatic hypotension. Most of the signal detection results from the FAERS database were verified in the JADER database. Drugs associated with orthostatic hypertension still focused on cardiovascular and nervous system drugs. This study employed the FAERS database to identify 33 drugs that may be potentially linked to orthostatic hypotension. Medical workers should remain vigilant regarding the risk of these drugs causing orthostatic hypotension.
Similar content being viewed by others
Introduction
Orthostatic hypotension (OH) is defined as a decrease in systolic blood pressure of more than 20 mmHg or a decrease in diastolic blood pressure of more than 10 mmHg after standing or tilting for 3 min, and the blood pressure remains in a reduced state1. The clinical manifestations of OH are quite varied, ranging from no significant symptoms to experiencing dizziness, blurred vision, general weakness, fatigue, and cognitive impairment. In severe cases, OH can lead to severely insufficient cerebral perfusion, which in turn can trigger loss of consciousness2,3. OH impacts a notable portion of adults with hypertension, with prevalence estimates reaching up to 10%, and is closely linked to the use of antihypertensive drugs4. A meta-analysis indicated that nearly one in five older adults living in the community are affected by OH5, significantly increasing the risk of falls among this population by threefold6. The falls and syncope associated with OH often hinder patients from exercising, leading to a decline in physical functioning and a severe reduction in overall quality of life7. Due to the insidious symptoms of OH, it has long been overlooked. However, numerous prospective cohort studies have established a connection between OH and various adverse health outcomes, including but not limited to coronary artery disease8, diabetes mellitus9, stroke10, and cognitive decline11. Early identification and intervention for OH may facilitate the optimization of antihypertensive treatment regimens, potentially reducing the necessity for multiple drugs and effectively lowering the risk of subsequent cardiovascular issues12,13.
Under normal physiological conditions, transitioning from a supine to an upright position can result in the gravitational shift of approximately 300–800 mL of blood from the chest to the lower extremity veins. This phenomenon leads to a rapid decrease in central venous blood volume, subsequently decreasing ventricular preload, stroke volume, and mean arterial pressure14. To maintain adequate perfusion pressure for vital organ blood flow, the body swiftly activates sympathetic nerves. This process depends on pressure receptors located in the carotid sinus and aortic arch, which detect changes in blood pressure and transmit afferent nerve impulses to the nucleus tractus solitarius in the brainstem. This action inhibits vasodilatory activity and regulates blood pressure accordingly15.
To better manage OH, it is essential, in the first place, to distinguish between its neurogenic and non-neurogenic forms. The maintenance of stable orthostatic blood pressure is, to a large extent, contingent upon the regulation of the autonomic nervous system. However, in patients with diabetes16, parkinson’s disease (PD)17, or multiple system atrophy18, there is often concomitant autonomic dysfunction. This can disrupt the conduction of the sympathetic reflex arc upon standing, leading to impaired vasoconstriction. This condition is termed neurogenic OH19. It typically exhibits disease-specificity, meaning that the autonomic nerve damage is caused by the disease itself, thereby affecting the stability of orthostatic blood pressure. However, the influence of drugs often leads to the occurrence of non - neurogenic OH20. Research indicates that more than 250 drugs can induce OH, encompassing cardiovascular drugs (such as diuretics, α - blockers, β - blockers) and neurological drugs (including antidepressants, benzodiazepines, and other psychoactive substances)21,22,23. Additionally, a systematic review has pointed out that the use of dopamine receptor agonists can also increase the risk of developing OH24. It is noteworthy that the American Heart Association has clearly stated that when multiple drugs are used in combination, additional synergistic harm may occur, resulting in a cumulative risk of hypotension25. Analyses by IQVIA, a British medical research database, of the risks of OH induced by different drug clusters across various age groups suggest that the overall cumulative risk of combination drug use is influenced by the risk of individual drugs, their mechanisms of action, and dosages26.
Most of the current studies concentrate mostly on the observational studies, case reports, and systematic evaluations, limiting the types of drugs and lacking a comprehensive systematic exploration of drugs that cause OH. The FDA Adverse Event Reporting System (FAERS) compiles adverse drug event (ADE) reports submitted by medical workers, patients, and drug manufacturers. The data from FAERS is extensively utilized in drug safety studies and evaluations27. To extract meaningful safety signals from this extensive dataset, we employed disproportionality analysis to investigate potential associations between reported drugs and ADEs28. We aimed to examine the drugs linked to OH within the FAERS database, provide physicians with a more comprehensive understanding of the safety profiles of these drugs, enable them to make more informed decisions regarding clinical medication administration and ultimately reduce the risk of drug-induced OH in patients.
Materials and methods
Study design
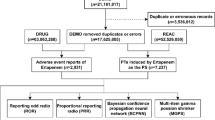
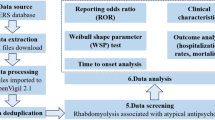
The objective of this study was to analyze the top 50 drugs associated with OH using disproportionate analysis from the FAERS database, in order to identify drugs with positive signals that contribute to OH. Given that the FAERS database compiles ADE reports from various sources, discrepancies in medical expertise between consumers and medical workers may result in potential false positive signals. To address this concern, we conducted subgroup analyses to evaluate this possible bias. In addition, we also conducted a time-to-onset (TTO) analysis for drugs with positive signals.The specific process is shown in Fig. 1.
Data sources and data cleaning
The data for this study was sourced from the FAERS database, which has been publicly accessible since 2004 and features a quarterly update mechanism (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html). A total of 83 quarters of data, spanning from Q1 2004 to Q3 2024, was downloaded and stored in ASCII format. Each quarterly dataset included patient demographics (DEMO), indication (INDI), drug use records (DRUG), therapy duration (THER), adverse event records (REAC), and patient outcomes (OUTC). To process the downloaded ASCII data in R (version 4.3.3), the dataset was initially cleaned in accordance with FDA recommendations for eliminating duplicate reports29. For reports sharing the same CASEID, the one with the latest FDA_DT value was preserved. In instances where both CASEID and FDA_DT were identical, the report with the highest PRIMARYID was retained. Subsequently, the dataset was further refined to include only reports where the target drug was designated as the Primary Suspect (PS), enhancing the reliability of the study. Additionally, reports lacking either a “drug start date” or “drug end date,” as well as those with a drug start date occurring after the ADE report, were excluded from the TTO analysis.
Adverse event reports from Q1 2004 to Q3 2024 were retrieved from the Japanese Adverse Drug Event Report (JADER) database (http://www.pmda.go.jp), which comprises four structured tables: the Demographic Information (DEMO) table containing patient gender and age, the Drug Information (DRUG) table documenting drug names and involvement roles, the Adverse Reaction (REAC) table recording adverse events and outcomes, and the Medical History (HIST) table detailing primary diseases. During data processing, duplicate entries were eliminated using the unique identification numbers in the DEMO table as the primary key, and only reports explicitly annotated with “suspected” drugs in the DRUG table were retained for subsequent analysis.
To standardize drug names, trade names and abbreviations were replaced with their corresponding generic names using the Medex_UIMA_1.3.8 tool developed by Vanderbilt University. Furthermore, this study adhered to the anatomical, therapeutic, and chemical (ATC) classification system, which is published and regularly updated by the World Health Organization’s Centre for the Integration of Methods in Pharmacometrics (https://www.who.int/tools/atc-ddd-toolkit/), for systematic drug classification.
Definition of variables
For the study population, based on the Medical Dictionary for Regulatory Activities (MedDRA) (https://www.meddra.org/), “orthostatic hypotension” was determined as the preferred term. The target population with OH was screened out. Definition of the reporting population: physicians, other health professionals, health professionals, pharmacists, and registered nurses were defined as medical workers. Due to the design architecture of the FAERS database, the same patient was allowed to have multiple ADE records, which may result in various different clinical outcomes. In this study, the most severe clinical outcome was selected as the final outcome.
Statistical analysis
Data processing and statistical analysis were performed using Microsoft Excel and R (4.3.3) software. Signal mining was based on the four-fold table (Table 1), and calculations were carried out using the reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network (BCPNN), and the multi-item gamma Poisson shrinker (MGPS). If a result met the requirements of all four algorithms simultaneously, it was defined as one risk signal. The calculation formulas and judgment criteria are shown in Supplementary Table 1. Signal strength was determined by the ROR value, with larger ROR values indicating stronger signals and a more significant statistical association between the drug and ADE. Subgroup analyses were performed to evaluate potential confounding factors related to ADEs reported by different groups and to validate the results of signal detection using the JADER database. Additionally, the time to onset characteristics of positively signaled drugs associated with OH were assessed using median values, interquartile ranges (IQR), and Weibull shape parameter (WSP) tests.
Results
Baseline characteristics of orthostatic hypotension
A total of 53,463,446 ADE reports were retrieved from the FAERS database, of which 15,737 were associated with OH. After processing, data from 15,480 patients was included for baseline analysis. Regarding gender distribution, the percentage of females (46.8%) surpassed that of males (43.5%), though there was 9.6% missing gender information. In terms of age demographics, 1.4% of patients were under 18 years old (212 cases), 24.8% were aged 18 to 65 years old (3,842 cases), and the highest percentage was of elderly patients over 65 years old (49.4%, or 7,654 cases). The majority of case reports originated from medical workers. Basic information is presented in Table 2.
The number of reported OH events peaked in 2019, with 1,341 cases, but has declined in recent years (Fig. 2A). The reports were primarily concentrated in the United States, France, and the United Kingdom, with a detailed distribution illustrated in Fig. 2B. Regarding clinical outcomes, the most frequent result was hospitalization, comprising 8,521 cases (55%). Serious adverse outcomes were less common, including 660 deaths (4.3%) and 508 cases categorized as life-threatening (3.3%), with a detailed distribution displayed in Fig. 2C.
(A) Annual number of reported adverse drug events related to orthostatic hypotension. (B) Top 8 reporting countries. US: The United States, FR: France, UK: The United Kingdom, CA: Canada, IT: Italy, JP: Japan, AUS: Australia, NL: Netherlands. (C) Proportion of clinical outcomes. HO: hospitalization, OT: other, NA: not available, DE: death, LT: life-threatening, DS: disability, RI: required intervention, CA: congenital anomaly.
Drugs associated with orthostatic hypotension
By counting the ADEs that caused OH, we identified the top 50 drugs that were the prime suspects for causing OH. These drugs included amlodipine (574 cases, 3.71%), levodopa (410 cases, 2.65%), furosemide (391 cases, 2.53%), clozapine (364 cases, 2.35%), tamsulosin (363 cases, 1.77%), terazosin ( 296 cases, 1.72%), ramipril (268 cases, 1.58%), bortezomib (256 cases, 1.51%), sacubitril/valsartan (247 cases, 1.51%), lenalidomide (221 cases, 1.50%), quetiapine (212 cases, 1.45%), bisoprolol (193 cases, 1.43%), apomorphine (185 cases, 1.41%), olanzapine (167 cases, (1) 29%), and duloxetine (148 cases, 1.21%), etc. For details, please refer to Supplementary Table (2) According to the results of the ATC systematic classification, which showed (Fig. 3) that the occurrence of OH was mainly associated with cardiovascular system drugs (2944 cases, 19.02%), followed by nervous system drugs (2279 cases,17.07%), antineoplastic and immunomodulating agents (732 cases,4.73%), genito urinary system and sex hormones drugs (559 cases, 3.61%), systemic hormonal preparations drugs (242 cases, 1.56%), musculo-skeletal system drugs (172 cases, 1.11%), and alimentary tract and metabolism drugs (93 cases, 0.60%), Blood system drugs (58 cases,0.37%).
Drug classification diagram based on ATC classification system. C: cardiovascular system drugs, N: nervous system drugs, L: antineoplastic and immunomodulating agents, H: systemic hormonal preparations drugs, G: genito urinary system and sex hormones drugs, M: musculo-skeletal system drugs, A: alimentary tract and metabolism drugs, B: Blood system drugs.
Disproportionality analysis and subgroup analysis
Disproportionality analysis of the top 50 drugs causing OH showed that 33 of them exhibited positive signals. Ranked according to the intensity of the ROR risk signal, the top 15 drugs were ranked as follows: terazosin [ROR (95% CI): 153.96 (124.57-190.28)], rasagiline [ROR (95% CI): 37.46 (29.99–46.78)], doxazosin [ROR (95% CI): 37.06 (31.32–43.86)], tamsulosin [ROR (95% CI): 33.87 (30.62–37.46)], apomorphine [ROR (95% CI): 28.18 (24.36–32.59)], nitroglycerine [ROR (95% CI): 26.85(21.67–33.25)], droxidopa [ROR (95% CI): 23.32 (20.79–26.17)], furosemide [ROR (95% CI): 18.51 (16.75–20.46)], spironolactone [ROR (95% CI): 15.7(12.93–19.06)], bisoprolol [ROR (95% CI): 15.21 (13.18–17.55)], ramipril [ROR (95% CI): 13.96(12.37–15.76)], diltiazem [ROR (95% CI): 13.49 (11.38-16)], irbesartan [ROR (95% CI): 13.43 (11.09–16.27)], amlodipine [ROR (95% CI): 11.05 (10.22–11.96)], and bortezomib [ROR (95% CI): 10.19 ( 9.01–11.52)]. For details, please refer to Fig. 4 and Supplementary Table 3. A query of FDA-approved drug labels revealed that three drugs (pimavanserin, cisplatin, dexamethasone) did not mention the risk of causing OH.
Based on the reported groups, they were classified into two categories, medical workers and consumers, and the 33 positive drugs were analyzed in subgroups using the ROR method. The results are shown in Fig. 5. OH events caused by abaloparatide [ROR (95% CI):0.56 (0.44–0.81)] were more common among consumers, and its reliability needs to be further verified. However, the vast majority of ADE reports for drugs originate from medical workers, thus ensuring the reliability of the analyzed results.
The top 50 drugs causing orthostatic hypotension in the JADER database
To mitigate the biases associated with incomplete reporting and causality of ADEs in the FAERS database, we collected ADE reports related to OH from the Japanese JADER database since 2004 (Supplementary Table 4). We then conducted signal detection on the top 50 drugs ranked by reporting frequency. The results are presented in Fig. 6. In the analysis of the JADER database, we found that the drugs predominantly associated with OH-related ADEs remained focused on cardiovascular and nervous system drugs. These included α-blockers, anti-Parkinson’s drugs, antipsychotics, β-blockers, and other categories, showing similarities to the drug categories reported in the FAERS database. Further verification revealed that the drug signal results from the majority of drugs in the FAERS database were confirmed in the JADER database, such as terazosin, doxazosin, levodopa, tamsulosin, and carvedilol. However, cisplatin and dexamethasone did not show positive signals in the JADER database, while duloxetine did. Notably, selegiline, the drug with the highest signal strength in the JADER database, and rasagiline in the FAERS database are classified within the same drug category. Through comparative analysis, we contend that the signal detection results in the FAERS database possess a certain level of reliability.
Time-to-onset analysis
After excluding the reports with a large number of missing date values (carbamazepine, nitroglycerin), a TTO analysis was performed on 31 drugs with positive signals. The results (Table 3) showed that the top 3 drugs with the shortest time to cause OH were: apomorphine, 5 days (IQR: 1–14), abaloparatide, 6.5 days (IQR: 3–11.5), and droxidopa, 12 days (IQR: 4–34.5). Conversely, atenolol had the longest median onset time, which was 1048.5 days (IQR: 255–1591). The evaluation results of the WSP analysis showed that abaloparatide, atenolol, bisoprolol, cisplatin, fluoxetine, pimavanserin, ramipril, rasagiline, and spironolactone met the definition of random failure. However, for most of the remaining drugs, the shape parameter β and the upper limit of its 95% confidence interval (CI) were less than 1, indicating that the probability of these drugs causing OH decreased over time.
Discussion
In this study, we conducted a signal mining analysis of drug-induced OH using data from the FAERS database spanning from Q1 2004 to Q3 2024. The correlation between the top 50 drugs and the risk of OH was evaluated, and clinical characteristics such as patient information and clinical outcomes were described. The proportion of serious adverse events caused by OH in this study was relatively low. Among them, the proportion of death cases was 4.3%, and that of life-threatening cases was 3.3%. Therefore, the treatment goals of OH are to reduce symptoms, improve the quality of life, and reduce the incidence of falls and syncope.Given the increasing use of drugs in current clinical practice, drug-induced OH has become an important issue that cannot be ignored and urgently needs to be addressed.
Population characteristics of drug-induced orthostatic hypotension
This study reveals that drug-induced OH events predominantly occur in females and the elderly, a finding consistent with previous research. Specifically, a cross-sectional study involving 4383 elderly individuals indicated that the prevalence of OH was higher in females than in males. Multivariable logistic regression analysis further confirmed an independent association between female gender and OH30. A clinical trial in South Korea also corroborated that females are more prone to OH31. Additionally, a subgroup analysis stratified by age demonstrated that, compared with other age groups, OH patients over 65 years old faced a significantly increased risk of all - cause mortality (RR 1.78; 95% CI 1.25–2.52)32. The results of the AHAP study conducted by Safarpour M et al. showed that the prevalence of OH was higher in females (13.7%) than in males (8.4%), and in the prediction model, female gender and age were identified as important predictors of OH33. Research suggests that, compared with males, females have relatively weaker sympathetic nervous system function, thus having a poorer ability to compensate for hypotension during postural changes34,35. For the elderly population, the decline in cardiac function, increased arterial stiffness, and blunted sympathetic regulation make the elderly more susceptible to OH36,37. This implies that in clinical practice, enhanced monitoring and management of these high - risk groups are necessary, along with a more cautious approach to drug selection.
Analysis of drug signal detection
In this study, the FAERS database was used for the first time to conduct a comprehensive analysis of drug-induced OH. After signal detection of the top 50 drugs, 33 drugs were identified as having a potential correlation with the occurrence of OH risk, while 17 drugs did not meet the definition of risk signals. Most of the associations between drugs and OH identified in this study are supported by multiple lines of evidence from high-quality clinical studies, extensive case reports, or official U.S. FDA approved drug inserts. Previous research has had certain limitations in detailing the specific frequency and risk signal strength of drugs that induce OH, which our study addresses. Furthermore, the reliability of our signaling results is bolstered by the fact that the majority of ADEs reported for these drugs came from medical workers, as evidenced by subgroup analyses.
Top 3 drugs based on reporting frequency
In this study, we identified the top 3 drugs most frequently associated with triggering OH: amlodipine, levodopa, and furosemide. Amlodipine, a commonly used dihydropyridine calcium channel blocker, is widely prescribed for the treatment of hypertension38. This aligns with previous studies that have identified calcium channel blockers (CCBs) as an independent risk factor for OH in elderly hypertensive patients in low-income countries, doubling their risk39. An outpatient survey targeting the elderly population also indicated an increased risk of OH with CCB usage (OR = 1.66, 95% CI: 1.11–2.48)40. Several clinical studies on hypertension treatment have demonstrated a potential risk of inducing OH with amlodipine41,42, possibly due to its vasodilatory effects43.
Levodopa is the most effective drug currently available for treating PD, significantly improving motor symptoms by converting to dopamine through decarboxylation in the brain44. A substantial body of literature suggests that levodopa may trigger or worsen OH in patients with PD45,46,47. A prospective cohort study involving 164 patients found that treatment with levodopa induced OH in 38% of the participants, with those having concurrent autonomic dysfunction being more likely to develop OH48. The underlying mechanism may involve the conversion of levodopa to dopamine in the kidneys, leading to increased urinary sodium excretion and a decrease in blood pressure49.
Furosemide, a potent diuretic, exerts its effects by reducing renal tubular concentrating ability and promoting the excretion of water, sodium, and chloride. A cross-sectional multifactorial analysis indicated that diuretic use is an independent risk factor for developing OH in elderly hypertensive patients50. Additionally, a retrospective study of 342 elderly veterans found that the prevalence of OH in patients treated with Furosemide was as high as 56%51. The mechanism may be related to the reduction in blood volume caused by furosemide52.
Top 3 drugs based on signal strength
A drug exhibiting a stronger risk signal typically signifies a more significant association with a specific ADE during actual use, indicating a higher potential risk53. In this study, the α1 receptor blocker terazosin was identified as having the highest risk signal [ROR (95% CI): 153.96 (124.57-190.28)], while the risk ratios (ROR [95% CI]) for the same class of drugs, doxazosin and tamsulosin, were 37.06 (31.32–43.86) and 33.87 (30.62–37.46), respectively, also indicating a higher risk association. These three drugs are frequently utilized in treating hypertension and benign prostatic hyperplasia. Data from the Eudra-Vigilance (EV) database corroborate our findings54. These α1-blockers function by significantly dilating peripheral blood vessels through the blockade of α1 receptors in vascular smooth muscle. Consequently, when a patient transitions quickly from a sitting or lying position to standing, it becomes challenging to maintain normal blood pressure regulation, leading to OH due to inadequate increases in blood return flow and a corresponding decline in cardiac output per heartbeat55. In our study, the median onset time for terazosin was observed to be just 14 days, indicating a relatively rapid onset of OH. Moreover, it was found that administering a lower dose of terazosin, specifically 5 mg daily, can significantly mitigate the risk of developing OH56. Therefore, in clinical practice, it is advisable to appropriately reduce the initial dose to prevent the onset of OH.
Rasagiline, a monoamine oxidase-B (MAO-B) inhibitor, ranked second in the risk signaling assay of this study [ROR (95% CI) 37.46 (29.99–46.78)]. In patients at the early stages of PD, a randomized controlled trial assessing therapeutic doses of rasagiline at 2 mg and 4 mg per day demonstrated a significant downward trend in blood pressure57. The PRESTO study further revealed that, compared to a placebo, a treatment dosage of 0.5 mg/day of rasagiline led to reductions in both systolic and diastolic blood pressure while standing58. Moreover, a Japanese post-marketing surveillance study indicated that 2.29% of PD patients receiving 1 mg of rasagiline developed OH59, with the prevalence reaching as high as 10% in the study conducted by Poewe W et al.60. These findings are highly consistent with those of the present study, both suggesting a significant association between rasagiline and OH. However, the underlying mechanisms by which rasagiline induces OH remain incompletely understood.
High-risk drug classesbased on the ATC classification
After categorizing and analyzing the top 50 drugs, we identified cardiovascular and nervous system drugs as the primary contributors to drug-induced OH. Within the category of cardiovascular drugs, doxazosin, nitroglycerin, and furosemide stand out due to their significant vasodilatory and diuretic effects, which pose a high risk for OH. Additionally, the use of β-blockers warrants considerable attention. The CRIC study demonstrated that β-blocker use was independently associated with the presence of OH in patients with chronic kidney disease through logistic regression modeling61. Furthermore, the Irish Longitudinal Study on Ageing further indicated that among hypertensive patients treated with β-blockers, significant OH symptoms can be observed 20 s after standing, and this increases the prevalence of OH by twofold62. The underlying mechanism may relate to β-blockers’ inhibition of sympathetic nerve activity, reduction of cardiac output, and disruption of normal vasoconstrictor reflexes63. In the field of nervous system drugs, antipsychotic drugs such as clozapine, quetiapine, and olanzapine have been proven to have OH as one of their common adverse reactions64. A study by Dyer AH et al.65 further revealed that long-term use of antipsychotics doubles the risk of developing OH in patients with Alzheimer’s disease. This adverse effect is explained by the ability of antipsychotics to block α-receptors, leading to vasodilation caused by norepinephrine’s impaired ability to exert its vasoconstrictive effects. Consequently, this vasodilation can result in a significant drop in blood pressure, particularly following sudden changes in body position66.
Time-to-onset analysis of positive signal drugs
We analyzed the TTO of the positive signaling drugs and found that apomorphine boasts a median TTO of just 5 days, making it the drug with the shortest onset time. Apomorphine is commonly employed in the acute treatment of fluctuating motor symptoms in patients with PD. While there has yet to be a dedicated study on the TTO of this medication, it is well-established that the long-term use of apomorphine can lead to OH in PD patients67,68,69. The precise mechanism behind this effect remains somewhat unclear; however, it may be linked to apomorphine’s stimulation of the emetic chemoreceptor area in the medulla oblongata, which triggers vomiting and subsequently reduces blood volume. Consequently, multiple studies have indicated that combining apomorphine with domperidone effectively prevents vomiting and reduces the incidence of OH70,71. Finally, our analysis of the WSP test results revealed that the incidence of most drug-induced OH tends to decrease over time. This significant finding emphasizes the need for clinicians to provide closer follow-up and monitoring for patients in the initial stages following medication administration.
Monitoring and management strategies for high-risk drugs
For patients suspected of having OH, when their clinical manifestations include symptoms such as dizziness, blurred vision, fatigue, and even syncope upon sudden standing, a series of systematic diagnostic measures should be adopted, including but not limited to supine - standing blood pressure measurement, head - up tilt test, and ambulatory blood pressure monitoring. Meanwhile, drug - induced factors should be highly emphasized, and a meticulous review of the types of drugs the patient is taking, any changes in dosage adjustment, and the duration of medication use should be carried out. To optimize the monitoring and management of patients taking high - risk drugs that may induce OH, we put forward the following specific recommendations: 1) Initiating with a low dose and slow titration is essential. For elderly patients or individuals with an inherent tendency towards hypotension, starting with a low initial dose and gradually and cautiously increasing the drug dose is of utmost importance. During this process, close tracking of the patient’s blood pressure changes and symptom manifestations is required. The dose-escalation strategy should be adjusted according to the individual’s tolerance to ensure the safety and effectiveness of treatment. 2)Choose low-risk drugs of the same class. For example, for patients with OH complicated by hypertension, angiotensin - converting enzyme inhibitors and angiotensin II receptor antagonists are recommended as the first choice, as these drugs carry a lower risk of causing OH72. 3) Strengthen risk communication and patient education: Comprehensively and elaborately explain to patients the potential OH risks and related symptoms of the drugs they are taking, enhancing patients’ awareness of the potential adverse drug reactions.
Multifactorial interactions and future directions
Notably, the pathogenesis of OH is far more complex than being solely attributed to drug factors. The disease-specificity and the complexity of drug-drug interactions are potential confounding factors that cannot be overlooked during the assessment of drug - induced OH. From the perspective of disease-specificity, multiple underlying pathological conditions, particularly the autonomic dysfunction associated with neurodegenerative disorders such as PD and multiple system atrophy, can inherently induce OH. When patients with these conditions receive drugs that may exacerbate the risk of OH, accurately discerning whether the symptoms stem from the direct effect of the drug, the pathological changes of the disease itself, or a synergistic effect of both becomes extremely challenging. The complexity of drug-drug interactions also poses challenges to the assessment of drug - induced OH. In clinical practice, elderly patients often need to take multiple drugs simultaneously, and complex interactions may occur among these drugs, affecting metabolism, pharmacodynamics, and blood pressure regulation. For instance, the combined use of antihypertensive drugs and antidepressants may enhance the antihypertensive effect, thus significantly increasing the risk of OH73. These intertwined factors may introduce confounding biases into the dataset of this study, affecting the accurate assessment of the strength of the association between drugs and OH. Future research needs to explore more rigorous study designs. For example, selecting patient populations with relatively homogeneous underlying disease types and drug profiles can reduce confounding effects. Meanwhile, by leveraging large real-world databases in combination with clinical case-control studies, systematically collecting and analyzing patients’ medical histories, drug records, and hypotensive events, and applying advanced statistical techniques such as multivariable analysis, it will be possible to precisely distinguish the independent effects of drugs, the disease itself, and drug-drug interactions on OH.
There are still some limitations in this study: (1) The FAERS database, which relies on a spontaneous reporting system, may be subject to differences in the identities of reporters, under - reporting, and a lack of data such as patients’ basic information and baseline medications. Consequently, this can affect the accuracy and reliability of signal detection; (2) This study did not take into account the impact of underlying diseases and concomitant drugs on signal detection; (3) The majority of the reporting countries are in Europe and America, with relatively few in Asia, suggesting a potential racial bias; (4) The time since a drug’s market launch and the level of media attention it receives can, to some extent, influence the number of ADE reports, potentially introducing certain subjective biases; (5) The detected positive signals indicate a statistical correlation between the drug and ADEs, but the clinical significance of these findings requires further validation through population-based observational studies. Despite these limitations, this research represents the first exploratory analysis using the FAERS database to identify drugs potentially associated with OH.
Conclusion
This study conducted a comprehensive analysis of drug-induced OH signals from the FAERS database between 2004 and 2024. It identified amlodipine, levodopa, and furosemide as the primary drugs responsible for OH. The findings revealed that OH was more prevalent among female and older individuals. Cardiovascular and nervous system drugs were identified as the main contributors to OH. Notably, the incidence of most drug-induced OH cases appeared to decrease over time, highlighting the clinical importance of early detection and monitoring. These insights not only enhance understanding of the pharmacological risk factors associated with OH but also serve as a crucial reference for drug selection and the monitoring of high-risk populations in clinical practice.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
References
Palma, J. A. & Kaufmann, H. Management of orthostatic hypotension. Continuum (Minneap Minn). 26, 154–177 (2020).
Wieling, W. et al. Diagnosis and treatment of orthostatic hypotension. Lancet Neurol. 21, 735–746 (2022).
Magkas, N. et al. Orthostatic hypotension: from pathophysiology to clinical applications and therapeutic considerations. J. Clin. Hypertens. (Greenwich) 21, 546–554 (2019).
Juraschek, S. P. et al. Orthostatic hypotension, hypertension treatment, and cardiovascular disease: an individual participant Meta-Analysis. JAMA 330, 1459–1471 (2023).
Saedon, N. I., Pin Tan, M. & Frith, J. The prevalence of orthostatic hypotension: A systematic review and Meta-Analysis. J. Gerontol. Biol. Sci. Med. Sci. 75, 117–122 (2020).
Alsayed Hassan, D. A., Chivese, T., Syed, M. A. & Alhussaini, N. W. Z. Prevalence and factors associated with falls in older adults in a middle Eastern population: a retrospective cross-sectional study. Public. Health 233, 54–59 (2024).
Christopoulos, E. M., Reijnierse, E. M., Lange, P. W., Meskers, C. G. M. & Maier, A. B. Orthostatic hypotension and orthostatic intolerance symptoms in geriatric rehabilitation inpatients, RESORT. J. Am. Med. Dir. Assoc. 22, 2468–2477 (2021).
Min, M. et al. Orthostatic hypotension and the risk of atrial fibrillation and other cardiovascular diseases: an updated meta-analysis of prospective cohort studies. J. Clin. Hypertens. (Greenwich) 21, 1221–1227 (2019).
D’Ippolito, I., Carlucci, M. A., D’Amato, C., Lauro, D. & Spallone, V. Determinants of orthostatic hypotension in type 2 diabetes: is cardiac autonomic neuropathy the main factor?? Endocr. Pract. 30, 802–809 (2024).
Min, M. et al. The association between orthostatic hypotension and cognition and stroke: a meta-analysis of prospective cohort studies. Blood Press. 29, 3–12 (2020).
Wiersinga, J. H. et al. Orthostatic hypotension and cerebral small vessel disease: A systematic review. J. Cereb. Blood Flow. Metab. 16, 271678X241283226 (2024).
Liang, J. et al. Associations between onset age of orthostatic hypotension and incident myocardial infarction, stroke, and dementia: A prospective cohort study. J. Gerontol. Biol. Sci. Med. Sci. 79, glae087 (2024).
Kaye, M. G. et al. Screening for orthostatic hypotension in the geriatric population in a real-world primary care setting reduces prescribed antihypertensive drugs. Blood Press. Monit. 28, 338–342 (2023).
Freeman, R. et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 21, 69–72 (2011).
Fedorowski, A. et al. Orthostatic hypotension: management of a complex, but common, medical problem. Circ. Arrhythm. Electrophysiol. 15, e010573 (2022).
Kominami, K., Akino, M. & Kanai, M. Efficacy of Neuro-muscular electrical stimulation for orthostatic hypotension associated with Long-term disuse and diabetic autonomic neuropathy: A case report. Phys. Ther. Res. 27, 180–185 (2024).
Grosu, C., Noea, O., Maștaleru, A., Ignat, E. B. & Leon, M. M. Neurogenic orthostatic hypotension in Parkinson Disease-A narrative review of diagnosis and management. J. Clin. Med. 14, 630 (2025).
Zhao, J. et al. Sustained therapeutic effect of spinal cord stimulation on improving severe neurogenic orthostatic hypotension in a patient with pure autonomic failure converting to multiple system atrophy. J. Neurol. 272, 177 (2025).
Idiaquez, J. F., Idiaquez, J., Casar, J. C. & Biaggioni, I. Neurogenic orthostatic hypotension. Lessons from synucleinopathies. Am. J. Hypertens. 34, 125–133 (2021).
Freeman, R. et al. Orthostatic hypotension: JACC State-of-the-Art review. J. Am. Coll. Cardiol. 72, 1294–1309 (2018).
Gibbon, J. R. & Frith, J. Orthostatic hypotension: a pragmatic guide to diagnosis and treatment. Drug Ther. Bull. 58, 166–171 (2020).
Rivasi, G., Rafanelli, M., Mossello, E., Brignole, M. & Ungar, A. Drug-Related orthostatic hypotension: beyond Anti-Hypertensive drugs. Drugs Aging 37, 725–738 (2020).
Pepersack, T. et al. Prevalence of orthostatic hypotension and relationship with drug use amongst older patients. Acta Clin. Belg. 68, 107–112 (2013).
Sako, W. et al. Comparative efficacy and safety of adjunctive drugs to Levodopa for fluctuating Parkinson’s disease - network meta-analysis. NPJ Parkinsons Dis. 9, 143 (2023).
Juraschek, S. P. et al. Orthostatic Hypotension in Adults With Hypertension: A Scientific Statement From the American Heart Association. Hypertension 81, e16-e30 (2024).
Bhanu, C. et al. Drug-induced orthostatic hypotension: cluster analysis of co-prescription patterns in older people in UK primary care. Pharmacoepidemiol Drug Saf. 33, e5730 (2024).
Ahdi, H. S., Wichelmann, T. A., Pandravada, S. & Ehrenpreis, E. D. Medication-induced osteonecrosis of the jaw: a review of cases from the food and drug administration adverse event reporting system (FAERS). BMC Pharmacol. Toxicol. 24, 15 (2023).
Zou, S. P. et al. A disproportionality analysis of adverse events associated to Pertuzumab in the FDA adverse event reporting system (FAERS). BMC Pharmacol. Toxicol. 24, 62 (2023).
Sakaeda, T., Tamon, A., Kadoyama, K. & Okuno, Y. Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10, 796–803 (2013).
Yang, M. et al. Epidemiology and risk factors for orthostatic hypotension and its severity in residents aged > 60 years: A Cross-Sectional study. Int. J. Hypertens. 27, 9945051 (2024).
Kim, K. T. et al. Blood pressure variability and ocular Vestibular-Evoked myogenic potentials are independently associated with orthostatic hypotension. J. Clin. Neurol. 20, 571–579 (2024).
Ricci, F. et al. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur. Heart J. 36, 1609–1617. https://doi.org/10.1093/eurheartj/ehv093 (2015).
Safarpour, M., Fotouhi, A., Hosseini, S. R., Mohamadzade, M. & Bijani, A. Predictors of orthostatic hypotension in the elderly: results from the Amirkola health and ageing project (AHAP) study. J. Tehran Heart Cent. 14, 165–170 (2019).
Evans, J. M. et al. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J. Appl. Physiol. 91, 2611–2618 (2001).
Shoemaker, J. K., Hogeman, C. S., Khan, M., Kimmerly, D. S. & Sinoway, L. I. Gender affects sympathetic and hemodynamic response to postural stress. Am. J. Physiol. Heart Circ. Physiol. 281, H2028–H2035 (2001).
Saz-Lara, A. et al. Association between arterial stiffness and orthostatic hypotension: A systematic review and meta-analysis. Front. Physiol. 14, 1164519 (2023).
Yuan, S. et al. Sympathetic activity is correlated with satellite cell aging and myogenesis via β2-adrenoceptor. Stem Cell. Res. Ther. 12, 505 (2021).
Wang, J. G., Palmer, B. F., Vogel Anderson, K. & Sever, P. Amlodipine in the current management of hypertension. J. Clin. Hypertens. (Greenwich) 25, 801–807 (2023).
Hailu, W., Tesfaye, T., Derseh, L., Hailu, A. & Clarfield, A. M. Prevalence of orthostatic hypotension and associated factors among older people with hypertension in Northern Ethiopia. BMC Geriatr. 24, 928 (2024).
Press, Y., Punchik, B. & Freud, T. Orthostatic hypotension and drug therapy in patients at an outpatient comprehensive geriatric assessment unit. J. Hypertens. 34, 351–358 (2016).
Brown, M. J., McInnes, G. T., Papst, C. C., Zhang, J. & MacDonald, T. M. Aliskiren and the calcium channel blocker amlodipine combination as an initial treatment strategy for hypertension control (ACCELERATE): a randomised, parallel-group trial. Lancet 377, 312–320 (2011).
Weir, M. R., Shojaee, A. & Maa, J. F. Efficacy of amlodipine/olmesartan medoxomil ± hydrochlorothiazide in patients aged ≥ 65 or < 65 years with uncontrolled hypertension on prior monotherapy. Postgrad. Med. 125, 124–134 (2013).
Simpson, M. D. & Cole, J. B. Developments in the epidemiology of calcium channel blocker poisoning and implications for management. Curr. Opin. Crit. Care 30, 603–610 (2024).
Murch, W. L., Spiridigliozzi, J., Heller, A. & Heller, E. Non-invasive, continuous oral delivery of solid levodopa-carbidopa for management of Parkinson’s disease. Sci. Rep. 14, 26826 (2024).
Liu, Z. et al. Acute effect of Levodopa on orthostatic hypotension and its association with motor responsiveness in Parkinson’s disease: results of acute Levodopa challenge test. Parkinsonism Relat. Disord. 115, 105860 (2023).
Earl, T. et al. Effect of Levodopa on postural blood pressure changes in Parkinson disease: a randomized crossover study. Clin. Auton. Res. 34, 117–124 (2024).
Su, D., Zhang, X., Su, Y., Chan, P. & Xu, E. Effects of different Levodopa doses on blood pressure in older patients with early and middle stages of Parkinson’s disease. Heliyon 9, e17876 (2023).
Cani, I. et al. Levodopa-induced orthostatic hypotension in parkinsonism: A red flag of autonomic failure. Eur. J. Neurol. 31, e16061 (2024).
Olivares-Hernández, A. et al. Dopamine Receptors and the Kidney: An Overview of Health- and Pharmacological-Targeted Implications. Biomolecules 11, 254 (2021).
Zhang, Q., Shen, S., Guan, H., Zhang, J. & Chen, X. Orthostatic hypotension is associated with malnutrition diagnosed by GLIM in elderly hypertensive patients. BMC Geriatr. 22, 866 (2022).
oon, I. O. & Braun, U. High prevalence of orthostatic hypotension and its correlation with potentially causative drugs among elderly veterans. J. Clin. Pharm. Ther. 30, 173–178 (2005).
Ordine, L., Losi, M. A., Canciello, G., Borrelli, F. & Esposito, G. Symptomatic orthostatic hypotension due to standing mid-left ventricular obstruction: a case report. Eur. Heart J. Case Rep. 8, ytae566 (2024).
Li, W. et al. Exploring the top 30 drugs associated with drug-induced constipation based on the FDA adverse event reporting system. Front. Pharmacol. 15, 1443555 (2024).
Cicione, A. et al. Adverse events related to alpha-blockers: analysis of real-life data from Eudra-Vigilance. Minerva Urol. Nephrol. 75, 479–485 (2023).
Bakr, A. M. & El-Sakka, A. I. Effects of alpha-1 adrenergic receptor blockers on erectile function: a systematic review. Expert Opin. Pharmacother. 26, 219–226 (2025).
Schultz, J. L. et al. A pilot dose-finding study of Terazosin in humans. medRxiv [Preprint] 22, 05.22.24307622 (2024). (2024).
Stern, M. B. et al. Double-blind, randomized, controlled trial of Rasagiline as monotherapy in early Parkinson’s disease patients. Mov. Disord. 19, 916–923 (2004).
Parkinson Study Group. A randomized Placebo-Controlled trial of Rasagiline in Levodopa-Treated patients with Parkinson disease and motor fluctuations: the PRESTO study. Arch. Neurol. 62, 241–248 (2005).
Hattori, N., Kajita, M., Fujimoto, S., Izutsu, M. & Fernandez, J. Safety and effectiveness of Rasagiline in patients with Parkinson’s disease in Japan: a post-marketing surveillance study. Expert Opin. Drug Saf. 23, 79–88 (2024).
Poewe, W. et al. Efficacy of Rasagiline in patients with the parkinsonian variant of multiple system atrophy: a randomised, placebo-controlled trial. Lancet Neurol. 14, 145–152 (2015).
Rouabhi, M. et al. Orthostatic hypertension and hypotension and outcomes in CKD: the CRIC (Chronic renal insufficiency Cohort) study. Kidney Med. 3, 206–215e1 (2021).
Canney, M. et al. Single agent antihypertensive therapy and orthostatic blood pressure behaviour in older adults using Beat-to-Beat measurements: the Irish longitudinal study on ageing. PLoS One 11, e0146156 (2016).
Monahan, K. D. Effect of aging on baroreflex function in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R3–R12 (2007).
Rivasi, G. & Ungar, A. Orthostatic hypotension in older adults: the role of drugs. Monaldi Arch. Chest Dis. 90, (2020).
Dyer, A. H. et al. Long-term antipsychotic use, orthostatic hypotension and falls in older adults with Alzheimer’s disease. Eur. Geriatr. Med. 15, 527–537 (2024).
Tanzer, T. D. et al. Treatment strategies for clozapine-induced hypotension: a systematic review. Ther. Adv. Psychopharmacol. 12, 20451253221092931 (2022).
Pepe, D. & Do, J. H. Analyzing Apomorphine-Mediated effects in a cell model for Parkinson’s disease with partial least squares structure equation modeling. J. Comput. Biol. 27, 1273–1282 (2020).
Thijssen, E. et al. Clinical trial evaluating apomorphine oromucosal solution in Parkinson’s disease patients. Clin. Transl Sci. 17, e13796 (2024).
Trosch, R. M., Silver, D. & Bottini, P. B. Intermittent subcutaneous apomorphine therapy for ‘off’ episodes in Parkinson’s disease: a 6-month open-label study. CNS Drugs 22, 519–527 (2008).
Bacchi, S., Chim, I., Kramer, P. & Postuma, R. B. Domperidone for hypotension in Parkinson’s disease: A systematic review. J. Parkinsons Dis. 7, 603–617 (2017).
Chen, J. J. Pharmacologic safety concerns in Parkinson’s disease: facts and insights. Int. J. Neurosci. 121, 45–52 (2011).
Whelton, P. K. et al. 2017 ACC/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71, 1269–1324 (2018).
Calvi, A. et al. Antidepressant drugs effects on blood pressure. Front. Cardiovasc. Med. 8, 704281 (2021).
Funding
This work was supported by the National Natural Science Foundation of China Youth Science Foundation (82104677) and Hospital capability enhancement project of Xiyuan Hospital, CACMS. (NO. XYZX0405-05).
Author information
Authors and Affiliations
Contributions
G.R. and X.C. designed the study. X.C., P.H., and L.G. collected the data. G.R., J.Z., and Y.D. downloaded and cleaned the data. G.R., Q.S., and X.M. analyzed the data and generated Figs. 1, 2, 3, 4, 5 and 6; Tables 1, 2 and 3. G.R., X.C., and X.M. wrote the main manuscript text. J.Z., Y.D., and L.G. provided professional advice and participated in result discussions. X.M. and Q.S. supervised the details of the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The data for this study was directly obtained from publicly available information in the US Food and Drug Administration Adverse Event Reporting System (FAERS), and therefore does not require ethics committee approval, nor does it involve the issue of participant consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ren, G., Chi, X., Huang, P. et al. Risk assessment of the top 50 drugs associated with drug-induced orthostatic hypotension: a disproportionality analysis of the FAERS and JADER databases. Sci Rep 15, 10359 (2025). https://doi.org/10.1038/s41598-025-95021-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95021-x