Abstract
Recently, adalimumab has become an important drug frequently used by dermatologists in the treatment of Hidradenitis suppurativa. While there are many publications by rheumatologists about the risk of hepatitis B and tuberculosis reactivation, the literature on reactivation in the treatment of hidradenitis is not extensive. With this study, we wanted to emphasize that adalimumab is a safe drug despite the risk of hepatitis B and tuberculosis reactivation and the importance of porphylaxis during the treatment of hidradenitis suppurativa. In this study, data from 462 HS patients followed up at the Dicle University Dermatology Clinic between 1 January 2017 and 30 June 2024 were retrospectively analyzed. Adalimumab use was detected in 56 of the 462 patients. Patients over 18 years of age and used adalimumab for at least 6 months were selected for this study. Two of these patients were not included in the study because they did not meet the criteria for age and duration of adalimumab use. All the participants were divided into 5 subgroups according to their HBV serological test results: natural immunity, chronic HBV infection, isolated anti-HBcIgG positivity, vaccinated and susceptible. Hepatitis B reactivation was detected only in those with positive anti-HBc IgG (chronic HBV infection, isolated anti-HBcIgG positivity, and natural immunity) tests who were at risk of reactivation. To investigate reactivation, HBV DNA test results before and after biological treatment and ALT-AST levels during the continuation of biological treatment were examined. Patients with a positive HBV DNA test before biological therapy and an increase of > 1 log10İU/ml in the HBV DNA titer during biological therapy and patients with a negative HBV DNA test before biological therapy and a positive HBV DNA test during the biological therapy use period were considered reactivation positive. Patients for whom no HBV DNA test was performed during the study period, those who had one positive HBV DNA test result before or after biological treatment and the other test result was not investigated, and those who had never been checked after starting biological treatment were not included in the reactivation investigation. For tuberculosis reactivation, those who had a latent tuberculosis test (quantitative test), did not report any symptoms and were chronic carriers were included. Among the patients with a positive latent TB test and suspicious clinical and radiological evaluation during biological treatment, those with a positive culture were considered reactivation positive. Of the 12 patients at risk of hepatitis B reactivation during adalimumab treatment, 8 received entecavir, and 4 received tenofovir prophylaxis. No hepatitis B reactivation was observed in any of the 12 patients during adalimumab treatment. Among the 54 patients, 4 were at risk of TB reactivation, and 4 received isoniazid as preophylactic treatment. None of the 4 patients were observed to have TB reactivation. Adalimumab has become a frequently preferred drug in the treatment of hidradenitis, and it is known that there is a risk of hepatitis b and TBc reactivation, which should be prevented. Despite these risks, we found that adalimumab can be safely used to treat hidradenitis suppurativa, especially with the use of prophylaxis.
Similar content being viewed by others
Introduction
Hidradenitis suppurativa (HS) is a chronic, inflammatory dermatosis that usually affects areas of flexural skin with dense hair follicles, such as the axillae, groin and genital area1. Depending on the severity of the disease, recurrent abscesses, nodules, fistulae and scars may occur throughout the disease process2. These can cause pain, bad odors and physical limitations in the affected area and can seriously affect quality of life2. The clinical management of HS and patient compliance are often challenging, and the efficacy and tolerability of current treatment options may be limited. In recent years, biological agents have gained importance in the treatment of dermatologists3. In particular, adalimumab, a TNF-alpha inhibitor, has been evaluated as a promising option for the treatment of HS and has become our treatment of choice in challenging cases of HS. However, the patient’s condition and risks should be carefully evaluated before this treatment is used. Adalimumab has been reported to cause hepatitis and tuberculosis reactivation because of its immunosuppressive effects, similar to those of other biological treatments4,5.
In our study, we aimed to investigate the rate of adalimumab use in HS patients followed up in our clinic and, contrary to other studies, hepatitis b and tuberculosis reactivation due to adalimumab only in HS treatment.
Methods
In this study, data from 462 HS patients followed up at Dicle University Dermatology Clinic between 1 January 2017 and 30 June 2024 were retrospectively analyzed. Adalimumab use was detected in 56 (12.12%) patients. Patients over 18 years of age and 54 (11.69%) patients who used adalimumab for at least 6 months were selected for this study. All the participants were divided into 5 subgroups according to their HBV serological test results: natural immunity, chronic HBV infection, isolated anti-HBcIgG positivity, vaccinated and susceptible. Hepatitis B reactivation was detected only in those with positive anti-HBc IgG (chronic HBV infection, isolated anti-HBcIgG positivity, and natural immunity) tests who were at risk of reactivation. To investigate reactivation, HBV DNA test results before and after biological treatment and ALT-AST levels during the continuation of biological treatment were examined. Patients with a positive HBV DNA test before biological therapy and an increase of > 1 log10İU/ml in the HBV DNA titer during biological therapy and patients with a negative HBV DNA test before biological therapy and a positive HBV DNA test during the biological therapy use period were considered reactivation positive. Patients for whom no HBV DNA test was performed during the study period, those who had one positive HBV DNA test result before or after biological treatment and the other test result was not investigated, and those who had never been checked after starting biological treatment were not included in the reactivation investigation. For tuberculosis reactivation, those who had a latent tuberculosis test (quantitative test), did not report any symptoms and were chronic carriers were included. Among the patients with a positive latent TB test and suspicious clinical and radiological evaluation during biological treatment, those with a positive culture were considered reactivation positive. Data were evaluated with the SPSS-21.0 statistical program, and the value, mean, median value, standard deviation, incidence and frequency of each parameter in all patients were recorded.
Findings
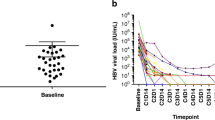
The mean age of the 54 patients receiving adalimumab was 32.11 years; 16 (29.63%) were female, and 38 (70.37%) were male. According to the hepatitis serological results of these patients, 27 (50%) were vaccinated, 15 (27.78%) were not vaccinated, and 12 (22.22%) were naturally immune, as shown in Fig. 1. Among the 12 patients at risk of hepatitis B reactivation during adalimumab treatment, 8 received entecavir, and 4 received tenofovir prophylaxis. No hepatitis B reactivation was observed in any of the 12 patients during adalimumab treatment.
In 54 patients, latent tuberculosis tests revealed that 4 (7.41%) were diagnosed with previous tuberculosis and received isoniazid prophylaxis for 9 months during treatment. No tuberculosis reactivation was detected in any of these 4 patients, as shown in Fig. 2. Although 1 patient was a carrier of both hepatitis B and tuberculosis, no reactivation was observed.
Discussion
Adalimumab, an anti-TNF drug, has recently come to the forefront as an important and frequently used treatment option for HS1,6.
However, owing to its immunosuppressive effects, it has been reported in many studies that it can cause reactivation in patients who are hepatitis B and tuberculosis carriers4,5.
Most of the studies on hepatitis b reactivation during adalimumab treatment have been published on rheumatological diseases.
Toshiyuki Watanabe et al. conducted a study on rheumatological patients and reported that 19 out of 152 patients used adalimumab and were at risk of hepatitis B reactivation. Hepatitis B reactivation was not observed in any of these 19 patients treated with adalimumab. However, unlike our study, detailed information on the use of prophylaxis was not included in this study7.
When the study by S. Piaserico et al. on psoriasis patients was analyzed, 17 patients treated with adalimumab were followed up for the risk of hepatitis B reactivation. None of the 7 patients at risk of reactivation developed reactivation, although they did not receive prophylactic treatment. Unlike in this study, although 10 of the 17 patients were HBsAg positive, no reactivation was observed during prophylactic treatment with adalimumab. This once again emphasizes the importance of prophylactic treatment during adalimumab treatment8.
A case series by D.J. No et al. revealed that adalimumab was preferred for the treatment of psoriasis lesions in 3 hepatitis B patients. Lamivudine was used prophylactically before adalimumab and throughout the treatment. Hepatitis B was controlled in these 3 patients, and no reactivation was detected in any of them. This case series demonstrates once again that prophylactic treatment with adalimumab is a safe choice, with no fear of hepatitis B reactivation during adalimumab treatment9.
When the study by Young Ho Lee et al. on 468 rheumatological patients at risk of hepatitis B reactivation was performed, 95 patients used adalimumab. Hepatitis B reactivation was detected in only one of these 95 patients. While it has been observed that this patient did not receive prophylactic treatment, the importance of prophylactic treatment is emphasized once again10.
-
Another treatment option recommended in international guidelines for the treatment of hidradenitis is infliximab, which shows TNF-alpha inhibitor effect like adalimumab11. If we look at the study conducted by Shunsuke Mori et al. in which 17 publications including 712 patients were analysed, including infliximab and adalimumab, reactivation rates were 2.4% for etanercept therapy, 0.6% for adalimumab, 0% for infliximab, 8.6% for tocilizumab, and 3.3% for rituximab12. The fact that the majority of patients with reactivation did not receive prophylactic treatment once again emphasises the importance of prophylaxis.
-
The study by Pérez-Alvarez et al. on 257 cases showed that the hepatitis B reactivation rate was 5% and that infliximab caused a higher rate of hepatitis B reactivation than adalimumab. Other information obtained in this study is that the percentage of reactivation was higher in patients previously treated with immunosuppressive agents (96% vs. 70%, p = 0.033) and lower in those who received antiviral prophylaxis (23% vs. 62%, p = 0.003)13. This once again emphasises the importance of prophylactic treatment like the data obtained in our study.
-
In the study conducted by Young Ho Lee et al. on 122 hepatitis B reactivation risk cases, TNF-alpha inhibitor etanercept was used in 56 cases, adalimumab in 25 cases and infliximab in 14 cases14. Hepatitis B reactivation was observed in 6 patients using TNF-alpha inhibitors, 4 of whom used etanercept and 2 of whom used infliximab. Antiviral prophylactic treatment was given only in one patient using etanercept. However, the American Gastroenterological Association recommends antiviral prophylaxis for patients at risk of hepatitis B reactivation during immunosuppressive therapy and for up to 6 months after discontinuation15.
Considering all these studies, although adalimumab has a risk of hepatitis B reactivation, this risk is very low and can be completely prevented with prophylactic treatment. However, when the literature is reviewed, the overwhelming majority of the studies are on rheumatological diseases and psoriasis. Studies on the risk of hepatitis B reactivation with adalimumab use during the treatment of hidradenitis suppurativa are very rare. With our study, we wanted to fill this large gap in the literature and contribute to future studies. Our study revealed that adalimumab is a safe choice in terms of hepatitis B reactivation in the treatment of hidradenitis and can be completely prevented with prophylactic treatment.
It has been reported in the literature that reactivation may develop with the use of adalimumab in latent tuberculosis carriers5.
In the study by Abdullah Al-Sohaim et al., 410 patients receiving adalimumab treatment, including only 6 patients with hidradenitis suppurativa, were investigated for tuberculosis reactivation. A total of 26 of 410 patients, the majority of whom were rheumatological patients, were at risk for tuberculosis reactivation, and all 26 of these patients had rheumatological diseases. All 26 patients received isoniazid prophylaxis, and no reactivation was observed in any of them. In parallel with our study, this study once again emphasized the importance of prophylaxis in preventing tuberculosis reactivation, but unlike our study, it cannot clearly demonstrate the risk during the treatment of hidradenitis suppurativa16.
In contrast to our study, the case of Freddy Caldera et al. involved a patient with Crohn’s disease who was at risk of tuberculosis recurrence and developed tuberculosis reactivation despite receiving prophylactic treatment17. However, since our study focused on hidradenitis suppurativa, risk similarity in terms of tuberculosis reactivation cannot be predicted.
When Ariana Ellis et al. examined hidradenitis suppurativa patients, 170 HS patients receiving biological treatment were included in the study. In the present study, reactivation was observed in 4 of the 9 high-risk patients, and 1 of them used adalimumab18. However, it is impossible to comment on adalimumab since it is not specified in detail which biologics all 9 risky patients received. Although our study was similarly based on patients with Hidradenitis suppurativa, unlike this study, tuberculosis reactivation was investigated only in patients treated with adalimumab. This is another piece of data that makes our study valuable.
When the literature is reviewed, to our knowledge, our study is the first study on tuberculosis reactivation in hidradenitis suppurativa patients receiving adalimumab treatment.
Conclusion
The TNF-alpha inhibitor adalimumab has recently been shown to be a safe and effective choice for the treatment of suppurative hydradenitis. In addition, HBV and latent TB screening should be performed before starting adalimumab treatment, and antiviral prophylaxis should be applied if necessary.
Of course, not only hepatitis B and tuberculosis but also other infectious agents should be taken into consideration in pre-treatment planning. Before starting adalimumab, the vaccination history should be reviewed, missing vaccines should be completed and, if possible, live-attenuated vaccines such as herpes zoster should be administered at least 4 weeks before the start of treatment19,20. If the patient is in the process of treatment, live vaccines should be strictly avoided and non-living vaccines (Shingrix®) should be preferred among both live (Zostavax®) and non-living vaccine (Shingrix®) options such as herpes zoster21,22. The current EULAR (European League Against Rheumatism) guideline on vaccination programme can be taken into consideration23.
In addition, hepatitis screening every 6 months and latent TB screening every year should not be neglected. In this way, possible complications during the treatment process can be minimized, early diagnosis and treatment opportunities can be obtained, and patient safety can be ensured.
Data availability
The data that support the findings of this study are available from the Dicle University Faculty of Medicine, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available, however, from the corresponding author upon reasonable request and with permission of the Dicle University Faculty of Medicine.
References
Cotton, C. H., Chen, S. X., Hussain, S. H., Lara-Corrales, I. & Zaenglein, A. L. Hidradenitis Suppurativa in Pediatric Patients. Pediatrics. ;151(5):e2022061049. (2023). https://doi.org/10.1542/peds.2022-061049. PMID: 37102307.
Goldburg, S. R., Strober, B. E. & Payette, M. J. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J. Am. Acad. Dermatol. 82 (5), 1045–1058. https://doi.org/10.1016/j.jaad.2019.08.090 (2020). Epub 2019 Oct 9. PMID: 31604104.
Maronese, C. A., Moltrasio, C., Genovese, G. & Marzano, A. V. Biologics for hidradenitis suppurativa: evolution of the treatment paradigm. Expert Rev. Clin. Immunol. 20 (5), 525–545 (2024). Epub 2024 Jan 2. PMID: 38130204.
Mori, S. & Fujiyama, S. Hepatitis B virus reactivation associated with antirheumatic therapy: risk and prophylaxis recommendations. World J. Gastroenterol. 21 (36), 10274–10289. https://doi.org/10.3748/wjg.v21.i36.10274 (2015). PMID: 26420955; PMCID: PMC4579875.
Robert, M. & Miossec, P. Reactivation of latent tuberculosis with TNF inhibitors: critical role of the beta 2 chain of the IL-12 receptor. Cell. Mol. Immunol. 18 (7), 1644–1651. https://doi.org/10.1038/s41423-021-00694-9 (2021). Epub 2021 May 21. PMID: 34021269; PMCID: PMC8245521.
Kimball, A. B. et al. Adalimumab for the treatment of moderate to severe Hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. ;157(12):846 – 55. (2012). https://doi.org/10.7326/0003-4819-157-12-201212180-00004. PMID: 23247938.
Watanabe, T. et al. Incidence and risk factors for reactivation from resolved hepatitis B virus in rheumatoid arthritis patients treated with biological disease-modifying antirheumatic drugs. Int. J. Rheum. Dis. 22 (4), 574–582 (2019). Epub 2018 Oct 18. PMID: 30338649.
Piaserico, S., Dapavo, P., Conti, A., Gisondi, P. & Russo, F. P. Adalimumab is a safe option for psoriasis patients with concomitant hepatitis B or C infection: a multicenter cohort study of 37 patients and review of the literature. J. Eur. Acad. Dermatol. Venereol. 31 (11), 1853–1859. https://doi.org/10.1111/jdv.14146 (2017). Epub 2017 Feb 21. PMID: 28146345.
No, D. J., Amin, M. & Wu, J. J. Adalimumab use in patients with psoriasis and hepatitis B: a case series. J. Eur. Acad. Dermatol. Venereol. 31 (12), e548–e550. https://doi.org/10.1111/jdv.14416 (2017). Epub 2017 Jul 12. PMID: 28622425.
Lee, Y. H., Bae, S. C. & Song, G. G. Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy. Clin. Exp. Rheumatol. 2013 Jan-Feb ;31(1):118–121. Epub 2012 Oct 30. PMID: 23111095.
Br, J. & Dermatol, V. 180, Issue: 5, Pages: 1009–1017, First published: 15 December 2018, DOI: (10.1111/bjd.17537).
Mori, S. & Fujiyama, S. Hepatitis B virus reactivation associated with antirheumatic therapy: risk and prophylaxis recommendations. World J. Gastroenterol. 21 (36), 10274–10289. https://doi.org/10.3748/wjg.v21.i36.10274 (2015).
Pérez-Alvarez, R. et al. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Med. (Baltim). 90 (6), 359–371. https://doi.org/10.1097/MD.0b013e3182380a76 (2011).
Lee, Y. H., Bae, S. C. & Song, G. G. Hepatitis B virus reactivation in HBsAg-positive patients with rheumatic diseases undergoing anti-tumor necrosis factor therapy or DMARDs. Int. J. Rheum. Dis. 16 (5), 527–531. https://doi.org/10.1111/1756-185X.12154 (2013).
ReddyKR et al. American gastroenterological association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy [published correction appears in gastroenterology. 2015;148(2):455. Multiple investigator names added]. Gastroenterology 148 (1), 215–e17. https://doi.org/10.1053/j.gastro.2014.10.039 (2015).
Al-Sohaim, A. et al. The risk of tuberculosis infection in 410 Saudipatients receiving adalimumab therapy. Ann. Saudi Med. 41 (5), 285–292. https://doi.org/10.5144/0256-4947.2021.285 (2021 Sep-Oct). Epub 2021 Oct 7. PMID: 34618606; PMCID: PMC8497010.
Caldera, F., Grimes, I. & Soni, A. Reactivation of latent tuberculosis in a Crohn’s patient after TB prophylaxis treated with adalimumab. Am J Gastroenterol. ;108(7):1181-2. (2013). https://doi.org/10.1038/ajg.2013.144. PMID: 23821003.
Ellis, A., Khanna, U., Galadari, A. & Fernandez, A. P. Conversion to positive latent tuberculosis infection status is low in patients with hidradenitis suppurativa taking biologic medications. J. Am. Acad. Dermatol. 83 (1), 246–248 (2020). Epub 2020 Jan 12. PMID: 31940465.
Rahier, J. F. et al. Vaccinations in patients with immune-mediated inflammatory diseases. Rheumatol. (Oxford). 49 (10), 1815–1827. https://doi.org/10.1093/rheumatology/keq183 (2010).
Ciccarese, G. et al. Anti-Herpes Zoster vaccination in patients with dermatologic diseases: a position statement from the Italian sidemast group of sexually transmitted, infectious and tropical diseases. Ital. J. Dermatol. Venerol. 159 (4), 375–379. https://doi.org/10.23736/S2784-8671.24.07895-2 (2024).
Winthrop, K. L. et al. Association between the initiation of anti-tumor necrosis factor therapy and the risk of herpes Zoster. JAMA 309 (9), 887–895. https://doi.org/10.1001/jama.2013.1099 (2013).
Maltz, F., Fidler, B. & Shingrix A new herpes Zoster vaccine. P T. 44 (7), 406–433 (2019).
Furer, V. et al. 2019 Update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 79 (1), 39–52. https://doi.org/10.1136/annrheumdis-2019-215882 (2020).
Acknowledgements
The authors acknowledge the patients included in this study, their clinicians and service providers.
Funding
No sources of funding were used to assist in the preparation of this letter.
Author information
Authors and Affiliations
Contributions
Ömer Karakoyun conceived of the presented idea. Ömer Karakoyun and Erhan Ayhan developed the theory and performed the computations. They verified the analytical methods together. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by institutional review boards at the University of Dicle. All participants provided informed consent by checking a box on a self-administered online informed consent form indicating their agreement to participate in the study. All stages of the research were carried out in accordance with the current guidelines and regulations of ethics in research with human beings.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Karakoyun, Ö., Ayhan, E. Risk of tuberculosis and hepatitis B reactivation during adalimumab use in the treatment of hidradenitis suppurativa. Sci Rep 15, 33737 (2025). https://doi.org/10.1038/s41598-025-95096-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95096-6