Abstract
The effect of prehospital advanced airway management (AAM) in patients with out-of-hospital cardiac arrest requiring extracorporeal cardiopulmonary resuscitation remains unclear. In this retrospective study, we analysed data from the Study of Advanced Cardiac Life Support for Ventricular Fibrillation with Extracorporeal Circulation in Japan II, encompassing 36 institutions in Japan (2013–2018). Patients were divided into two groups: those who received prehospital AAM and those who did not. The primary outcome was a favourable neurological outcome at discharge, and secondary outcomes included 30-day survival, estimated low-flow time, duration of mechanical ventilation, and length of stay in the intensive care unit. A generalised estimating equation model adjusted for prehospital confounders was used for between-group comparisons. Sensitivity analysis was performed using propensity score matching. Among 1,789 patients, those who received prehospital AAM had significantly lower favourable neurological outcomes at discharge, lower 30-day survival, longer low-flow time, shorter mechanical ventilation days, and shorter intensive care unit stay days than those who did not receive prehospital AAM. Propensity score-matched analysis confirmed these findings. Prehospital AAM in patients with out-of-hospital cardiac arrest undergoing extracorporeal cardiopulmonary resuscitation was associated with poorer clinical outcomes. Further studies are needed to optimise prehospital care for these patients.
Similar content being viewed by others
Introduction
Out-of-hospital cardiac arrest (OHCA) is a major public health concern with high morbidity and mortality rates. Over 356,000 OHCA cases occur annually in the United States, with approximately 120,000 in Japan1,2. Survival rates after OHCA remain low worldwide3. To improve outcomes, the use of extracorporeal cardiopulmonary resuscitation (ECPR), which involves veno-arterial extracorporeal membrane oxygenation (ECMO), has become widespread4.
In Japan, managing OHCA typically involves either bag-valve mask (BVM) ventilation or advanced airway management (AAM) using supraglottic airway devices or endotracheal intubation. Although BVM ventilation is simple, it carries risks, such as stomach inflation, reflux, aspiration, and difficulty in maintaining an effective seal3,5,6 AAM may help mitigate these risks.
Furthermore, BVM ventilation requires interruptions in chest compressions, with reports indicating that approximately half of the interruptions during cardiopulmonary resuscitation (CPR) are owing to BVM ventilation, with 29% of the interruption time dedicated to it7. AAM can reduce these interruptions, potentially improving cerebral blood flow during CPR. However, AAM complications, including tube misplacement, aspiration, regurgitation, airway injury, and time consumption have been noted3,8,9,10.
For patients receiving ECPR, minimising low-flow time with appropriate chest compressions is essential, as shorter low-flow time is associated with better clinical outcomes11.
Given these factors, whether prehospital AAM benefits patients with OHCA requiring ECPR treatment is a crucial question, yet evidence is limited. This study aimed to determine the effect of prehospital AAM on the outcomes of patients who undergo ECPR.
Methods
Study design and setting
This was a secondary examination of a nationwide, retrospective, multicentre registry—the Study of Advanced Cardiac Life Support for Ventricular Fibrillation with Extracorporeal Circulation in Japan (SAVE-J) II12. The study analysed the effect of prehospital AAM on the outcomes of patients resuscitated with ECPR across 36 institutions in Japan. The SAVE-J II, conducted from 2013 to 2018, aimed to examine the effectiveness of ECPR on clinical outcomes.
Ethics statement
This study adhered to the 1964 Declaration of Helsinki and its amendments and received approval from the Institutional Review Boards of the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000036490, registered date: April 12th, 2019) and Tokyo Medical and Dental University (M2019-018). The requirement for informed consent was waived owing to the retrospective design of the study and the use of anonymised patient and hospital data.
Study population
The inclusion criteria were as follows: (1) age ≥ 18 years and (2) OHCA treated with ECPR. Exclusion criteria included ECMO implementation after intensive care unit (ICU) admission, cardiac arrest owing to external causes (e.g., trauma, accidental hypothermia, asphyxia, drowning, toxicity, heat stroke), and missing data on the type of airway management.
Data collection
Clinical patient data and hospitalisation medical records were obtained from the SAVE-J II database. The following information was retrospectively collected from the patients’ medical records: age, sex, witnessed status, incidence of bystander CPR, initial cardiac rhythm, prehospital procedure, arterial partial pressure of oxygen (paO2) level at hospital arrival, duration of mechanical ventilation, length of ICU and hospital stay, 30-day survival rate, and cerebral performance category score at hospital discharge. Furthermore, we collected data on signs of life at hospital arrival, which comprised spontaneous respiration/gasping, any body movement, and bilateral pupillary reflexes12.
Definitions and outcomes
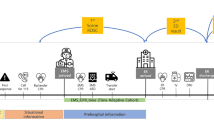
The time from emergency medical service (EMS) activation to hospital admission was defined as the time from EMS call to patient arrival at the hospital. The time from EMS activation to ECMO initiation was defined as the time from EMS call to ECMO initiation. The time from arrival at the scene to ECMO initiation was defined as the time from EMS arrival at the scene of occurrence to the time of ECMO initiation. Estimated low-flow time was defined as the time from (1) cardiac arrest to ECMO initiation if cardiac arrest occurred in front of the EMS crew, (2) EMS call to ECMO initiation in patients with bystander CPR, or (3) EMS arrival to ECMO initiation in patients without bystander CPR if cardiac arrest did not occur in front of the EMS crew. Estimated no-flow time was defined as the time from EMS call to arrival in the case of patients without bystander CPR. The estimated no-flow time was considered zero min in patients who received bystander CPR. In this study, return of spontaneous circulation (ROSC) was defined as at least one minute of sustained and confirmed pulsation13.
Prehospital AAM is an invasive technique used for airway management, including endotracheal intubation or use of supraglottic airway devices. In the Japanese EMS system, paramedics are authorized to perform epinephrine administration and prehospital AAM, such as endotracheal intubation or supraglottic airway device insertion, for OHCA patients. Furthermore, paramedics must seek instructions from the local medical control doctor and council before performing these treatments14.
The primary outcome was favourable neurological status at discharge, defined as a Cerebral Performance Category score of 1 or 2. Secondary outcomes included 30-day survival, estimated low-flow time, duration of mechanical ventilation (days), and length of ICU stay.
Statistical analysis
Patients were divided into AAM and no AAM groups, and the differences in baseline characteristics and outcomes between the two groups were evaluated. Furthermore, the AAM was divided into two subgroups (endotracheal intubation and supraglottic airway devices) to analyse their outcomes separately. In univariate analysis, we used the Student’s t test or Mann–Whitney U test to compare continuous variables and the χ2 test or Fisher’s exact to compare categorical variables as appropriate. Categorical variables are reported as numbers (percentages), whereas continuous variables are reported as means (standard deviations) or medians (interquartile [IQR] ranges), as appropriate. Multivariable logistic regression analyses were also performed to determine whether AAM was associated with study outcomes after adjusting for potential confounders. The models were adjusted for the following variables that were not influenced by prehospital AAM: age, incidence of witnessed cardiac arrest, bystander CPR, initial rhythm at the scene, epinephrine administration, and estimated no-flow time, which were selected based on clinical perspective and previous reports15,16,17,18. The Kolmogorov–Smirnov test and quantile-quantile plot were used to assess whether a dataset of outcomes followed a normal distribution. We also performed a sensitivity analysis using a propensity score matching method to create matched groups: AAM and no AAM. Propensity scores were calculated using logistic regression analysis, which included variables from the mixed-effects model. The nearest neighbour method was used with a calliper of 0.25 (i.e., the largest allowable difference in the propensity scores of matched participants was 25%) to match the logit-transformed propensity scores at a ratio of 1:1 between the two groups. The absolute standardised mean difference was used to assess the matching balance for the different variables between the two groups, and matching was considered acceptable at values < 0.1. Inter-group comparisons of the outcomes among the propensity score-matched subjects were performed using the χ2 test.
Statistical significance was set at a two-sided p-value < 0.05. All statistical analyses were performed using R (version 4.3.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
Figure 1 shows the patient selection process. Of the 2157 potentially eligible patients, 1789 who received ECPR in the emergency department were included in this analysis. Among them, 817 (45.7%) and 972 (54.3%) patients were in the AAM and no AAM groups, respectively.
The baseline characteristics of the two groups are summarised in Table 1. The median age of all participants was 61.0 (IQR, 50.0–69.0) years, with 83.3% being men. In the AAM group, endotracheal intubation was used in 23.9% of cases, and supraglottic airway devices were used in 76.1%.
The rates of witnessed arrests (75.5% vs. 81.9%, p = 0.001) and bystander CPR (55.9% vs. 61.6%, p = 0.015) were significantly lower in the AAM group. Although the rate of initial shockable rhythm was similar in both groups, the rates of prehospital defibrillation (66.4% vs. 56.8%, p < 0.001) and epinephrine administration by EMS crew (53.7% vs.21.8%, p < 0.001) were significantly higher in the AAM group. All signs of life were significantly less prevalent in the AAM group. PaO2 level on hospital arrival was significantly higher in the AAM group (69 mmHg vs. 57 mmHg, p = 0.002). In the AAM group, paO2 level was significantly higher in the endotracheal intubation subgroup than in the supraglottic airway subgroup (86.5 mmHg vs. 64.2 mmHg, p < 0.001). There was no significant difference in no-flow time between both groups. The median time from EMS activation to patient hospital arrival (33 [IQR, 28–41] min vs. 30 [IQR, 25–37] min; p < 0.001) and to ECMO initiation (58 [IQR, 49–70] min vs. 56 [IQR, 46–70] min, p = 0.002) were significantly longer in the AAM group, although there was no significant difference regarding the median time from hospital arrival to ECMO initiation between both groups (22 [IQR, 16–33] min vs. 23 [IQR, 16–35] min, p = 0.224).
The analysis results of the primary and secondary outcomes in both groups are shown in Table 2. Compared with the no AAM group, the rates of favourable neurological outcomes at hospital discharge (10.2 vs. 17.7%, p < 0.001) and 30-day survival (24.1 vs. 29.6%, p = 0.009) were significantly lower in the AAM group. The duration of mechanical ventilation (2 [IQR, 1–9] days vs. 4 [IQR, 1–11] days, p < 0.001) and length of ICU stay (2 [IQR, 1–9] days vs.4 [IQR, 1–11] days, p < 0.001) were significantly shorter in the AAM group. The estimated low-flow time was significantly longer in the AAM group (59 min [IQR, 49–71] vs. 55 min [IQR, 44–69], p < 0.001). After adjusting for potential confounders, prehospital AAM was associated with significantly lower odds of favourable neurological outcomes at hospital discharge (adjusted odds ratio [AOR]: 0.59, 95% confidence interval [CI]: 0.42–0.81; p = 0.001) and a lower, but non-significant, likelihood of 30-day survival (AOR: 0.82, 95% CI: 0.64–1.05; p = 0.120). As datasets regarding the duration of mechanical ventilation, length of ICU stay, and estimated low-flow time were not normally distributed, multivariable logistic regression analysis was not performed.
Propensity score matching generated 719 matched pairs, whose baseline characteristics and outcomes are shown in Table 3 and Supplementary Table 1. The absolute standardised mean difference values for each variable were < 0.1, indicating that a well-matched balance was achieved. The propensity score–matched analysis confirmed the multivariate finding of significantly lower favorable neurological outcomes at hospital discharge in the AAM group. It also revealed a significant reduction in 30-day survival, which was not observed in the multivariate analysis.
Supplementary Table 2 shows the comparison of the time-course and outcomes between the endotracheal intubation (195 patients) and supraglottic device airway insertion (622 patients) subgroups. Although there were no significant differences in the time from EMS activation to ECMO initiation or from patient hospital arrival to ECMO initiation, the time from EMS activation to patient hospital arrival was significantly longer (36 min [28–43] vs. 33 min [IQR, 27–40], p = 0.012). Furthermore, the rate of favourable neurological outcomes at discharge tended to be higher in the endotracheal intubation group than the supraglottic airway device group (13.8% vs. 9.0%; p = 0.057). The differences were not statistically significant for all outcomes.
Discussion
This retrospective multicentre observational study investigated the effect of prehospital AAM on the outcomes of patients with OHCA who underwent ECPR. As a result, the prehospital AAM was significantly associated with lower rates of favourable neurological outcomes at discharge. The AAM group tended to have longer low-flow times and lower 30‐day survival rates, although there was no significant difference between the AAM and no-AAM groups.
While no prior studies specifically addressed the effects of AAM in ECPR patients, several large observational studies have revealed an association between prehospital AAM and clinical outcomes in patients with OHCA19,20,21,22,23,24. However, the results of these studies are controversial, and the clinical benefits of prehospital AAM are still being debated. In contrast with the findings of our study, the findings of a secondary analysis of the Pragmatic Airway Resuscitation Trial, in which the clinical benefit of prehospital AAM was compared with that of BVM ventilation during transportation in patients with OHCA in an RCT, revealed that patients who underwent AAM had higher rates of survival and favourable neurological outcomes than patients who underwent BVM ventilation19. The differences in patient backgrounds between both studies may explain this discrepancy. In our study, the rate of shockable initial rhythms was 63.5%, possibly because all included patients underwent ECPR, whereas the rate was 20.2% in the aforementioned RCT19. Additionally, although the rate of prehospital adrenaline administration was 36.3% in our study, the rate was 93.3% in that study19. Furthermore, the Japanese EMS system, which includes restrictions on the decision to terminate resuscitation attempts in the field, might also have affected clinical outcomes25,26. In the present study, similar to prehospital AAM, no protocol was established for ECPR implementation and intensive care after ECPR implementation. Therefore, the possibility of selection bias affecting the patient population cannot be ruled out. Although the Extracorporeal Life Support Organization guideline has recommended the optimal target population for ECPR27and ECPR administration should be based on the guideline criteria, decision-making for ECPR is often critical and relies on incomplete information (e.g., age and time) in real-world practice. Further prospective studies with strict protocols regarding patient selection and methods of prehospital airway management are needed to elucidate the association between prehospital AAM and the outcomes of patients receiving ECPR. Our study demonstrated that median time from EMS activation to ECMO initiation in the AAM group was two minutes longer than in the no-AAM group, and the median estimated low-flow time in the AAM group was four minutes longer, although paO2 levels were significantly higher in the AAM group. These findings suggest that performing AAM in the prehospital setting could achieve a more effective ventilation but prolong the time to hospital arrival, potentially delaying critical in-hospital interventions such as ECMO or percutaneous coronary intervention. Previous studies have shown that increased low-flow time worsens clinical and neurological outcomes in OHCA patients, indicating that delays caused by prehospital AAM could adversely affect outcomes9,28. It has also been reported that a no-flow time exceeding five minutes significantly correlates with poor neurological prognosis in patients receiving ECPR29,30,31,32. Although a causal relationship between prehospital AAM and long transport time could not be established because of the study design, it is plausible that prehospital AAM contributes to extended transport times, impacting clinical outcomes in patients receiving ECPR. Therefore, for possible ECPR candidates, careful consideration should be given to balancing prehospital procedures to minimize delays in hospital transport, as extended prehospital care times may delay critical in-hospital intervention, including ECPR. Further prospective studies are required to better understand timing optimization and determine the suitable prehospital AAM method, particularly in ECPR candidates.
In addition to the time factors, prehospital AAM during transportation, which could be owing to unstable settings such as car swaying, a limited number of paramedical staff, and limited activity space, could also lead to the interruption of chest compressions and/or unstable chest compressions. A retrospective study reported that prehospital endotracheal intubation led to a median chest compression interruption of 109.5 s, likely due to the procedural demands and required personnel6. Furthermore, reports indicate that interruptions in chest compressions negatively impact prognosis in patients with OHCA33,34and that each additional 5-second pause (no chest compression time) before ECMO cannulation decreased the likelihood of survival or favourable neurological outcomes35. These findings suggest that prolonged chest compression interruptions may have contributed to the worse outcomes, as observed in the AAM group. Furthermore, in some cases of airway difficulty, multiple AAM attempts might lead to the loss of oxygenation and increased transport time36. Although in our study, data were unavailable regarding the number of times AAM was attempted by the EMS crew or regarding the chest compression interruption time, repeated and/or failed intubation attempts could have led to significant interruptions in chest compressions. In addition to the effect of prehospital AAM in patients who are candidates for ECPR, differences in clinical benefits between the methods of AAM (intubation or supraglottic airway) should be discussed. In our subgroup analysis, no significant differences were observed in patient outcomes based on the method of AAM. However, the significantly longer time from EMS activation to patient hospital arrival in the endotracheal intubation group suggests that intubation may be more time-consuming than use of supraglottic devices. In the subgroup analysis, the endotracheal intubation group demonstrated a significantly longer time from EMS activation to hospital arrival, while paO2 levels at hospital arrival were significantly higher. Endotracheal intubation is inherently more challenging than supraglottic airway device insertion and requires training for proficiency9, suggesting that in prehospital care by EMS crew, simple supraglottic airway device insertion could be more effective than endotracheal intubation in patients who require rapid in-hospital interventions. However, it may be clinically challenging for EMS crews to decide whether to prioritise transport and/or which device to use for prehospital AAM. Future research is required to investigate the difference in impact on clinical outcomes between the devices used for AAM.
The strengths of our study include its large sample size and use of a comprehensive database from multiple hospitals. However, several limitations should be considered when interpreting our findings. First, our study exclusively included patients who underwent ECPR. This selection criterion may have introduced collider bias37, as both the exposure (advanced airway management) and outcomes (patient survival or favourable neurological outcomes) could influence the likelihood of receiving ECPR. Owing to the design of the SAVE-J II study, we lacked data on patients with OHCA who did not undergo ECPR, which could have influenced the outcomes. Second, although the sample size was substantial, it was not large enough to ensure complete homogeneity in patient backgrounds; therefore, residual confounding factors may have been present. Third, we could not collect data regarding the time of AAM initiation and the duration of AAM attempts, reasons for AAM failure, and number of AAM attempts, which could affect the time-course and patient outcomes. Furthermore, it was impossible to evaluate the quality of prehospital resuscitation, including detailed information regarding prehospital defibrillations, chest compression interruption times, the quality of ventilation before and after AAM, and proficiency of the EMS crew who administered AAM, as we did not have access to information regarding bystander’s occupation (i.e., whether they were healthcare providers). Fourth, in the Japanese system, paramedics are required to explain the patient’s condition and receive instructions from a local medical control doctor before performing AAM or administering epinephrine. Therefore, different regions might have different protocols for prehospital treatments, which may have introduced selection bias. Although patients were eligible for AAM, BVM ventilation was chosen based on field judgment in some cases. Future studies including both patients who received and did not receive prehospital AAM are warranted to better understand the impact of prehospital AAM strategies. Fifth, although Utstein recommended an ROSC duration ≥ 30 s38, in the present study, any duration was considered, as clinical data for ROSC duration was missing. Sixth, in this study, there was no uniform protocol concerning blood sampling (timing, part, etc.), making it challenging to assess the impact of blood test results. Seventh, data on the implementation of automated CPR devices and cannulation techniques were not available. Eighth, the differences in facilities, time of onset (hour, day, and month), and geographical factors, such as suburban or urban areas, were not adjusted for. Finally, we could not obtain data regarding the number of patients transferred early, which might have influenced the duration of hospital stay. Furthermore, when evaluating outcomes according to the duration of mechanical ventilation and the length of ICU stay, patients who died shortly after hospital arrival were likely to have shorter hospital stays, eliciting a survival bias. Despite these limitations, we demonstrated that the implementation of prehospital AAM was associated with prolonged low-flow time and poor outcomes. Therefore, it is desirable to develop protocols that consider individual patients’ situations to decide whether to prioritise prehospital AAM or early transportation. Future studies with robust protocols are crucial to validate these findings and enhance clinical guidelines for prehospital care.
Conclusions
Among patients with OHCA who underwent ECPR, prehospital AAM was associated with poorer neurological and clinical outcomes and longer transport time. Prospective studies are needed to establish a protocol to determine whether early transport or AAM should be prioritised in prehospital settings, based on patient characteristics and situations.
Data availability
The datasets analysed in this study are not publicly available because of privacy issues but are available from the corresponding author upon reasonable request.
References
Tsao, C. W. et al. Heart disease and stroke Statistics-2023 update: A report from the American heart association. Circulation 147, e93–e621 (2023).
Ohashi-Fukuda, N., Fukuda, T., Doi, K. & Morimura, N. Effect of prehospital advanced airway management for pediatric out-of-hospital cardiac arrest. Resuscitation 114, 66–72 (2017).
Jung, E., Ro, Y. S., Ryu, H. H. & Shin, S. D. Association of prehospital airway management technique with survival outcomes of out-of-hospital cardiac arrest patients. PLoS One. 17, e0269599 (2022).
Inoue, A., Hifumi, T., Sakamoto, T. & Kuroda, Y. Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest in adult patients. J. Am. Heart Assoc. 9, e015291 (2022).
Aufderheide, T. P. & Lurie, K. G. Death by hyperventilation: a common and life-threatening problem during cardiopulmonary resuscitation. Crit. Care Med. 32, S345–S351 (2004).
Wang, H. E., Simeone, S. J., Weaver, M. D. & Callaway, C. W. Interruptions in cardiopulmonary resuscitation from paramedic endotracheal intubation. Ann. Emerg. Med. 54, 645–652e1 (2009).
Odegaard, S., Pillgram, M., Berg, N. E., Olasveengen, T. & Kramer-Johansen, J. Time used for ventilation in two-rescuer CPR with a bag-valve-mask device during out-of-hospital cardiac arrest. Resuscitation 77, 57–62 (2008).
Lockey, D. J. et al. Advanced airway management is necessary in prehospital trauma patients. Br. J. Anaesth. 114, 657–662 (2015).
Benger, J. R. et al. Effect of a strategy of a supraglottic airway device vs tracheal intubation during out-of-hospital cardiac arrest on functional outcome: the AIRWAYS-2 randomized clinical trial. JAMA 320, 779–791 (2018).
Jabre, P. et al. Effect of bag-mask ventilation vs endotracheal intubation during cardiopulmonary resuscitation on neurological outcome after out-of-hospital cardiorespiratory arrest: a randomized clinical trial. JAMA 319, 779–787 (2018).
Mandigers, L. et al. Systematic review and meta-analysis comparing low-flow duration of extracorporeal and conventional cardiopulmonary resuscitation. Interact. Cardiovasc. Thorac. Surg. 35, ivac219 (2022).
Bunya, N. et al. Prognostic significance of signs of life in out-of-hospital cardiac arrest patients undergoing extracorporeal cardiopulmonary resuscitation. Crit. Care Med. 52 (4), 542–550 (2024).
Inoue, A. et al. Extracorporeal cardiopulmonary resuscitation in adult patients with out-of-hospital cardiac arrest: a retrospective large cohort multicenter study in Japan. Crit. Care. 26, 129 (2022).
Fire and Disaster Management Agency (FDMA). Report of the study group on the ideal way of emergency services [Internet]. Tokyo: KDMA; 2018 [cited 2024 Dec 18]. Available from: https://www.ajha.or.jp/topics/admininfo/pdf/2017/170410_1.pdf?fbclid=IwAR2Epjgnj9xxZLF1Kf N7O4uQHRCTbjMcZGyaHfnN8FWeHww-PxvfcnXzpw. Japan.
Oh, S. H. et al. The impact of sex and age on neurological outcomes in out-of-hospital cardiac arrest patients with targeted temperature management. Crit. Care. 21, 272 (2017).
Sasson, C., Rogers, M. A., Dahl, J. & Kellermann, A. L. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes. 3, 63–81 (2010).
Debaty, G. et al. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation 112, 1–10 (2017).
Martinell, L. et al. Early predictors of poor outcome after out-of-hospital cardiac arrest. Crit. Care. 21, 96 (2017).
Lupton, J. R. et al. Outcomes with the use of bag-valve-mask ventilation during out-of-hospital cardiac arrest in the pragmatic airway resuscitation trial. Acad. Emerg. Med. 27, 366–374 (2020).
Wang, Y. et al. Effects of prehospital management in out-of-hospital cardiac arrest: advanced airway and adrenaline administration. BMC Health Serv. Res. 22, 546 (2022).
Kang, K. et al. Prehospital endotracheal intubation and survival after out-of-hospital cardiac arrest: results from the Korean nationwide registry. Am. J. Emerg. Med. 34, 128–132 (2016).
Katabami, K., Kimura, T., Hirata, T. & Tamakoshi, A. Association between advanced airway management with adrenaline injection and prognosis in adult patients with Asystole asphyxia out-of-hospital cardiac arrest. J. Epidemiol. 34, 31–37 (2024).
McMullan, J. et al. Airway management and out-of-hospital cardiac arrest outcome in the CARES registry. Resuscitation 85, 617–622 (2014).
Hasegawa, K., Hiraide, A., Chang, Y. & Brown, D. F. Association of prehospital advanced airway management with neurologic outcome and survival in patients with out-of-hospital cardiac arrest. JAMA 309, 257–266 (2013).
Kajino, K. et al. Current termination of resuscitation (TOR) guidelines predict neurologically favorable outcome in Japan. Resuscitation 84, 54–59 (2013).
Goto, Y., Maeda, T. & Goto, Y. N. Termination-of-resuscitation rule for emergency department physicians treating out-of-hospital cardiac arrest patients: an observational cohort study. Crit. Care. 17, R235 (2013).
Richardson, A. C. et al. Extracorporeal cardiopulmonary resuscitation in adults: interim guideline consensus statement from the extracorporeal life support organization. ASAIO J. 67, 221–228 (2021).
Yang, J. H. Clinical significance of low-flow time in patients treated with extracorporeal cardiopulmonary resuscitation. Korean Circ. J. 48, 716–718 (2018).
Murakami, N. et al. Prognostic impact of no-flow time on 30-day neurological outcomes in patients with out-of-hospital cardiac arrest who received extracorporeal cardiopulmonary resuscitation. Circ. J. 84, 1097–1104 (2020).
Yannopoulos, D. et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet 396, 1807–1816 (2020).
Nakashima, T. et al. Patients with refractory out-of-cardiac arrest and sustained ventricular fibrillation as candidates for extracorporeal cardiopulmonary resuscitation - prospective multi-centre observational study. Circ. J. 83, 1011–1018 (2019).
Matsuoka, Y. et al. Hospitals’ extracorporeal cardiopulmonary resuscitation capabilities and outcomes in out-of-hospital cardiac arrest: a population-based study. Resuscitation 136, 85–92 (2019).
Souchtchenko, S. S., Benner, J. P., Allen, J. L. & Brady, W. J. A review of chest compression interruptions during out-of-hospital cardiac arrest and strategies for the future. J. Emerg. Med. 45, 458–466 (2013).
Leo, W. Z., Chua, D., Tan, H. C. & Ho, V. K. Chest compression quality and patient outcomes with the use of a CPR feedback device: A retrospective study. Sci. Rep. 13, 19852 (2023).
Lauridsen, K. G. et al. Association of chest compression pause duration prior to E-CPR cannulation with cardiac arrest survival outcomes. Resuscitation 177, 85–92 (2022).
Benoit, J. L., Prince, D. K. & Wang, H. E. Mechanisms linking advanced airway management and cardiac arrest outcomes. Resuscitation 93, 124–127 (2015).
Holmberg, M. J. & Andersen, L. W. Collider bias. JAMA 327, 1282–1283 (2022).
Jacobs, I. & task force of the International Liaison Committee on Resuscitation (American Heart Association. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa). Circulation. 110, 3385-97 (2004).
Acknowledgements
We thank all members of the SAVE-J II study group and all the reviewers and for their assistance and support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
N.K. participated in the study design, data collection, drafting of the manuscript, and statistical analyses. W.T. participated in the statistical analyses and helped draft the manuscript. K.M., Y.O., A.I., T.H., T.S., and Y.K. participated in study conception and design, data collection, and drafting of the manuscript. All the authors have read the manuscript, discussed the results, and approved this submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kawahara, N., Takayama, W., Morishita, K. et al. Effects of prehospital advanced airway management on cardiac arrest patients who underwent extracorporeal cardiopulmonary resuscitation. Sci Rep 15, 10670 (2025). https://doi.org/10.1038/s41598-025-95176-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95176-7