Abstract
This study assessed initial stability using Resonance Frequency Analysis (RFA) and Damping Capacity Analysis (DCA) devices in three different bone conditions: a control group without bone defects, a cortical bone defect model, and a cancellous bone defect model. Additionally, the correlation between Implant Stability Quotient (ISQ) and Implant Stability Test (IST) values was investigated. This in vitro study utilized a controlled experimental design with bovine rib specimens. Thirty implants were placed in specimens divided into three groups (n = 10): no defect (Group A), cortical bone defect (Group B), and cancellous bone defect (Group C). Implant stability was measured using RFA and DCA devices. ISQ and IST values in Group A were 84.83 ± 1.38 and 86.52 ± 1.73, respectively; in Group B, they were 64.20 ± 4.87 and 64.25 ± 5.26, respectively; and in Group C, they were 82.80 ± 5.74 and 86.47 ± 4.01, respectively. Statistical analyses were performed using Kruskal-Wallis test with Bonferroni test to compare differences among groups, and Spearman’s correlation analysis was used to evaluate the relationship between ISQ and IST values. A significant reduction in ISQ(p < 0.001) and IST(p < 0.001) values was observed in the cortical bone defect group. In contrast, the cancellous bone defect group showed no significant difference from the control group (ISQ: p = 0.493, IST: p = 0.594). ISQ and IST values exhibited a significant positive correlation (r = 0.798, p < 0.001), and stability values varied significantly with measurement direction. This study confirmed that cortical bone defects significantly reduced primary stability, whereas cancellous bone defects had no significant impact when cortical bone was sufficient. Additionally, ISQ and IST values showed a strong positive correlation, indicating that both methods provide consistent assessments of implant stability.
Similar content being viewed by others
Introduction
Primary stability of dental implants, defined as the mechanical stability obtained immediately after implant placement, is a crucial factor for successful osseointegration1. Adequate primary stability requires sufficient bone volume and quality. Several studies have reported differences in implant stability depending on bone quality, with a significant decrease in implant stability observed in low density bone2,3,4,5,6. Primary stability is primarily derived from the mechanical interlocking of the implant with the cortical bone, with increasing cortical bone thickness correlating with increased implant stability and larger cortical bone defects correlating with decreased stability5,7,8,9,10,11. Several methods exist to measure implant stability, with Resonance Frequency Analysis (RFA) and Damping Capacity Analysis (DCA) being the most widely used non-invasive, objective diagnostic techniques. RFA measures the resonance frequency of a smart peg transducer attached to the implant, yielding an Implant Stability Quotient (ISQ) value ranging from 0 to 100, with higher values indicating greater stability12,13. An implant is considered to have low stability when the ISQ is below 60, medium stability when the ISQ is between 60 and 70, and high stability when the ISQ is above 70.
In comparison, DCA involves a percussive rod that strikes the implant, and the deceleration of the rod is measured to determine implant stability. This method does not require the removal of the healing abutment, making it quick and convenient, but patient discomfort can be a drawback14,15. Unlike previous DCA devices (e.g., Periotest), the newly developed DCA device has been modified to enhance patient comfort and measurement accuracy. The percussion force has been reduced, and an integrated safety control system adjusts the number of percussion strikes based on implant stability. The measurement values (Implant Stability Test; IST) are displayed in a format similar to that of Osstell, ranging from 1 to 99, with higher values indicating greater stability15,16. An implant is considered to have low stability when the IST is below 60, medium stability when the IST is between 60 and 65, and high stability when the IST is above 65.
Previous studies have predominantly utilized ISQ and Periotest values (PTVs) to measure implant stability relative to bone density and defect size2,14,17,18,19. However, few studies have compared ISQ and IST values in relation to different types of bone defects. This study aims to measure and compare initial stability in cortical and cancellous bone defect models using RFA and DCA devices and investigate the correlation between ISQ and IST values. Additionally, the effect of measurement direction on implant stability was evaluated.
Materials and methods
Bone models preparation
Refrigerated bovine ribs with a diameter of 6 mm or more were prepared, and CBCT (DENTRI-S, HDXWILL, Seoul, Korea) were taken to select ribs that satisfied the Hounsfield unit(HU) of 350–850 corresponding to the D3 bone quality classification according to the Misch classification20. According to Misch’s classification, D1(HU > 1250) bone consists of dense cortical bone; D 2(HU 850–1250) bone has porous cortical bone and coarse cancellous bone; D 3(HU 350–850) bone has porous cortical bone and fine cancellous bone; and D 4(HU150-350) bone has fine cancellous bone.
Drilling procedure, defect preparation and implant placement
Samples were divided into three groups based on the type of defect (Fig. 1): Group A had no peri-implant defect and served as the control; Group B had a cortical bone defect with a diameter of 6 mm and a height of 4 mm; and Group C had a cancellous bone defect, where the implant was fixed only in cortical bone. A total of 30 implants were placed, with 10 implants in each group. The centers of the implant beds were spaced approximately 15 to 20 mm apart.
Schematic drawing of defect types. (a) Control; no defect. (b) Cortical defect (diameter 6 mm x height 4 mm). (c) Cancellous defect (diameter 6 mm); to be fixed only in cortical bone. The average thickness of bovine cortical bone was 3–4 mm. The light-shaded region represents cancellous bone, while the darker-shaded area indicates cortical bone. The darkest-shaded region corresponds to the defect area created for each experimental condition.
Bone-level implants (4.5 × 8.5 mm, IS, Neobiotech, Seoul, Korea) were placed following the manufacturer’s protocol. The drilling sequence was as follows: point Lindemann drill (Ø2.5), initial drill (Ø2.2), Ø2.9 twist drill, Ø3.4 twist drill, Ø3.9 twist drill, Ø4.5 countersink (Fig. 2a). For Group B, defects were created using a wide-diameter to form a 6 mm diameter, 4 mm height defect in the cortical bone (Fig. 2b). For Group C, longitudinal sectioning of the bovine bone was performed to measure cancellous bone height. The defect was created using a wide-diameter drill from the cancellous bone side towards the implant apex, ensuring that the implant was stabilized only in the cortical bone (Fig. 2c). Implants were placed with a maximum insertion torque(IT) of 40 N⋅cm (Fig. 2d-f). The insertion torque values varied among groups: Group A (control) showed 35.00 ± 4.47 N⋅cm (range: 30–40 N⋅cm), Group B (cortical defect) exhibited lower values of 15.50 ± 1.50 N⋅cm (range: 15–20 N⋅cm), while Group C (cancellous defect) had a broader range with values of 27.00 ± 5.57 N⋅cm (range: 20–35 N⋅cm).
Photo of bovine bone after defect formation and implant placement. (a, d) Control; no defect. (b, e) Cortical defect. (c, f) Cancellous defect. (a, b) View at the coronal side after defect formation. (c) View at the apex side after defect formation. (d, e) view at the coronal side after implant placement. (f) view at the apex side after implant placement.
Primary stability measurement
The longitudinal direction of the rib (parallel to the rib axis) was defined as mesiodistal, and the perpendicular direction as buccolingual. For implant stability measurements, SmartPeg transducers were attached to the implants fixtures, and ISQ values were measured using a RFA device (Osstell Mentor®, Osstell AB, Gothenburg, Sweden) three times in the buccal, lingual, mesial, and distal directions, and the mean value for each direction was calculated for analysis. Subsequently, 4.0 × 4.0 mm healing abutments (IS, Neobiotech, Seoul, Korea) were connected. IST values were measured using a DCA device (AnyCheck, Neobiotech, Seoul, Korea). The device was positioned perpendicularly to the healing abutment surface, and IST values were recorded in the same four directions (buccal, lingual, mesial, and distal). Each measurement was performed three times, and the mean value was used for statistical analysis.
Statistical analysis
Statistical analyses were conducted using SPSS Statistics 21.0 (IBM Corp., Armonk, NY, USA). Differences in stability due to bone defects and measurement directions were evaluated using the Kruskal-Wallis test. Post-hoc comparisons between groups were performed using the Bonferroni test. The correlation between implant stability (ISQ, IST) and insertion torque was assessed using Spearman’s rank correlation analysis, as the data did not follow a normal distribution based on the Shapiro-Wilk normality test. Spearman’s correlation was conducted for the following variable pairs: ISQ and IST (primary correlation analysis); ISQ and IT; IST and IT. The agreement between the two implant stability measurement methods was evaluated using a Bland-Altman analysis performed with Excel 2016 (Microsoft Corp., Redmond, WA, USA). Statistical significance was set at P < 0.05.
Results
In the cortical bone defect group (Group B), both ISQ and IST values significantly decreased (p < 0.001), while the cancellous bone defect group (Group C) showed no significant difference compared to the control group (Group A) (ISQ: p = 0.493, IST: p = 0.594). ISQ and IST values in Group A were 84.83 ± 1.38 and 86.52 ± 1.73, respectively; in Group B, they were 64.20 ± 4.87 and 64.25 ± 5.26, respectively; and in Group C, they were 82.80 ± 5.74 and 86.47 ± 4.01, respectively (Table 1).
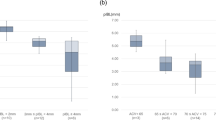
Figure 3 showed the ISQ and IST measurements according to the measurement direction. The results of the Kruskal-Wallis test indicated that there were significant differences in some groups based on the measurement direction in Groups A and C. In the ISQ measurements, significant differences were found between the buccal-mesial(p = 0.019), buccal-distal(p = 0.010), lingual-mesial(p = 0.015), and lingual-distal(p = 0.008) directions in Group C. In Group A, although not significant, similar directional differences were observed. Similarly, for the IST measurements, significant differences were found between the buccal-distal(p = 0.006) and lingual-distal(p = 0.002) directions in Group A and between the buccal-lingual(p < 0.001) and lingual-mesial(p = 0.012) directions in Group C.
ISQ and IST measurements for Group A (control), Group B (cortical defect), and Group C (cancellous defect). Data are presented as means ± standard deviations (SD) of implant stability measured in different directions: buccal (B), lingual (L), mesial (M), and distal (D). Statistical analysis was performed using the Kruskal-Wallis test, followed by Bonferroni post hoc test for multiple comparisons. The statistical significance level is p < 0.05. Asterisks (*) indicate statistically significant differences between groups. ISQ, Implant Stability Quotient; IST, Implant Stability Test.
ISQ and IST measurements exhibited a significant positive correlation (Spearman’s correlation coefficient: 0.798, p < 0.001) (Fig. 4). The insertion torque and stability showed a significant positive correlation regardless of the type of measurement device used (Fig. 5). The Spearman correlation coefficients for each group were as follows: Both-IT: 0.619 (p < 0.001), ISQ-IT: 0.599 (p < 0.001), IST-IT: 0.610 (p < 0.001). Analysis using the Bland-Altman plot (Fig. 6) to compare the consistency between ISQ and IST measurements showed a mean absolute difference of 1.80 (-3.59 to 11.39, P = 0.014).
Correlation between the ISQ and IST values. Scatter plot showing the correlation between ISQ and IST measurements. Each dot represents a single measurement pair. The Spearman correlation coefficient is r = 0.798, indicating a strong positive correlation. ISQ, Implant Stability Quotient; IST, Implant Stability Test.
Correlation between the IT and implant stability. (A) ISQ – IT, (B) IST – IT. Scatter plots showing the correlation between insertion torque (IT) and implant stability as measured by ISQ and IST. Each dot represents a single measurement pair. The Spearman correlation coefficients are (a) r = 0.599 for ISQ-IT and (b) r = 0.610 for IST-IT, indicating moderate positive correlations. ISQ, Implant Stability Quotient; IST, Implant Stability Test; IT, Insertion Torque.
Bland-Altman plot for Implant stability. This plot illustrates the agreement between two different measurements of implant stability. The x-axis represents the average of the two measurements, while the y-axis represents the difference between the two measurements. The solid orange line represents the mean difference (bias), which is 1.80. The dashed lines represent the limits of agreement, calculated as mean difference ± 1.96 standard deviations (SD), which are 11.39 (+ 1.96 SD) and − 3.59 (-1.96 SD). The p-value for the mean difference is 0.014, indicating a statistically significant bias.
Discussion
This study compared the initial stability using RFA and DCA devices in cortical and trabecular bone defect models. The experimental results presented that implant stability significantly decreased in cortical bone defect models. These results were consistent with previous studies, which reported that the ISQ value increased with the thickness of the cortical bone7,8,11,21,22. Additionally, other studies reported the correlation between ISQ and both the cortical bone thickness and the cortical bone-to-trabecular bone ratio, as measured by CBCT21,22.
In this study, the ISQ and IST values measured in cortical bone defect model (Group B) were 64.20 ± 4.87 and 64.25 ± 5.26, respectively, indicating moderate stability. Implant stability was shown to be influenced by the degree of supporting bone support around implant. The height of the placed implant was 8.5 mm, and the cortical bone defect was 4 mm high, resulting in implant exposure not exceeding 50%. Previous studies using box-type bone defect models of varying widths reported low stability with ISQ values below 60 when 50% of the implant surface was exposed18. Considering these results, it was inferred that if cancellous bone alone could support more than 50% of the implant surface even without cortical bone, moderate stability could be achieved. This might serve as a predictive criterion for implant placement in cases where cortical bone is insufficient, such as in sites with inadequate healing after tooth extraction or immediate implant placement. These conditions align with Group B (cortical bone defect model), where cortical bone is insufficient around the implant, necessitating initial stability from a limited amount of surrounding cancellous bone.
While many studies have reported the association between cortical bone and implant stability, there are few reports on the relationship between cancellous bone and implant stability. In this study, the cancellous bone defects showed a tendency to decrease implant stability compared to no defects. This finding was consistent with previous finite element analysis studies that reported a decrease in implant stability with reduced proximal support of cancellous bone23. However, in our experimental model of cancellous bone defects, the difference in implant stability was not significant, which could be attributed to the location of the defects. In our experimental model, the cancellous bone defect was located at the apex, whereas in previous finite element analysis studies, the cancellous bone defect was located at the neck of the implant23. Similarly, other studies that measured implant stability based on location of fixation reported that fixation at the neck of the implant had a greater impact on stability than fixation at the bottom24. These findings suggested that cancellous bone defects located at the apex did not significantly affect primary stability in cases where cortical bone was preserved. This condition was similar to maxillary posterior implant placement with sinus lift, where the absence of cancellous bone at the apical region was compensated by cortical bone support at the implant neck, contributing to primary stability. Another reason why trabecular bone defects had no significant impact in this experiment could be the presence of thick cortical bone. A previous experiment, which used an epoxy resin model simulating cortical bone, trabecular bone, and connective tissue, found that when the cortical bone thickness was 4 mm or more, elastic changes in the underlying trabecular bone region could not be detected by both RFA and DCA measurement methods17. Similarly, in this experiment, the cortical bone thickness was also approximately 3–4 mm, which might have reduced the impact of the underlying trabecular bone defect.
This study also analyzed the differences in implant stability based on the direction of measurement. Specifically, the measurements were taken in four directions: buccal, lingual, mesial, and distal. The results indicated that the direction of measurement significantly influenced the ISQ and IST values, particularly in the control and cortical bone defect groups. For instance, in the control group, significant differences in IST values were observed between the buccal-distal and lingual-distal directions, while in the cortical bone defect group, significant differences were found between the buccal-lingual and lingual-mesial directions. Additionally, ISQ values in the cortical bone defect group showed significant differences in all directions except for buccal-lingual and mesial-distal. Similarly, as in previous studies9,25,26, the ISQ values tended to be higher when measured parallel to the long axis of the rib (mesial and distal) compared to the perpendicular direction (buccal and lingual). These results indicated that implant stability measurements are influenced by the direction of measurement. In this experiment, IST did not show any tendency, which is presumed to be due to the measurement principle of IST. Previous studies using a box-shaped bone defect model reported differences in PTV depending on the measurement direction, and showed lower implant stability when measured in the direction opposite to the bone defect compared to the same direction as the bone defect18. The study attributed the difference to the percussion method of the PTV measurement, which exerts dislodgement forces on the implant and does not obtain sufficient support from the bone wall due to bone defects. Similarly, the IST in our experiment, which also uses a percussive measurement method like PTV, did not show a specific tendency because our bone defect model is circular, resulting in no differences in bone wall support depending on the location. Taking this into account, implant stability values might be varied depending on the bone defect location than the measurement direction. In clinical practice, IST measurement was often difficult to measure in the mesial, distal, and lingual directions, excluding the buccal direction, due to anatomical structures such as adjacent teeth. Therefore, when measuring implant stability, it is necessary to carefully review the measured implant stability values, especially if the bone defect is located on one side.
Both RFA and DCA methods are widely used non-invasive techniques for measuring implant stability. Several studies have compared implant stability using these methods. In this study, ISQ and IST values showed a significant positive correlation of 0.798, consistent with previous in vitro studies, which reported a high correlation coefficient between ISQ and IST15. Additionally, Bland-Altman analysis was performed to compare the reliability of the two measurements, and since most of the differences were within the limits of agreement, it could be said that the consistency between the methods is maintained. This suggested the potential compatibility of ISQ and IST measurements for clinical use. During the healing process after one-stage surgery, ISQ required the removal of the healing abutment to attach the smart peg, which might influence bone resorption27,28. Therefore, IST measurements, which did not require removing the healing abutment, might be more beneficial. However, the Bland-Altman analysis results showed a significant mean difference of 1.80 (P = 0.014) between ISQ and IST, and previous studies using Bland-Altman analysis to compare ISQ and IST also confirmed a significant difference between the two values15. Therefore, this difference should be considered when using both devices to measure implant stability. Identified differences in implant primary stability according to bone defects, assessed through RFA and DCA, suggest the potential compatibility of ISQ and IST measurements for clinical use in evaluating implant stability, highlighting their usefulness in improving the prediction and assessment of implant prognosis.
Insertion torque could also be used to measure implant stability. Previous study29 reported significant correlations between IST and IT, and other studies4,15 reported significant correlations between ISQ and IT. Likewise, this study confirmed significant positive correlations of greater than 0.5 between IT and both ISQ and IST values.
Since this study utilized an experimental model based on D3 bone quality, an under-drilling protocol was applied to enhance primary stability. Under-drilling has been widely used in low-density bone to improve implant stability by increasing bone compression during placement30. However, in the cortical bone defect model (Group B), the absence of cortical support limited the effectiveness of under-drilling, resulting in lower insertion torque values and reduced implant stability. Conversely, in the cancellous bone defect model (Group C), under-drilling partially compensated for the lack of cancellous bone support, leading to moderate insertion torque values. These findings suggested that while under-drilling improved stability in low-density bone, its effectiveness was influenced by the availability of cortical and trabecular bone support, necessitating careful selection of drilling protocols based on bone quality.
The limitations of this study included it’s in vitro nature, the use of bovine ribs, and the relatively thick cortical bone. Therefore, the trend of ISQ and IST values observed in this study might not align with those in human alveolar bone. This indicated the necessity for additional experiments with cortical bone thickness similar to that of humans and clinical studies.
Conclussion
-
- Implant stability significantly decreased in both RFA and DCA methods when cortical bone is deficient, whereas cancellous bone defects did not have a significant impact when cortical bone is sufficient.
-
- ISQ and IST values showed a strong positive correlation, providing a consistent assessment of implant stability.
-
- Both ISQ and IST measurements were influenced by the direction of measurement.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Albrektsson, T. & Zarb, G. A. Current interpretations of the osseointegrated response: Clinical significance. Int. J. Prosthodont. 6, 95–105 (1993).
Oh, J. S. & Kim, S. G. Clinical study of the relationship between implant stability measurements using Periotest and osstell mentor and bone quality assessment. Or. Surg. Or. Med. Or. Pa. 113, E35–E40. https://doi.org/10.1016/j.tripleo.2011.07.003 (2012).
Yoon, H. G., Heo, S. J., Koak, J. Y., Kim, S. K. & Lee, S. Y. Effect of bone quality and implant surgical technique on implant stability quotient (ISQ) value. J. Adv. Prosthodont. 3, 10–15. https://doi.org/10.4047/jap.2011.3.1.10 (2011).
Lee, S. Y. et al. A comparison of bone bed Preparation with laser and conventional drill on the relationship between implant stability quotient (ISQ) values and implant insertion variables. J. Adv. Prosthodont. 2, 148–153. https://doi.org/10.4047/jap.2010.2.4.148 (2010).
Putra, R. H. et al. Radiographic alveolar bone assessment in correlation with primary implant stability: A systematic review and meta-analysis. Clin. Oral Implants Res. 35, 1–20. https://doi.org/10.1111/clr.14195 (2024).
Bera, T. et al. Relative contribution of trabecular and cortical bone to primary implant stability: an in vitro model study. J. Pharm. Bioallied Sci. 16, S3427–S3429. https://doi.org/10.4103/jpbs.jpbs_899_24 (2024).
Winter, W., Möhrle, S., Holst, S. & Karl, M. Parameters of implant stability measurements based on resonance frequency and damping capacity: A comparative finite element analysis. Int. J. Oral Max Impl. 25, 532–539 (2010).
Shin, S. Y. et al. Bone cement grafting increases implant primary stability in circumferential cortical bone defects. J. Periodontal Implant Sci. 45, 30–35. https://doi.org/10.5051/jpis.2015.45.1.30 (2015).
Shin, S. Y. et al. The effects of defect type and depth, and measurement direction on the implant stability quotient value. J. Oral Implantol. 41, 652–656. https://doi.org/10.1563/AAID-JOI-D-13-0031 (2015).
Di Stefano, D. A., Arosio, P., Cappare, P., Barbon, S. & Gherlone, E. F. Stability of dental implants and thickness of cortical bone: clinical research and future perspectives. A systematic review. Mater. (Basel). https://doi.org/10.3390/ma14237183 (2021).
Barros, L. A. et al. In vitro evaluation of the influence of bone cortical thickness on the primary stability of conventional- and short-sized implants. J. Clin. Exp. Dent. 14, e138–e143. https://doi.org/10.4317/jced.58886 (2022).
Becker, W., Hujoel, P. & Becker, B. E. Resonance frequency analysis: comparing two clinical instruments. Clin. Implant Dent. Relat. Res. 20, 308–312. https://doi.org/10.1111/cid.12598 (2018).
Lages, F. S., Douglas-de Oliveira, D. W. & Costa, F. O. Relationship between implant stability measurements obtained by insertion torque and resonance frequency analysis: A systematic review. Clin. Implant Dent. R. 20, 26–33. https://doi.org/10.1111/cid.12565 (2018).
Zix, J., Hug, S., Kessler-Liechti, G. & Mericske-Stern, R. Measurement of dental implant stability by resonance frequency analysis and damping capacity assessment: comparison of both techniques in a clinical trial. Int. J. Oral Max Impl. 23, 525–530 (2008).
Lee, J. et al. Comparison of implant stability measurements between a resonance frequency analysis device and a modified damping capacity analysis device: an study. J. Periodontal Implan. 50, 56–66. https://doi.org/10.5051/jpis.2020.50.1.56 (2020).
Lee, D. H. et al. The reliability of anycheck device related to healing abutment diameter. J. Adv. Prosthodont. 12, 83–88. https://doi.org/10.4047/jap.2020.12.2.83 (2020).
Feng, S. W. et al. Influence of simulated bone quality and cortical bone thickness on implant stability detection using resonance frequency and damping factor analysis. Int. J. Oral Max Impl. 29, 105–112. https://doi.org/10.11607/jomi.3181 (2014).
Yim, H. J. et al. Primary stability of implants with peri-implant bone defects of various widths: An investigation. J. Periodontal Implan. 49, 39–46. https://doi.org/10.5051/jpis.2019.49.1.39 (2019).
Choi, H. H., Chung, C. H., Kim, S. G. & Son, M. K. Reliability of 2 implant stability measuring methods in assessment of various periimplant bone loss: an in vitro study with the Periotest and osstell mentor. Implant Dent. 23, 51–56. https://doi.org/10.1097/ID.0000000000000000 (2014).
Misch, C. E. Contemporary Implant Dentistry 2nd edn (Mosby, 1999).
Chatvaratthana, K., Thaworanunta, S., Seriwatanachai, D. & Wongsirichat, N. Correlation between the thickness of the crestal and buccolingual cortical bone at varying depths and implant stability quotients. PLoS One. 12, e0190293. https://doi.org/10.1371/journal.pone.0190293 (2017).
Talluri, S. et al. Evaluating cortico-cancellous ratio using virtual implant planning and its relation with immediate and long-term stability of a dental implant- A CBCT-assisted prospective observational clinical study. Niger J. Clin. Pract. 22, 982–987. https://doi.org/10.4103/njcp.njcp_22_19 (2019).
Gomes, C., Mesnard, M. & Ramos, A. Bone density and proximal support effects on dental implant stability - Finite element analysis and in vitro experiments. J Stomatol Oral Maxi 124. (2023).
Ito, Y. et al. Relevance of resonance frequency analysis to evaluate dental implant stability: Simulation and histomorphometrical animal experiments. Clin. Oral Implants Res. 19, 9–14. https://doi.org/10.1111/j.1600-0501.2007.01419.x (2008).
Fischer, K., Stenberg, T., Hedin, M. & Sennerby, L. Five-year results from a randomized, controlled trial on early and delayed loading of implants supporting full-arch prosthesis in the edentulous maxilla. Clin. Oral Implan Res. 19, 433–441. https://doi.org/10.1111/j.1600-0501.2007.01510.x (2008).
Veltri, M., Balleri, P. & Ferrari, M. Influence of transducer orientation on osstell™ stability measurements of osseointegrated implants. Clin. Implant Dent. R. 9, 60–64. https://doi.org/10.1111/j.1708-8208.2007.00035.x (2007).
Degidi, M., Nardi, D. & Piattelli, A. One abutment at one time: Non-removal of an immediate abutment and its effect on bone healing around subcrestal tapered implants. Clin. Oral Implants Res. 22, 1303–1307. https://doi.org/10.1111/j.1600-0501.2010.02111.x (2011).
Koutouzis, T., Koutouzis, G., Gadalla, H. & Neiva, R. The effect of healing abutment reconnection and Disconnection on soft and hard peri-implant tissues: A short-term randomized controlled clinical trial. Int. J. Oral Maxillofac. Implants. 28, 807–814. https://doi.org/10.11607/jomi.3022 (2013).
Al-Jamal, M. F. J. & Al-Jumaily, H. A. Can the bone density estimated by CBCT predict the primary stability of dental implants?? A new measurement protocol. J. Craniofac. Surg. 32, E171–E174. https://doi.org/10.1097/Scs.0000000000006991 (2021).
Delgado-Ruiz, R., Gold, J., Somohano Marquez, T. & Romanos, G. Under-Drilling versus hybrid osseodensification technique: differences in implant primary stability and bone density of the implant bed walls. Mater. (Basel). 13. https://doi.org/10.3390/ma13020390 (2020).
Funding
None.
Author information
Authors and Affiliations
Contributions
S.L, J.L, M.K and H.K had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: S.L and J.L Acquisition, analysis, or interpretation of data: M.K and H.K Drafting of the manuscript: M.K and H.K Critical revision of the manuscript for important intellectual content: S.L and J.L Study supervision: S.L All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, M., Lee, JH., Kim, Hs. et al. Evaluation of primary stability of implants in bovine bone defects models. Sci Rep 15, 10237 (2025). https://doi.org/10.1038/s41598-025-95318-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95318-x