Abstract
Monitoring intra-abdominal pressure (IAP) in critical care patients is crucial for preventing intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS), with their severe consequences. The muscle contraction sensor (MC) introduced in this study offers a novel, non-invasive method with promising accuracy based on previous findings. This study further evaluates the MC accuracy and reproducibility and examines its correlation with objective IAP measurements obtained through a CO2 insufflator. We enrolled 41 patients undergoing elective laparoscopic gallbladder removal under general anesthesia with complete muscle relaxation. Two MC sensors were placed on the right and left sides of the abdomen, and elevated IAP was induced by insufflating CO2 into the peritoneal cavity. IAP measurements from the MC sensors were compared to the randomized IAP values set on the CO2 insufflator. Data from both methods were analyzed to assess the accuracy and agreement with the insufflator measurements. The MC sensor provided continuous and accurate detection of IAP changes. A Pearson correlation coefficient of 0.963 indicated a strong positive linear correlation between the MC sensor readings and the IAP values set on the insufflator. The coefficient of determination (R2) was 0.927, showing that the model explains 92.7% of the variation in IAP values based on the MC sensor signals. Receiver operating characteristic analysis demonstrated that the MC sensor system performed exceptionally well in identifying both IAH and ACS cases, with an area under the curve of 0.996 for IAH and 0.981 for ACS. The study introduces a transcutaneous pressure measuring device as an innovative, non-invasive method for assessing IAP. The system strongly correlates with IAP values measured by CO2 insufflation, indicating its accuracy. It thus could present an alternative to conventional IAP measurement in the future. The MC capability to deliver real-time, continuous data holds substantial potential for proactive patient care. By incorporating advanced analytics like machine learning, the system could detect trends and provide early warnings of dangerous IAP changes, enabling timely, targeted interventions to enhance outcomes for critically ill patients.
Similar content being viewed by others
Introduction
Intraabdominal pressure (IAP) is the steady-state pressure concealed within the abdominal cavity1. Since the abdominal cavity is an enclosed space with partially compliant borders, such as the diaphragm and abdominal musculature, its volume changes due to body position, muscle contractions, or breathing can influence IAP. The contents of the abdominal cavity follow the principles of static fluid, so pressure applied to any part of the abdominal cavity is expected to be transmitted throughout the cavity without reduction. Therefore, a pressure measured at a single point within the abdomen can be presumed to reflect the pressure across the entire abdominal region2,3. Strictly speaking, IAP in healthy individuals ranges from subatmospheric to 0 mmHg. However, certain medical conditions (pregnancy, morbid obesity) can result in chronically elevated IAP of 10–15 mmHg, which has no negative effects due to adaptation mechanisms. In critically ill patients, normal IAP is up to 5–7 mmHg4,5.
Abdominal perfusion pressure (APP) is determined as the difference between mean arterial pressure (MAP) and IAP and has been proposed as a precise indicator of abdominal visceral perfusion in the setting of resuscitation of critically ill patients1,6.
Intraabdominal hypertension (IAH), defined as a sustained or repeated IAP elevation above 12 mmHg in three consecutive measurements1, can, if not treated timely, progress to abdominal compartment syndrome (ACS). At critical IAP values between 10 and 15 mmHg, reductions in microcirculatory blood flow occur, and the initial onset of organ dysfunction and failure becomes apparent. The effects of IAH are not limited to the intraabdominal organs but have a direct or indirect impact on every organ system7. Therefore, ACS is characterized as continuous IAP above 20 mmHg associated with newly developed organ dysfunction or failure1. Given the potential for clinical deterioration in patients with IAH and the imperative to prevent and treat ACS, monitoring IAP is necessary for all critically ill patients. Timely action and prevention of irreversible organ damage hinge on the early identification of risk factors for the abovementioned conditions8,9,10. The World Society of the Abdominal Compartment Syndrome (WSACS) suggests measuring IAP every 4 h with the system inserted in the bladder. The IAP has to be measured at end-expiration with the patient fully supine and under complete muscle relaxation. Risk factors for ACS can be categorized into four main groups: (a) intra-abdominal fluid collections (such as ascites, hematoperitoneum, peritonitis, and collections associated with pancreatitis), (b) substantial fluid resuscitation and increased capillary permeability (including extensive burns, sepsis, and massive transfusion), (c) elevated intraluminal contents of the digestive tract (such as ileus), and (d) decreased compliance of the abdominal wall (related to mechanical ventilation with high final expiratory pressures, obesity, and extensive body burns). Frequently, patients in intensive care units simultaneously exhibit multiple risk factors that exert a synergistic impact11.
The preferred method for assessing IAP in critically ill patients currently involves using a closed measuring system inserted into the bladder. This indirect measuring method relies on the principle that the pressure in the urinary bladder, owing to its intra-abdominal location, is approximately equivalent to the IAP. Measurement is conducted on a fully relaxed supine patient, assessing the water column height along the mid-axillary line at the level of the anterior superior iliac spine. The drawback lies in its relative imprecision and limited reproducibility of measurement outcomes, primarily stemming from the complexity of the procedure and the skill-dependent nature of the method12,13,14. In addition, the technique is quite labor-intensive. It is typically employed intermittently, resulting in a delayed diagnosis of elevated IAP15. Studies have indicated that elevated bladder volumes can lead to increased bladder pressure, particularly at higher IAP, causing measurements to deviate from accurate results of abdominal pressure1,16.
The muscle contraction sensor (MC) utilized in this study was first described in 2011 by Đordević et al.17 and was initially used to assess muscle tension during skeletal muscle contractions. It identifies alterations in tension within the elastic material beneath the surface, whether in the longitudinal or transverse direction, not exclusively from muscle contraction. These tension changes result in indentation force variations and, consequently, the degree of bending in the sensor. The piezoresistive silicon strain gauge element records these changes, consistently recording electrical signals18,19. Furthermore, the sensor has been previously used in a study by Kušar et al.20, where it was compared with an intravesical pressure measurement system in the early postoperative course after an abdominal operation. The authors attempted to determine whether alterations in tension within the abdominal wall, as detected by the transcutaneous sensor, can be employed to evaluate changes in IAP. An approximately linear correlation between intravesical pressure and pressure on the sensor tip within each subject was observed, however, the slopes and intercepts of the regression lines exhibited variation. The data indicated that the specificity in detecting IAP of 20 mmHg and above is 88%, accompanied by a negative predictive value of 96%. A potential limitation of the study is its comparison of results obtained by the MC sensors with those of the intravesical measurement system, which might exhibit a lack of precision and reproducibility in its outcomes.
Our study aimed to assess the accuracy of MC sensor measurements compared to IAP values from a CO2 insufflator as the reference standard. We also explored its potential for continuous monitoring in critically ill patients, where real-time IAP data could aid early detection of IAH and ACS, enabling timely interventions and improving outcomes.
Methods
Patients
We conducted a study that juxtaposed MC sensor readings with objective intraabdominal pressure measurements obtained through CO2 insufflator in patients undergoing elective laparoscopic gallbladder removal. The study included 42 patients hospitalised in the Clinical Department for Abdominal Surgery, University Medical Centre Ljubljana, Slovenia. However, results of measurements in the first patient were excluded due to sensor-related issues encountered during measurements and the statistical analysis and results processing considered data from the subsequent 41 patients.Given the necessity of general anesthesia with complete muscle relaxation required for the surgical procedure, our study gained an advantage from the absence of voluntary or involuntary muscle contractions, which might otherwise have influenced the results.
Inclusion criteria
-
Patients aged 18 years or older.
-
Elective laparoscopic gallbladder removal (cholecystectomy) due to gallbladder polyps or symptomatic cholecystolithiasis.
Exclusion criteria
-
History of heart or lung disease that could deteriorate with increased IAP.
-
Inability or unwillingness to provide informed consent.
-
Prior surgeries in the upper abdomen.
-
Injuries or complications from establishing pneumoperitoneum or placing the first trocar during the procedure.
-
Expected non-cooperation with the study protocol.
Ethical considerations
The study was approved by the National Medical Ethics Committee of Slovenia and conducted in compliance with the World Medical Association’s Declaration of Helsinki (2013). This latest version establishes the ethical principles governing medical research involving human subjects, ensuring the highest patient safety standards and ethical conduct in clinical trials globally. Participating patients received a detailed explanation of the study protocol, and informed consent was obtained from all participants.
Equipment
We used transcutaneous sensors (MC-System, TMG-BMC, Ljubljana, Slovenia) to assess the tension on the abdominal wall, which were previously proved to correlate with IAP measured with intravesical pressure measurement system20. The indented tip of the sensor protrudes towards the skin surface, where the force on the tip varies with the tension of the surface (Fig. 1). The piezoresistive silicon force sensor generates an output signal proportionate to the applied indenting force. The relationship between surface tension and the force registered at the sensor’s tip has been previously confirmed17,19,20.
IAP was measured directly via the ENDOFLATOR® 50 (KARL STORZ SE & Co. KG, Tuttlingen, Germany) CO2 insufflator. The insufflation system was used during the initial establishment of the pneumoperitoneum with a Veress needle and, when attached to the first 11 mm laparoscopic trocar, providing high flow rates displayed objective real-time value of IAP.
The subcutaneous layer and rectus muscle thickness were obtained by ultrasound with a linear probe in the 18–6 MHz frequency range (High Frequency Linear 8870, FlexFocus 500, BK Medical, Denmark).
Measurements
The study protocol was designed in consideration of standard laparoscopic cholecystectomy in order not to prolong the procedure or in any way endanger participants. Patients were placed in a supine position on the operating table and given general anesthesia with complete muscle relaxation, which was monitored throughout measurement by the anesthesiology team with Train of Four (TOF) monitoring.
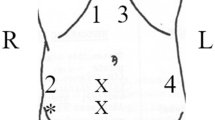
First, the line connecting the left and right anterior superior iliac spine was determined as this was the height of later sensor placement. The rectus muscle was identified using a linear ultrasound probe, and the thickness of the abdominal wall and rectus muscle was measured. Subtraction of rectus muscle from abdominal wall thickness resulted in the extent of subcutaneous fat. This information could be potentialy useful in the further investigation, but was not used in the current study. The measurement site was marked, and the skin was thoroughly cleaned and degreased using alcohol swabs, which provided excellent sensor-skin contact. Sensors were attached to a previously determined line 5 cm from the midline using adhesive patches (Fig. 2).
(a) 2 MC sensors were placed on the line connecting the left and right anterior superior iliac spine, 5 cm from the midline, over the rectus muscle, (b) as depicted on an exemplary photo of a patient. The sensors were then covered with surgical drapes, maintaining the sterile conditions required for laparoscopic cholecystectomy. Note that the initial 11 mm supraumbilical port was used to connect the CO2 insufflator.
Following sterile preparation of the operative field, sensors were attached entirely outside of the sterile field and covered with standard surgical drapes. The transverse umbilical skin incision was followed by initial abdominal insufflation using the Veress needle technique to standard 12 mmHg. An 11 mm laparoscopic trocar was subsequently inserted and connected to a CO2 insufflator via an insufflation tube. The abdominal cavity was checked for any possible viscus injury during the initial steps using the laparoscopic camera. The IAP value on the CO2 insufflator was reduced to 5 mmHg, and after stabilization of the IAP value, the first measurement using sensors for 60 s was performed. A 60-s interval was applied for skin viscoelasticity adjustment and temperature calibration. This period enables the sensors to stabilize against the skin’s viscoelastic properties and adapt to body temperature, ensuring accurate and consistent measurements by establishing an adequately calibrated baseline. After that, we performed 10 consecutive measurements with sensors for IAP values set on the CO2 insufflator ranging from 7 to 25 mmHg, each value lasting 15 s after stabilization of the IAP value on the insufflator. IAP sequence was patient-unique and upfront randomized using a computer randomization program. During the survey, synchronization of IAP value with MC sensor reading was obtained using a high-definition camera with 60 frames per second. After 10 consecutive IAP values, the last measurement was performed at 21 mmHg IAP for 60 s. This value was used as IAP above 20 mmHg, which was proven to be clinically significant in the setting of ACS and, therefore, subject to deeper investigation. Throughout the survey, the anesthesiology team carefully monitored the patient’s vital signs and was instructed to stop the measurement procedure if there were indications of patient instability.
With study measurements completed, the surgeon in charge proceeded to elective laparoscopic cholecystectomy. The MC sensors were removed at the end of laparoscopic cholecystectomy as they were outside of the sterile operative field and did not interfere with the planned procedure.
Signal processing
To monitor IAP continuously, we used two synchronized MC sensors that recorded the raw MC signals for 300 s. The measurement duration was prolonged to account for the initial effects of indentation and temperature on the skin. The skin adapted to the applied pressure during this period, resulting in higher tension values. Additionally, the piezoresistive silicon-based sensor adjusted to the skin temperature, ensuring consistent readings throughout the measurement process.
The sampling rate was 1 kHz. We applied a forwarder-backward 3rd-order low-pass Butterworth filter with a cut-off frequency of 4 Hz to each raw MC signal to remove high-frequency noise. The sensor system was set to detect IAP variations during random changes of IAP from 5 to 25 mmHg with a step size of 2 mmHg. For the purpose of analysis, the signals were decimated by a factor of ten and segmented into 15 s intervals corresponding to the different IAP levels, for each of the 41 recordings from the patients. We removed the respiratory and pulse effects (local maxima) from the signal and computed the average value for each 15 s interval. To enable direct comparability between the MC sensor outputs (15 s averages) and the 5–25 mmHg range measured by the insufflator, we applied a simple min–max linear scaling to each sensor reading, where the lowest reading was mapped to 5 mmHg and the highest reading was mapped to 25 mmHg. These data were used for statistical analysis.
Statistical methods
The linear correlation between the IAP value set on the endoscopic insufflator and the IAP value detected with MC sensors was assessed using Pearson correlation coefficients. The strength of this relationship was further quantified by calculating the coefficient of determination (R2 values). A Receiver Operating Characteristic (ROC) analysis was conducted to assess the ability of the MC sensor system to identify abnormal IAP conditions such as intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS). The ROC curve compares Sensitivity (True Positive Fraction, TPF) and Specificity (False Positive Fraction, FPF) for various MC sensor signal cut-off values. The Bland–Altman plot was used to assess the agreement between the MC sensor results and the gold-standard method using an insufflator, demonstrating the consistency of the measurements across the range.
Results
We enrolled 41 patients in the study, with the majority being female (71%, n = 29) and 29% male (n = 12). The age, the body height and the body mass were not significantly different from the normal distribution, while Shapiro–Wilk test showed significant departure from normality for BMI (p = 0.006). Table 1 summarizes patient characteristics.
All data from the 41 study participants were pooled for the final analysis.
We used the Pearson correlation coefficient to analyze the relationship between the IAP values and the MC sensor measurements, as the scatter plot indicated a linear relationship. Although the data distribution was not normal (negative Shapiro–Wilk test, p < 0.001), the use of the Pearson coefficient was justified due to the large sample size (41 study participants × 11 measurments = 451)) and the linear nature of the relationship. Additionally, we calculated the Spearman correlation coefficient, which was very similar to the Pearson coefficient (Pearson r = 0.963, Spearman ρ = 0.969), confirming that the relationship is almost entirely linear. Both coefficients were statistically highly significant (p < 0.001).
The Pearson correlation coefficient r = 0.963 (Fig. 3) indicates a strong positive linear correlation between the MC sensor signals and the IAP values set on the insufflator. These results suggest that the MC sensor system accurately detects changes in IAP. The calculated coefficient of determination (R2) of 0.927 demonstrates that 92.7% of the variation in IAP values is explained by the MC sensor signal, confirming the accuracy of the sensor system in predicting IAP values measured by the insufflator.
The ROC analysis demonstrated the excellent performance of the MC sensor system in identifying both intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) cases. For IAH, defined as an IAP > 12 mmHg, the AUC was 0.996 (Fig. 4), with a 95% confidence interval of 0.993 to 0.999 (asymptotic probability < 0.0001). This result indicates a very low probability of misclassification, meaning that the MC sensor system performs exceptionally well in distinguishing IAH from normal IAP. The optimal cut-off value for IAH detection was 12.591, determined by maximizing the Youden index (TPF + TNF-1, where TNF is the True Negative Fraction or Specificity). At this cut-off value, the sensitivity was 0.995, and the specificity was 0.949, confirming the sensor’s high diagnostic accuracy.
Similarly, for ACS, defined as an IAP > 20 mmHg, the AUC was 0.981, with a 95% confidence interval of 0.969–0.992 (asymptotic probability < 0.0001) (Fig. 5). These results indicate that the MC sensor system is also highly accurate in detecting ACS. The optimal cut-off value for ACS detection was 20.53, with a sensitivity of 0.975 and a specificity of 0.900, further highlighting the system’s reliability in distinguishing ACS from other conditions.
The Bland–Altman plot (Fig. 6) assessed the agreement between the MC sensor system and the insufflator. The bias (mean difference) was -1.51 mmHg, the limits of agreement were − 5.00 mmHg and 1.98 mmHg. These findings indicate that the MC sensor may provide assessment of IAP consistent with the insufflator measurements, with over 95% of the differences falling within these limits.
Discussion
This study is the first to compare MC sensor IAP measurements with objectively set IAP values using a CO2 insufflator during elective cholecystectomy. The MC sensor is a novel device for continuous IAP monitoring that has been proven to assess IAP accurately.
The accuracy of the MC sensor was validated by its strong correlation with accurate intraperitoneal pressure measured by the CO2 insufflator. Pearson’s correlation coefficient (r = 0.963) demonstrates a robust positive linear association between IAP measurements obtained via an insufflator and MC34 signals recorded with an MC sensor. Concurrently, the Spearman rank correlation coefficient (ρ = 0.969) reveals an almost perfect monotonic relationship, indicating that as one variable increases, the other consistently rises, independent of a strictly linear form. Thus, in our evaluation of IAP measurements and MC sensor-captured signals, Pearson’s r and Spearman’s ρ provide complementary statistical insights into the strength and nature of the association between the variables. Furthermore, the coefficient of determination (R2 = 0.927) from linear regression analysis indicates that the MC sensor signals explain 92.7% of the variation in IAP values, underscoring its high predictive value for IAP measurement.
ROC analysis further confirmed the MC sensor’s effectiveness in identifying IAH and ACS. The sensor achieved an AUC value of 0.996 for IAH, with a sensitivity of 99.5% and a specificity of 94.9%, reflecting nearly perfect performance in detecting elevated IAP (above 12 mmHg) and distinguishing it from normal levels. Similarly, an AUC value of 0.981 for ACS, with a sensitivity of 97.5% and a specificity of 90%, underscores the sensor’s excellent ability to detect ACS, partially defined by IAP exceeding 20 mmHg.
The Bland–Altman analysis revealed a rather wide lower limit of agreement, − 5.0 mmHg, which may indicate possible underestimating of IAP. However, several factors mitigate this impact. First, the threshold for abdominal compartment syndrome (ACS) is typically higher than IAP 20 mmHg, rendering ± 5 mmHg deviation around lower IAP values less clinically significant. Second, the MC sensor’s continuous monitoring approach allows trends to be detected promptly, reducing reliance on sporadic measurements and improving the early recognition of critical IAP elevations. Finally, this degree of variation aligns with existing methods of IAP assessment, including intravesical techniques. Despite the − 5 mmHg discrepancy, the MC sensor’s real-time predictive capability appears advantageous for early intervention and enhanced patient outcomes.
The MC sensor system is entirely non-invasive, with the electrodes attached to the skin similar to electrocardiogram (ECG) electrodes, making it possible to monitor IAP in intensive care unit patients continuously. Intermittent IAP measurement is widely accepted as routine practice in these patients and patients with a high clinical suspicion of developing ACS21. ACS, and to some extent IAH, in critically ill patients, is associated with increased morbidity and mortality, and prompt identification and treatment are essential to mitigate severe outcomes and improve patients’ survival. Elevated IAP not only affects the abdominal organs but also has far-reaching effects on other organ systems. The kidneys are typically the first organs to exhibit signs of acute injury related to ACS due to venous congestion, parenchymal compression, and declined renal blood flow. Increased IAP also causes an upward shift of the diaphragm and lowers chest wall compliance, ultimately reducing lung volumes and causing hypoxia and hypercarbia4,22,23,24.
The current gold standard for measuring IAP involves assessing the hydrostatic pressure inside the urinary bladder, which correlates well with IAP when the proper technique is applied. The method’s major advantages include low cost, relative non-invasiveness, and low complication risks. However, in some situations, such as bladder trauma, neurogenic bladder dysfunction, outflow obstruction, or pelvic hematomas, this technique is contraindicated or unreliable21,25.
The MC sensor offers several benefits for managing patients at risk of increased IAP. Its patch-like, transcutaneous design allows for completely non-invasive measurement, avoiding the need for bladder fluid instillation and reducing the risk of the urinary tract infection. The continuous, real-time data stream from the MC sensor to the patient monitor provides IAP values at all times, enhancing patient monitoring. Additionally, it offers insights into pressure changes and helps tailor treatment protocols to individual needs. Unlike intermittent measurements, the MC sensor can better track IAP trends, which is crucial for early detection of IAH and timely therapeutic interventions. This feature improves overall patient care quality. Furthermore, the automated nature of the MC sensor system reduces the workload on healthcare providers and minimizes potential errors associated with examiner skills.
In the study by Kušar et al.20 the specificity of detecting IAP of 20 mmHg and above was 88%, with a negative predictive value of 96%. However, patients increased their IAP using the Valsalva maneuver with voluntary abdominal muscle contractions, which may affect the transcutaneous signals more than the actual change in IAP. In our study, patients were under general anesthesia with complete muscle relaxation. This ensured that muscle contractions did not influence sensor readings.
This study presents a novel transcutaneous monitoring system for detecting IAP, demonstrating the feasibility and accuracy of MC sensors for continuous, real-time pressure monitoring. Moreover, the continuous, real-time nature of MC sensor monitoring offers the potential to detect emerging trends in IAP, allowing for predictive and preventive actions. With sufficient data, advanced algorithms, including machine learning, can be developed to recognize patterns in time-series measurements, enabling early intervention before critical IAP thresholds are reached. This approach could significantly improve patient outcomes by identifying subtle changes in pressure dynamics that may not be apparent through intermittent measurement methods.
Despite the advantages highlighted, several limitations must be acknowledged. First, all patients in the study were under general anesthesia with complete muscle relaxation, similar to critically ill patients in intensive care units. However, we cannot generalize the system accuracy to non-anesthetized patients whose voluntary muscle contractions might interfere with measurements. Although this issue also affects contemporary intravesical measurement systems, further studies are needed to address this limitation. Second, while the current MC sensor system effectively detects changes and trends in IAP, it cannot independently ascertain absolute pressure values. Consequently, further research is required to calibrate the sensor for full clinical autonomy. In parallel, the simultaneous determination of absolute values via the MC sensor and deformation measurement through multifrequency bioimpedance27 holds promise for facilitating the clinical application of this novel method. Third, IAP changes in this study were achieved by setting the desired IAP value on a CO2 insufflator. While an error margin less than 15% of the preset intra-abdominal pressure setting was previously reported, using an insufflator likely remains the most accurate method for establishing true intra-abdominal pressure. This approach allowed us to avoid the potential inaccuracies associated with the intravesical system, which, despite its limitations, remains the current reference standard for IAP measurement. To validate the new method, the next phase of our study will include a comparison of all three methods: the CO2 insufflator, MC sensors, and the intravesical system.
Conclusion
The study confirms that the MC sensor system is a novel and effective method for continuously monitoring IAP in a non-invasive manner. The system strongly correlates with IAP values measured by CO2 insufflation, indicating its accuracy.
The MC sensor demonstrates exceptional sensitivity, specificity, and AUC values in scaled signal measurements, critical attributes for reliably monitoring abdominal wall pressure variations. While the sensor currently accurately tracks intra-abdominal pressure (IAP) fluctuations, entire clinical viability hinges on calibration to ensure precise absolute IAP measurements. Further research is needed to optimize the calibration protocol and evaluate performance in non-anesthetized patients, where motion artifacts and physiological variability may challenge accuracy. Additionally, integrating advanced analytical techniques such as machine learning and iterative data-driven refinements could empower the system to detect emerging trends and provide early alerts for hazardous IAP elevations. This advancement would enable timely, targeted interventions, ultimately enhancing outcomes for critically ill patients.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at TMG-BMC Ltd.
References
Malbrain, M. L. et al. Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. I. Definitions. J Intensive Care Med. 32(11), 1722–1732 (2006).
Stokes, I. A. F., Gardner-Morse, M. G. & Henry, S. M. Intra-abdominal pressure and abdominal wall muscular function: Spinal unloading mechanism. Clin. Biomech. (Bristol, Avon). 25(9), 859 (2010).
Rogers, W. K. & Garcia, L. Intraabdominal hypertension, abdominal compartment syndrome, and the open abdomen. Chest. 153(1), 238–250 (2018).
Kirkpatrick, A. W. et al. Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 39(7), 1190 (2013).
Leon, M., Chavez, L. & Surani, S. Abdominal compartment syndrome among surgical patients. World J. Gastrointest. Surg. 13(4), 330 (2021).
Cheatham, M. L. et al. Abdominal perfusion pressure: A superior parameter in the assessment of intra-abdominal hypertension. J. Trauma 49(4), 621–627 (2000).
Cheatham, M. L. Abdominal compartment syndrome: Pathophysiology and definitions. Scand. J. Trauma Resusc. Emerg. Med. 17, 10 (2009).
Ertel, W., Oberholzer, A., Platz, A., Stocker, R. & Trentz, O. Incidence and clinical pattern of the abdominal compartment syndrome after “damage-control” laparotomy in 311 patients with severe abdominal and/or pelvic trauma. Crit. Care Med. 28(6), 1747–53 (2000).
Morken, J. & West, M. A. Abdominal compartment syndrome in the intensive care unit. Curr Opin. Crit Care 7(4), 268–274 (2001).
Malbrain, M. L. N. G. & De laet, I. E. Intra-abdominal hypertension: Evolving concepts. Clin. Chest Med. 30(1), 45–70 (2009).
Reintam Blaser, A. et al. Incidence, risk factors, and outcomes of intra-abdominal hypertension in critically Ill patients—A prospective multicenter study (IROI Study). Crit. Care Med. 47(4), 535 (2019).
Hecker, A. et al. Acute abdominal compartment syndrome: Current diagnostic and therapeutic options. Langenbeck’s Arch. Surg. 401(1), 15–24 (2016).
Rogers, W. K. & Garcia, L. Intraabdominal hypertension, abdominal compartment syndrome, and the open abdomen. Chest 153(1), 238–250 (2018).
Liao, C. H. et al. Systematic review of diagnostic sensors for intra-abdominal pressure monitoring. Sensors 21(14), 4824 (2021).
Kirkpatrick, A. W. et al. Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 39(7), 1190 (2013).
Gudmundsson, F., Viste, A., Gislason, H. & Svanes, K. Comparison of different methods for measuring intra-abdominal pressure. Intensive Care Med. 28(4), 509–514 (2002).
Dordević, S., Stančin, S., Meglič, A., Milutinović, V. & Tomažič, S. MC sensor: A novel method for measurement of muscle tension. Sensors (Basel) 11(10), 9411–9425 (2011).
Meglič, A., Uršič, M., Škorjanc, A., Đorđević, S. & Belušič, G. The Piezo-resistive MC sensor is a fast and accurate sensor for the measurement of mechanical muscle activity. Sensors (Basel). 19(9), 2108 (2019).
Đorđević, S., Tomažič, S., Narici, M., Pišot, R. & Meglič, A. In-vivo measurement of muscle tension: Dynamic properties of the MC sensor during isometric muscle contraction. Sensors (Basel). 14(9), 17848–17863 (2014).
Kušar, M., Djokić, M., Djordjević, S., Hribernik, M., Krašna, S. & Trotovšek, B. Preliminary study of reliability of transcutaneous sensors in measuring intraabdominal pressure. Sci. Rep. 12(1). Available from https://pubmed.ncbi.nlm.nih.gov/35585106/ (2022).
Sugrue, M., De Waele, J. J., De Keulenaer, B. L., Roberts, D. J. & Malbrain, M. L. N. G. A user’s guide to intra-abdominal pressure measurement. Anaesthesiol. Intensive Ther. 47(3), 241–251 (2015).
Regli, A., Pelosi, P. & Malbrain, MLNG. Ventilation in patients with intra-abdominal hypertension: What every critical care physician needs to know. Ann. Intensive Care 9(1).
Malbrain, M. L. N. G., De Keulenaer, B. L. & Khanna, A. K. Continuous intra-abdominal pressure: Is it ready for prime time?. Intensive Care Med. 48(10), 1501 (2022).
Łagosz, P., Sokolski, M., Biegus, J., Tycinska, A. & Zymlinski, R. Elevated intra-abdominal pressure: A review of current knowledge. World J. Clin. Cases 10(10), 3005 (2022).
Malbrain, M. L. N. G. Different techniques to measure intra-abdominal pressure (IAP): Time for a critical re-appraisal. Intensive Care Med. 30(3), 357–371 (2004).
Jacobs, V. R. & Morrison, J. E. The real intraabdominal pressure during laparoscopy: Comparison of different insufflators. J. Minim. Invasive Gynecol. 14(1), 103–107 (2007).
Wacker, J. et al. Non-invasive continuous measurement of the intra-abdominal pressure. EMBEC 2024. IFMBE Proc. 113, 160–168. https://doi.org/10.1007/978-3-031-61628-0_18 (2024).
Funding
The authors acknowledge the financial support from the Slovenian Research and Innovation Agency (research core funding No. P2-0109).
Author information
Authors and Affiliations
Contributions
All authors equally participated in the study design and study execution. S.D, S.K. and J.Ž. did the statistical analysis and prepared Fig. 2–6, B.P. prepared Fig. 1. B.P. an S.D. wrote the main manuscipt. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Plešnik, B., Djokić, M., Djordjević, S. et al. Non-invasive and continuous intra-abdominal pressure assessment using MC sensors. Sci Rep 15, 10775 (2025). https://doi.org/10.1038/s41598-025-95512-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95512-x
Keywords
This article is cited by
-
Variation and accuracy of intra-abdominal pressure measurement in different body positions: a prospective study
World Journal of Emergency Surgery (2025)