Abstract
Research on neuronal and astroglial markers for predicting outcomes in aquaporin-4 antibody-seropositive neuromyelitis optica spectrum disorders (NMOSD) remains limited. We aimed to evaluate the prognostic value of blood biomarkers for neuronal and astroglial damage in NMOSD compared with multiple sclerosis (MS). Patients with NMOSD and MS were prospectively recruited, and baseline serum levels of neurofilament light (sNfL) and glial fibrillary acidic protein (sGFAP) were measured. The correlations between these biomarkers and neurological disability (Expanded Disability Status Scale, EDSS) and cognitive function (iPad-based processing speed test, PST) were analyzed at baseline and two years later. In this cohort of 41 NMOSD and 92 MS patients, blood biomarkers demonstrated distinct patterns of association with current and future outcomes. In NMOSD, sGFAP was consistently linked to neurological disability and cognitive impairment over time, reflecting astrocytopathy with minimal silent neurodegeneration. In MS, sGFAP did not correlate with baseline EDSS but showed associations with future scores. Notably, sNfL was more strongly associated with future PST scores than baseline scores (p = 0.005), suggesting ongoing neurodegeneration. These results underscore that blood biomarkers are predictive of both current and future outcomes in NMOSD and MS, with differing patterns reflecting the unique pathogenesis of each disease.
Similar content being viewed by others
Introduction
Neuromyelitis optica spectrum disorders (NMOSD) and multiple sclerosis (MS) are autoimmune demyelinating diseases of the central nervous system (CNS), but they differ significantly in their underlying mechanisms and pathology1,2,3,4. NMOSD is primarily associated with autoantibodies targeting the aquaporin-4 (AQP4) protein expressed on astrocytes, leading to astrocyte damage and secondary demyelination5,6. Meanwhile, MS is characterized by immune-mediated damage to the myelin sheath and oligodendrocytes, although the specific antigen driving the immune response remains unidentified7,8,9. Despite these differences, both conditions are characterized by relapses and deterioration throughout life, necessitating the development of biomarkers to facilitate long-term prognostication10.
Recent advancements have highlighted the potential of serum glial fibrillary acidic protein (sGFAP) and serum neurofilament light chain (sNfL) as promising biomarkers for assessing disease activity and predicting disability in neurological diseases including NMOSD and MS11,12,13. These markers reflect different types of CNS damage, with sGFAP indicating astroglial injury and sNfL reflecting neuronal damage. In MS, studies have established a strong correlation between elevated levels of sGFAP and sNfL with future disease progression, including brain atrophy and increasing disability, suggesting their utility in long-term prognostication14,15,16,17,18,19,20. Similarly, in NMOSD, these biomarkers—particularly sGFAP—have been associated with current disease disability, relapse activity, and the future occurrence of relapses21,22,23,24,25,26. However, there have been limited studies investigating the long-term prognostic value of these biomarkers simultaneously in cohorts of both MS and NMOSD patients.
Given the distinct pathogenic mechanisms underlying NMOSD and MS, we hypothesized that the prognostic significance of sGFAP and sNfL would differ between these diseases. To test this hypothesis, we aimed to prospectively investigate the prognostic value of sGFAP and sNfL in predicting future outcomes, including neurological disability, cognitive function, clinical relapses, and disability progression, in NMOSD compared to MS.
Results
Baseline characteristics of participants
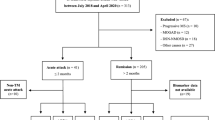
During the study period, a total of 260 patients were initially considered, including 192 with MS and 68 with NMOSD. Of these, 92 patients with relapsing-remitting MS and 41 with NMOSD consented to participate and met the criteria for enrollment, as they were not experiencing a recent clinical attack within 2 months prior to enrollment at the time (Fig. 1)21. NMOSD patients were significantly older and more likely to be female compared to MS patients (Table 1). They also had fewer years of education and a shorter duration of the disease than those with MS. After adjusting for age, baseline Expanded Disability Status Scale (EDSS) and Processing Speed Test (PST) scores were comparable between the groups. As for blood biomarkers, while sNfL levels did not differ between the groups, sGFAP levels were significantly higher in patients with NMOSD.
Correlation of blood biomarkers with clinical outcomes
A total of 37 patients with NMOSD and 87 with MS completed the follow-up EDSS evaluation, while 31 with NMOSD and 61 with MS completed the follow-up PST evaluation (Fig. 2). Over the 2-year follow-up period, EDSS scores in patients with MS increased (baseline vs. 2-year, median (IQR): 2.0 (1.0–3.5) vs. 2.0 (1.0–3.5), p = 0.024), indicating a decline in disability, while those in patients with NMOSD did not exhibit significant changes (2.5 (2.0–4.3) vs. 3.0 (2.0–3.5), p = 0.362). Meanwhile, PST scores improved in both NMOSD (36.0 (19.0–53.0) vs. 41.0 (23.0–54.0), p = 0.009) and MS (44.0 (30.5–60.0) vs. 49.0 (38.5–64.5), p < 0.001).
Correlations between blood biomarkers and clinical outcomes were assessed. Before the analysis, we evaluated the influence of treatment type on baseline blood biomarker levels. No significant differences were observed between B-cell depleting treatment (BCDT) and non-BCDT in patients with NMOSD (sGFAP: 125.9 vs. 126.8 pg/mL, p = 0.850; sNfL: 11.56 vs. 14.89 pg/mL, p = 0.172), nor between high-efficacy and low-efficacy treatments in patients with MS (sGFAP: 106.90 vs. 84.49 pg/mL, p = 0.101; sNfL: 9.93 vs. 9.58 pg/mL, p = 0.534). In patients with NMOSD, sGFAP was consistently positively correlated with both baseline EDSS scores (r = 0.43, p = 0.005) and future EDSS scores (r = 0.44, p = 0.006). In contrast, sNfL showed a positive association with EDSS scores that did not reach statistical significance (r = 0.31, p = 0.066) (Fig. 3a). Conversely, in patients with MS, sNfL was positively correlated with both baseline EDSS scores (r = 0.25, p = 0.018) and future EDSS scores (r = 0.32, p = 0.003). Meanwhile, sGFAP did not show a significant association with baseline EDSS scores but exhibited a trend toward a positive correlation (r = 0.19, p = 0.079) and demonstrated a significant correlation with future EDSS scores (r = 0.28, p = 0.010) (Fig. 3b).
Correlations between blood biomarkers and clinical outcomes at baseline and 2-year later. (a) sGFAP, sNfL–EDSS correlations in NMOSD, (b) sGFAP, sNfL–EDSS correlations in MS, (c) sGFAP, sNfL–PST correlations in NMOSD, (d) sGFAP, sNfL–PST correlations in MS. P values for comparisons of correlation coefficients between baseline and 2-year outcomes were as follows: (a) sGFAP: p = 0.958, sNfL: p = 0.682, (b) sGFAP: p = 0.532, sNfL: p = 0.617, (c) sGFAP: p = 0.754, sNfL: p = 0.757, (d) sGFAP: p = 0.180, sNfL: p = 0.005. ** p < 0.01 for correlation coefficients, baseline vs. 2-year outcomes. EDSS = Expanded disability status scale; MS = Multiple sclerosis; NMOSD = Neuromyelitis optica spectrum disorder; PST = Processing speed test; sGFAP = Serum glial fibrillary acidic protein, sNfL = Serum neurofilament light chain.
For PST scores in NMOSD patients, sGFAP correlated negatively with baseline (r = −0.51, p < 0.001) and future (r = −0.45, p = 0.010) scores. Notably, sNfL also showed significant negative associations with baseline (r = −0.50, p < 0.001) and future (r = −0.44, p = 0.012) scores (Fig. 3c). For PST scores in MS patients, sGFAP correlated with both baseline PST scores (r = −0.30, p = 0.004) and future scores (r = −0.49, p < 0.001). Conversely, sNfL did not show a significant correlation with baseline scores, but did correlate with future scores (r = −0.51, p < 0.001) (Fig. 3d). No significant differences were found between baseline and future correlations of serum biomarkers and clinical outcomes in NMOSD patients. However, in MS patients, significant differences were observed in the correlations between sNfL and PST scores from baseline to future values (p = 0.005) (Table 2).
Finally, we assessed whether baseline sGFAP and sNfL levels could predict changes in EDSS and PST scores during the study period (ΔEDSS and ΔPST). However, no significant associations were identified in these predictive analyses (Fig. 4).
Correlations between blood biomarkers and changes in clinical parameters. (a) sGFAP, sNfL–ΔEDSS correlations in NMOSD, (b) sGFAP, sNfL–ΔEDSS correlations in MS, (c) sGFAP, sNfL–ΔPST correlations in NMOSD, (d) sGFAP, sNfL–ΔPST correlations in MS. EDSS = Expanded disability status scale; MS = Multiple sclerosis; NMOSD = Neuromyelitis optica spectrum disorder; PST = Processing speed test; sGFAP = Serum glial fibrillary acidic protein, sNfL = Serum neurofilament light chain.
Clinical attacks and disease progression
During the follow-up period, a clinical attack occurred in four NMOSD patients (9.8%) and six MS patients (6.5%). Baseline sGFAP and sNfL levels showed no significant difference between patients with and without attacks in either the NMOSD (sGFAP: 121.9 vs. 128.2 pg/mL, p = 0.911; sNfL: 12.22 vs. 16.49 pg/mL, p = 0.608) or MS (sGFAP: 90.66 vs. 82.71 pg/mL, p = 0.849; sNfL: 9.83 vs. 8.42 pg/mL, p = 0.194) groups.
Additionally, six-month confirmed disability progression (6 m-CDP) was observed in two NMOSD patients (4.9%) and six MS patients (6.5%). The ages of NMOSD patients with 6 m-CDP were 69 and 64 years, while the median age [interquartile range] of MS patients with 6 m-CDP was 49.5 [43–59] years (Table 3). In NMOSD, all patients with 6 m-CDP experienced worsening ambulation as the primary manifestation of disease progression. Notably, one patient (patient #1) also showed a decline in PST scores during this period. However, MRI evaluations were not conducted at the time of progression to confirm CNS pathology changes. Baseline sGFAP and sNfL levels did not differ between patients with and without 6 m-CDP in either group, with NMOSD showing sGFAP levels of 229.3 pg/mL versus 121.9 pg/mL (p = 0.243) and sNfL levels of 32.75 pg/mL versus 12.22 pg/mL (p = 0.108), while in MS, sGFAP was 70.34 pg/mL versus 90.66 pg/mL (p = 0.502), and sNfL was 9.66 pg/mL versus 9.83 pg/mL (p = 0.772).
Discussion
This prospective longitudinal study assessed the prognostic value of sGFAP and sNfL in predicting two-year clinical outcomes in patients with NMOSD and MS. The baseline levels of these biomarkers correlated with both concurrent and future outcomes, showing distinct patterns between the two conditions.
Both sNfL and sGFAP have been recognized in recent years as disease biomarkers for MS and NMOSD. In MS, sNfL levels are valuable for predicting subclinical disease activity, assessing relapse risk, and identifying the development of gadolinium-enhancing lesions20,27,28,29,30,31. Conversely, sGFAP offers additional insights into disease progression, particularly due to its enhanced ability to detect disability progression independent of relapse activity (PIRA)16,19,32. The differential associations of these biomarkers in MS have been attributed to their specific roles: higher baseline sNfL levels are indicative of white matter volume loss, while baseline sGFAP levels correlate specifically with gray matter (GM) loss19,33,34. sGFAP is considered more reflective of a diffuse disease process in the brain, as its elevation is associated with astrocyte proliferation, activation, and potential injury35,36. Meanwhile, sNfL levels increase to a lesser extent in progressive MS, with these elevations attributed to neuronal loss as part of the progression pathogenesis, subclinical neuroinflammation in chronic active lesions, and changes in normal-appearing white matter19,37. In NMOSD, sGFAP, as a marker of astrocyte damage, is more closely associated with disease status, including disease disability, relapse activity, treatment response, and future relapse risk21,22,23,24,25,26. While sNfL also correlates with disease disability, this association is relatively weaker compared to sGFAP21,23,25.
In this study, simultaneous analysis of these biomarkers in NMOSD and MS patients revealed distinct correlations with clinical parameters, highlighting their differing pathogenesis1,2,3,4,10. In MS, sNfL showed a strong correlation with EDSS scores, consistent with its prognostic ability for white matter volume loss. In contrast, in NMOSD, EDSS scores were predominantly associated with sGFAP, reflecting its astrocytopathic nature of the disease, while this was not observed in MS. Aligning its role as a marker for disease progression and potential GM loss16,19,32, sGFAP correlated with both concurrent and future cognitive function in MS and NMOSD, although the association patterns differed between the two diseases. While patients with NMOSD exhibited comparable correlations between blood biomarkers and PST scores, patients with MS demonstrated significantly stronger NfL–PST correlations at future time points than at baseline. This suggests that neurodegeneration in MS may progress more rapidly in patients with severe underlying neuronal damage.
Although NMOSD and MS are both associated with chronic autoimmune inflammation, their progression patterns differ significantly4. NMOSD primarily involves attack-related progression with limited inter-attack neurodegeneration38,39, unlike MS, which involves ongoing, silent neurodegenerative processes that contribute to worsening clinical outcomes over time, independent of clinical relapses. These differing progression patterns may be attributed to distinct brain pathology and pathogenic mechanisms4. Cortical GM lesions, a hallmark of neurodegeneration in MS, are also present in NMOSD but differ significantly in structure and composition. In NMOSD, cortical GM lesions are defined by scattered loss of cortical neurons in layers II‒IV, loss of AQP4 immunoreactivity on astrocytes in layer I without complement deposition, microglial activation in layer II, and lymphocytic meningeal infiltration40,41. Importantly, NMOSD lesions lack cortical demyelination and meningeal B-cell lymphoid follicle-like infiltrates, which are characteristic features of MS brains40,41. Moreover, astrocyte loss may also explain the relatively limited neurodegeneration in NMOSD. While astrocyte loss initially causes excitotoxic neuronal and axonal damage, activated astrocytes in MS promote inflammation, tissue injury, and chronic neurodegeneration42,43. In NMOSD, acute astrocyte loss contributes to focal neurodegeneration in active lesions44, whereas chronic astrocyte activation in MS drives progressive neurodegeneration.
Clinical progression outcomes in real-world settings also differ between NMOSD and MS38. Recent studies report a PIRA prevalence of less than 3% in NMOSD39,45, significantly lower than the approximately 5% annual PIRA rate in MS, where PIRA accounts for over 50% of disability accrual events46. In our cohort, the prevalence of 6 m-CDP was lower in NMOSD patients (4.9%, 2 patients) than in MS patients (6.5%, 6 patients) but exceeded rates reported in prior studies39,45. This elevated rate should be interpreted with caution, given the small sample size and potential confounding factors, including age-related changes such as muscle disuse atrophy. Notably, NMOSD patients who developed 6 m-CDP exhibited higher baseline levels of sGFAP (229.3 pg/mL vs. 121.9 pg/mL) and sNfL (32.75 pg/mL vs. 12.22 pg/mL) compared to those without 6 m-CDP. Further research with larger cohorts and extended follow-up is needed to validate these observations.
The correlation of PST scores with both sGFAP and sNfL in NMOSD is noteworthy. Cognitive dysfunction in NMOSD has received less attention, and the usefulness of PST or symbol-digit modality tests in NMOSD is not well established as in MS47. However, the consistent associations of PST scores with neuronal and astroglial damage markers emphasize the importance of incorporating these tests in NMOSD care. The observed improvement in PST scores over time may be attributed to a practice effect from repeated testing48,49, rather than genuine cognitive improvement. Longer-term studies with larger sample sizes are needed to clarify the clinical significance and implications of these findings.
There are several limitations to this study. The relatively small number of participants may have influenced the strength and reliability of the correlations between baseline sGFAP and sNfL with the occurrence of clinical relapses or 6 m-CDP in both NMOSD and MS patients. The limited number of participants and the infrequent occurrence of significant clinical deterioration may have contributed to the absence of significant associations between baseline blood biomarkers and clinical events or changes in clinical parameters (ΔEDSS and ΔPST) during the study period. Nevertheless, distinct biomarker correlations based on disease type and specific time points were still identified. Another limitation is the exclusion of patients with progressive MS, which may have reduced the ability to detect stronger associations between the biomarkers and future clinical outcomes in the MS group. An age imbalance between two disease groups should also be noted. Considering that age can impact neurological status, cognitive function tests, and the relationship between disease biomarkers and clinical parameters10, the 6-year median age difference between the groups may have influenced the observed differences between NMOSD and MS, warranting a cautious interpretation. Finally, the lack of analysis correlating MRI findings with blood biomarkers represents another constraint of the study.
In conclusion, sGFAP and sNfL were associated with both concurrent and future outcomes in NMOSD and MS patients, but the patterns differed, suggesting distinct pathogenesis and neurodegeneration in each disease. These findings provide further insights into the mechanisms of CNS demyelinating diseases and underscore the importance of disease-specific interpretations of biomarkers.
Methods
Participants and study design
From October 2019 to July 2021, we prospectively enrolled patients with either MS or NMOSD who visited the department of Neurology at a tertiary medical center (Asan Medical Center, Seoul, Korea). The study adhered to the principles of the Declaration of Helsinki and was approved by the institutional review boards and ethics committee (approval No. 2020 − 1678). Written informed consent was obtained from all participants before enrollment.
The diagnoses of NMOSD and MS were made in accordance with established consensus criteria50,51. Specifically, NMOSD diagnosis required the presence of aquaporin-4 (AQP4) antibodies detected through a cell-based assay. Patients who had experienced a clinical relapse within 2 months prior to enrolment or had neurological impairments that could interfere with cognitive testing, such as severe visual impairment (20/200 or worse) or significant limb paralysis, were excluded from the study.
Blood samples were collected at enrolment, and neurological disability was evaluated using the Expanded Disability Status Scale (EDSS). The Processing Speed Test (PST), an iPad-based tool simulating the Symbol Digit Modalities Test was also conducted with additional informed consents52. EDSS and PST evaluations occurred at 6-month intervals. Clinical relapses and 6-months confirmed disability progression (6mCDP), defined as a sustained increase in the EDSS score for at least 180 days since the previous visit of ≥ 1.0 point from an EDSS score of 1.0–5.0 or ≥ 0.5 point from an EDSS score of ≥ 5.553, were also investigated.
Blood biomarkers
Serum samples were gathered and preserved at a temperature of −80 °C following standardized protocols. Only samples that had been thawed once were used in the experiments, and they were carefully thawed immediately before analysis. NfL and GFAP serum levels were assessed in duplicate using a Simoa™ HD-1 Analyzer (Quanterix, MA, USA) at PrismCDX (Gyeonggi-do, South Korea) by a clinical data blinded investigator.
Statistical analysis
We depicted the clinical traits of the patients using the median and interquartile range for continuous variables and frequency (%) for categorical variables. We compared clinical variables between MS and NMOSD patients using the Mann-Whitney U test for continuous variables and the Chi-square test for categorical variables. EDSS, PST scores, and serum biomarker levels for each group were compared using ANCOVA with age as a covariate. Longitudinal analyses were performed on patients who underwent evaluation for both EDSS and PST at baseline and approximately two years later. Paired values of EDSS and PST scores (baseline and two-year) were compared using the Wilcoxon signed-rank test due to their non-parametric distributions. Pearson’s correlation test was used to examine the relationships between log-transformed serum biomarker levels and EDSS or PST scores. We compared the statistical differences between baseline correlations and those at the two-year follow-up using Fisher’s Z transformation. We also evaluated the correlations between log-transformed serum biomarker levels and changes in clinical parameters (EDSS and PST scores) over the study period. All statistical analyses were performed using GraphPad Prism version 10.1 and SPSS Statistics version 28.0.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Weinshenker, B. G. Natural history of multiple sclerosis. Ann. Neurol. 36 Suppl, 6–11. https://doi.org/10.1002/ana.410360704 (1994).
Lublin, F. D. & Reingold, S. C. Defining the clinical course of multiple sclerosis: results of an international survey. National multiple sclerosis society (USA) advisory committee on clinical trials of new agents in multiple sclerosis. Neurology 46, 907–911. https://doi.org/10.1212/wnl.46.4.907 (1996).
Lublin, F. D. et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 83, 278–286. https://doi.org/10.1212/WNL.0000000000000560 (2014).
Kawachi, I. & Lassmann, H. Neurodegeneration in multiple sclerosis and neuromyelitis Optica. J. Neurol. Neurosurg. Psychiatry. 88, 137–145. https://doi.org/10.1136/jnnp-2016-313300 (2017).
Lennon, V. A. et al. A serum autoantibody marker of neuromyelitis Optica: distinction from multiple sclerosis. Lancet 364, 2106–2112. https://doi.org/10.1016/s0140-6736(04)17551-x (2004).
Jarius, S. & Wildemann, B. The history of neuromyelitis Optica. J. Neuroinflammation. 10 https://doi.org/10.1186/1742-2094-10-8 (2013).
Kuerten, S. et al. Autoantibodies against central nervous system antigens in a subset of B cell-dominant multiple sclerosis patients. Proc. Natl. Acad. Sci. U S A. 117, 21512–21518. https://doi.org/10.1073/pnas.2011249117 (2020).
Hoftberger, R., Lassmann, H., Berger, T. & Reindl, M. Pathogenic autoantibodies in multiple sclerosis - from a simple Idea to a complex concept. Nat. Rev. Neurol. 18, 681–688. https://doi.org/10.1038/s41582-022-00700-2 (2022).
Zamecnik, C. R. et al. An autoantibody signature predictive for multiple sclerosis. Nat. Med. 30, 1300–1308. https://doi.org/10.1038/s41591-024-02938-3 (2024).
Lee, E. J. et al. Clinical implication of serum biomarkers and patient age in inflammatory demyelinating diseases. Ann. Clin. Transl Neurol. 7, 992–1001. https://doi.org/10.1002/acn3.51070 (2020).
Kim, H. et al. Serum biomarkers in Myelin oligodendrocyte glycoprotein antibody-associated disease. Neurol. Neuroimmunol. Neuroinflamm. 7 https://doi.org/10.1212/NXI.0000000000000708 (2020).
Kim, H. J. et al. Serum proteins for monitoring and predicting visual function in patients with recent optic neuritis. Sci. Rep. 13, 5609. https://doi.org/10.1038/s41598-023-32748-5 (2023).
Kim, K. W. et al. Disease characteristics of idiopathic transverse myelitis with serum neuronal and astroglial damage biomarkers. Sci. Rep. 13, 3988. https://doi.org/10.1038/s41598-023-30755-0 (2023).
Kuhle, J. et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology 88, 826–831. https://doi.org/10.1212/WNL.0000000000003653 (2017).
Uher, T. et al. Measurement of neurofilaments improves stratification of future disease activity in early multiple sclerosis. Mult Scler. 27, 2001–2013. https://doi.org/10.1177/13524585211047977 (2021).
Barro, C. et al. Serum GFAP and NfL levels differentiate subsequent progression and disease activity in patients with progressive multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 10 https://doi.org/10.1212/NXI.0000000000200052 (2023).
Canto, E. et al. Association between serum neurofilament light chain levels and Long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol. 76, 1359–1366. https://doi.org/10.1001/jamaneurol.2019.2137 (2019).
Bhan, A. et al. Neurofilaments and 10-year follow-up in multiple sclerosis. Mult Scler. 24, 1301–1307. https://doi.org/10.1177/1352458518782005 (2018).
Meier, S. et al. Serum glial fibrillary acidic protein compared with neurofilament light chain as a biomarker for disease progression in multiple sclerosis. JAMA Neurol. 80, 287–297. https://doi.org/10.1001/jamaneurol.2022.5250 (2023).
Barro, C. et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 141, 2382–2391. https://doi.org/10.1093/brain/awy154 (2018).
Watanabe, M. et al. Serum GFAP and neurofilament light as biomarkers of disease activity and disability in NMOSD. Neurology 93, e1299–e1311. https://doi.org/10.1212/WNL.0000000000008160 (2019).
Aktas, O. et al. Serum glial fibrillary acidic protein: A neuromyelitis Optica spectrum disorder biomarker. Ann. Neurol. 89, 895–910. https://doi.org/10.1002/ana.26067 (2021).
Schindler, P. et al. Serum GFAP and NfL as disease severity and prognostic biomarkers in patients with aquaporin-4 antibody-positive neuromyelitis Optica spectrum disorder. J. Neuroinflammation. 18, 105. https://doi.org/10.1186/s12974-021-02138-7 (2021).
Kim, H., Lee, E. J., Lim, Y. M. & Kim, K. K. Glial fibrillary acidic protein in blood as a disease biomarker of neuromyelitis Optica spectrum disorders. Front. Neurol. 13, 865730. https://doi.org/10.3389/fneur.2022.865730 (2022).
Kim, H. et al. Longitudinal follow-up of serum biomarkers in patients with neuromyelitis Optica spectrum disorder. Mult Scler. 28, 512–521. https://doi.org/10.1177/13524585211024978 (2022).
Schindler, P. et al. Glial fibrillary acidic protein as a biomarker in neuromyelitis Optica spectrum disorder: a current review. Expert Rev. Clin. Immunol. 19, 71–91. https://doi.org/10.1080/1744666X.2023.2148657 (2023).
Disanto, G. et al. Serum neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 81, 857–870. https://doi.org/10.1002/ana.24954 (2017).
Kuhle, J. et al. Neurofilament light levels are associated with long-term outcomes in multiple sclerosis. Mult Scler. 26, 1691–1699. https://doi.org/10.1177/1352458519885613 (2020).
Kapoor, R. et al. Serum neurofilament light as a biomarker in progressive multiple sclerosis. Neurology 95, 436–444. https://doi.org/10.1212/WNL.0000000000010346 (2020).
Benkert, P. et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 21, 246–257. https://doi.org/10.1016/S1474-4422(22)00009-6 (2022).
Freedman, M. S. et al. Guidance for use of neurofilament light chain as a cerebrospinal fluid and blood biomarker in multiple sclerosis management. EBioMedicine 101, 104970. https://doi.org/10.1016/j.ebiom.2024.104970 (2024).
Rosenstein, I. et al. Association of serum glial fibrillary acidic protein with progression independent of relapse activity in multiple sclerosis. J. Neurol. 271, 4412–4422. https://doi.org/10.1007/s00415-024-12389-y (2024).
Calabrese, M. et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch. Neurol. 66, 1144–1150. https://doi.org/10.1001/archneurol.2009.174 (2009).
Cagol, A. et al. Association of brain atrophy with disease progression independent of relapse activity in patients with relapsing multiple sclerosis. JAMA Neurol. 79, 682–692. https://doi.org/10.1001/jamaneurol.2022.1025 (2022).
Prineas, J. W. & Lee, S. Multiple sclerosis: destruction and regeneration of astrocytes in acute lesions. J. Neuropathol. Exp. Neurol. 78, 140–156. https://doi.org/10.1093/jnen/nly121 (2019).
Saraste, M. et al. Increased serum glial fibrillary acidic protein associates with microstructural white matter damage in multiple sclerosis: GFAP and DTI. Mult Scler. Relat. Disord. 50, 102810. https://doi.org/10.1016/j.msard.2021.102810 (2021).
Maggi, P. et al. Chronic white matter inflammation and serum neurofilament levels in multiple sclerosis. Neurology 97, e543–e553. https://doi.org/10.1212/WNL.0000000000012326 (2021).
Molazadeh, N. et al. Progression independent of relapses in aquaporin4-IgG-seropositive neuromyelitis Optica spectrum disorder, Myelin oligodendrocyte glycoprotein antibody-associated disease, and multiple sclerosis. Mult Scler. Relat. Disord. 80, 105093. https://doi.org/10.1016/j.msard.2023.105093 (2023).
Siriratnam, P. et al. Prevalence of progression independent of relapse activity and relapse-Associated worsening in patients with AQP4-IgG-Positive NMOSD. Neurology 103, e209940. https://doi.org/10.1212/WNL.0000000000209940 (2024).
Popescu, B. F. et al. Absence of cortical demyelination in neuromyelitis Optica. Neurology 75, 2103–2109. https://doi.org/10.1212/WNL.0b013e318200d80c (2010).
Saji, E. et al. Cognitive impairment and cortical degeneration in neuromyelitis Optica. Ann. Neurol. 73, 65–76. https://doi.org/10.1002/ana.23721 (2013).
Bruck, W. et al. Reduced astrocytic NF-kappaB activation by Laquinimod protects from cuprizone-induced demyelination. Acta Neuropathol. 124, 411–424. https://doi.org/10.1007/s00401-012-1009-1 (2012).
Brosnan, C. F. & Raine, C. S. The astrocyte in multiple sclerosis revisited. Glia 61, 453–465. https://doi.org/10.1002/glia.22443 (2013).
Hokari, M. et al. Clinicopathological features in anterior visual pathway in neuromyelitis Optica. Ann. Neurol. 79, 605–624. https://doi.org/10.1002/ana.24608 (2016).
Wingerchuk, D. M., Pittock, S. J., Lucchinetti, C. F., Lennon, V. A. & Weinshenker, B. G. A secondary progressive clinical course is uncommon in neuromyelitis Optica. Neurology 68, 603–605. https://doi.org/10.1212/01.wnl.0000254502.87233.9a (2007).
Muller, J. et al. Harmonizing definitions for progression independent of relapse activity in multiple sclerosis: A systematic review. JAMA Neurol. 80, 1232–1245. https://doi.org/10.1001/jamaneurol.2023.3331 (2023).
Seo, D. et al. Digital symbol-digit modalities test with modified flexible protocols in patients with CNS demyelinating diseases. Sci. Rep. 14, 14649. https://doi.org/10.1038/s41598-024-65486-3 (2024).
Fuchs, T. A. et al. Repeated forms, testing intervals, and SDMT performance in a large multiple sclerosis dataset. Mult Scler. Relat. Disord. 68, 104375. https://doi.org/10.1016/j.msard.2022.104375 (2022).
Castrogiovanni, N. et al. Longitudinal changes in cognitive test scores in patients with Relapsing-Remitting multiple sclerosis: an analysis of the DECIDE dataset. Neurology 101, e1–e11. https://doi.org/10.1212/WNL.0000000000207301 (2023).
Wingerchuk, D. M. et al. International consensus diagnostic criteria for neuromyelitis Optica spectrum disorders. Neurology 85, 177–189. https://doi.org/10.1212/WNL.0000000000001729 (2015).
Thompson, A. J. et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 17, 162–173. https://doi.org/10.1016/S1474-4422(17)30470-2 (2018).
Rao, S. M. et al. Processing speed test: validation of a self-administered, iPad((R))-based tool for screening cognitive dysfunction in a clinic setting. Mult Scler. 23, 1929–1937. https://doi.org/10.1177/1352458516688955 (2017).
Sharmin, S. et al. Confirmed disability progression as a marker of permanent disability in multiple sclerosis. Eur. J. Neurol. 29, 2321–2334. https://doi.org/10.1111/ene.15406 (2022).
Acknowledgements
This study was funded by grants from the Asan Medical Center (2023IP0077-1), the National Research Foundation of Korea (NRF), funded by the Korean government (MSIT) (No. 2023R1A2C2007798), and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HR21C0198).
Author information
Authors and Affiliations
Contributions
W.S., E.-J.L., J.-Y.K., H.-J.J., H.K. and Y.-M.L. contributed to conception and design of the study. W.S., D.S., I.J., J.H.K., L.C., K.K. and H.L. contributed to acquisition and analysis of data. W.S., E.-J.L., B.J.C., H.K. and Y.-M.L. contributed to drafting the manuscript and figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Tables.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shin, W., Lee, EJ., Seo, D. et al. Distinct prognostic implications of blood neuronal and astroglial biomarkers in neuromyelitis optica spectrum disorders versus multiple sclerosis. Sci Rep 15, 11326 (2025). https://doi.org/10.1038/s41598-025-95773-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95773-6
Keywords
This article is cited by
-
Longitudinal changes in sleep quality, and their predictors in patients with multiple sclerosis
Scientific Reports (2025)