Abstract
Sarcopenia is a prevalent condition in tumor patients and can potentially impact the prognosis of tumor treatment. This retrospective study aimed to evaluate the correlations between sarcopenia and the prognosis of patients with unresectable colorectal liver metastases (CRLM) received drug-eluting beads transcatheter arterial chemoembolization (DEB-TACE) therapy. From December 2018 to December 2023, unresectable CRLM patients who had already received second-line therapy from the Wuhan Union Hospital were involved in our study. Skeletal muscle mass was evaluated on CT at the L3 vertebra, and the optimal cut-off point for skeletal muscle index classification was determined using x-tile software. Overall survival (OS) and progression-free survival (PFS) were estimated using Kaplan–Meier analysis and Cox regression analysis. Seventy-one patients were included in the study, 34 with sarcopenia (sarcopenia group) and 37 without sarcopenia (non-sarcopenia group), respectively. The median PFS and OS was elevated in the non-sarcopenia group compared with the sarcopenia group (6.1 months versus 4.3 months, p = 0.012; 14.8 months versus 10.2 months, p < 0.001). The multivariate Cox regression analysis revealed that sarcopenia, extrahepatic metastases, and neutrophil-to-lymphocyte ratio (NLR) ≥ 5 were identified as independent risk factors for both PFS and OS. The advantages of non-sarcopenia in terms of OS were consistent across all subgroups examined. Additionally, the sarcopenia group exhibited a higher incidence of vomiting/nausea, fatigue, and abdominal pain following the DEB-TACE operation compared to the non-sarcopenia group. Sarcopenia demonstrated a substantial predictive value for both PFS and OS in unresectable CRLM patients who underwent DEB-TACE treatments. Besides, NLR > 5 and extrahepatic metastases were independent risk factors linked to a poorer prognosis. Furthermore, patients with sarcopenia may face an increased likelihood of experiencing adverse events following DEB-TACE treatments.
Similar content being viewed by others
Introduction
Liver metastasis is a crucial determinant of the prognosis for patients with colorectal cancer (CRC), often resulting in organ failure and high mortality rates1,2. The majority of patients with colorectal liver metastases (CRLM) are not eligible for surgical removal and hence receive systemic chemotherapy3. While systemic therapy has demonstrated some efficacy, achieving a long-term response is challenging due to chemotherapy toxicity and treatment resistance4. For unresectable CRLM patients who had failed systematic treatments, drug-eluting beads transcatheter arterial chemoembolization (DEB-TACE), a regional therapy for liver metastases, was considered an alternative optional treatment5. Several studies have shown that DEB-TACE treatment could improve survival rates in patients with unresectable CRLM and yield promising outcomes6,7,8. However, despite these encouraging findings, there remains a lack of identified indicators that can reliably predict patients’ response to DEB-TACE treatment. The development of such measures would be instrumental in stratifying patients and optimizing treatment efficiency.
Sarcopenia refers to a syndrome that is characterized by the gradual and widespread reduction of both skeletal muscle mass and its corresponding functionality9. Previous research indicated that elevated inflammatory markers within the circulatory system of cancer patients may constitute a risk factor for the development of sarcopenia in these patients10,11. The systemic inflammatory response disrupts protein turnover and cellular growth, potentially compromising skeletal muscle quality. The neutrophil-to-lymphocyte ratio (NLR), a widely recognized indicator of systemic inflammation, is higher in patients with sarcopenia compared to those without the condition12. To diagnose sarcopenia, L3 pyramidal analysis on computed tomography (CT) images is commonly utilized to quantify skeletal muscle and fat mass13. Although there is no consensus on the cut-off point for sarcopenia due to differences in clinical features, various studies have demonstrated that sarcopenia is linked to a poorer prognosis in patients with colorectal, lung, and pancreatic cancer14,15,16. Sevgilioglu et al. found that sarcopenia played a significant role as a prognostic element in the survival of CRLM patients who underwent chemotherapy17. Similarly, Yang et al. observed that sarcopenia influenced the overall survival (OS) of patients with CRLM undergoing hepatectomy, and it was also associated with more complications18. Nevertheless, there is limited research available on the impact of sarcopenia specifically on DEB-TACE treatment in patients with unresectable CRLM.
Hence, the objective of this study was to examine the relationship between sarcopenia and long-term prognosis as well as the complications in patients with unresectable CRLM who received DEB-TACE treatments following chemotherapy failure.
Methods
Patients
This retrospective cohort study included unresectable CRLM patients who had already received second-line therapy (FOLFIRI or FOLFOX, with or without bevacizumab or cetuximab) from the Wuhan Union Hospital between December 2018 to December 2023. The study was approved by the institutional review board of our local hospital (2018-S505). The Ethics Committee of Wuhan Union Hospital waived the requirement for informed consent due to the retrospective study design.
The inclusion criteria were as follow: (1) patients diagnosed with colorectal cancer liver metastases confirmed histologically; (2) patients treated with DEB-TACE treatment; (3) Eastern Cooperative Oncology Group (ECOG) performance status scores of 0 or 1; (4) aged > 18 years. The following were the exclusion criteria: (1) prior treatment with hepatectomy, radiation, or thermoablation of the liver; (2) liver metastases burden exceeding 70% of liver volume; (3) severe hepatic failure or renal impairment; (4) patients with incomplete medical records.
Imaging analysis of skeletal muscle mass
CT images of patients taken within one week prior to DEB-TACE operation were utilized for quantifying skeletal muscle mass at the level of the third lumbar vertebra (L3). The skeletal muscle index (SMI, cm2/m2) was employed to evaluate the status of skeletal muscle quality, which is determined by calculating the square of skeletal muscle area (cm2) divided by the square of height (m2)19. The images were analyzed by two trained radiologists, G.F.Z. and C.S.Z., who have 25 and 31 years of imaging experience, respectively. Tomovision SliceOmatic version 4.3 software (Toronto, Canada) were used to delineate the area of interest of the skeletal muscle on axial CT at the middle of the L3 vertebral body (Fig. 1). The muscles include the psoas, rectus, transverse abdominis, quadratus lumborum, internal obliques, longissimus pectoralis, and pectoral spinous muscles. The entire muscle area was measured, and the average of the two radiologists’ measurements was calculated for subsequent analysis.
The optimal cut-off point for SMI classification was confirmed using X-tile version 3.6.1 software (Yale University School of Medicine; New Haven, USA). This software offers a straightforward and well-rounded approach to classify the cohort into low and high levels of marker expression according to progression-free survival (PFS) or OS results20. Additionally, there are variations in the distribution of skeletal muscle and adipose tissue between male and female patients. Consequently, patients were categorized into sarcopenia group and non-sarcopenia group based on these defined cut-off values.
Treatment
The DEB-TACE operations were performed under the supervision of several experienced interventional radiologists. A 5-F catheter was introduced into the common hepatic artery and superior mesenteric artery to distinguish the tumor-supplying arteries. After confirming the artery, a 2.7-F coaxial microcatheter was used to perform super-selective arterial catheterization. Then, the 80 mg irinotecan loaded into one vial (2 ml) beads mixed with non-ionic contrast agents were delivered via the microcatheter. When the angiography showed that the staining of the tumor disappeared or almost disappeared, the operation ceased.
Follow-up and assessment
All patients were followed up until 31 December 2023. Abdominal contrast-enhanced CT, chest and pelvis-enhanced CT, and laboratory were carried out before treatment and every treatment course (6–8 weeks). The primary endpoint was OS. PFS, overall response rate (ORR), disease control rate (DCR), and adverse events were considered as secondary objectives. The treatment response including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), was evaluated according to the Response Evaluation Criteria in Solid Tumors 1.1 protocol (RECIST 1.1)21. PFS was defined as the duration from the first DEB-TACE treatment of the patient to either tumor progression according to the RECIST 1.1 criteria or death. The time from the start of DEB-TACE treatment to the last follow-up or patient death was defined as OS. ORR was defined to the percentage of patients who attained CR or PR. DCR was defined as the sum of CR, PR, and SD.
Statistical analysis
The IBM SPSS version 25.0 software (San Jose, New York, USA) and R version 4.1.1 software (Vienna, Austria) were applied to fundamental statistical analysis. Data are expressed in the count (%), median, or mean ± standard deviation. Kaplan–Meier curves were plotted to assess patients’ OS and PFS, while a log-rank test was employed to compare the two groups. Univariate analysis was applied, while the factors with p value < 0.1 were further assessed by multivariate analysis to confirm the potential prognostic factors impacting PFS and OS. P value < 0.05 (two-tailed) was regarded as statistically significant.
Results
Patient characteristics
From December 2018 to December 2023, 96 patients with unresectable CRLM were enrolled in this study. After applying the inclusion and exclusion criteria, we included 71 patients for analysis. Among them, 34 patients were classified to the sarcopenia group, while 37 patients were non-sarcopenia group (Fig. 2). The baseline characteristics of the two groups with unresectable CRLM are displayed in Table 1. There were no significant differences between the baseline data, except for SMA and SMI. The median follow-up period was 11 months (range 2–28 months) in the sarcopenia group and 15 months (range 2–33 months) in the non-sarcopenia group.
The optimum cutoff value of SMI
X-tile plot analysis was conducted using X-tile version 3.6.1 software to confirm the optimal cut-off based on the patient’s SMI index as well as OS. Prior researches indicated notable distinctions in skeletal muscle quality between females and males22.Therefore, we calculated separate cut-off values for each gender. The SMI cut-off value for 44 male patients was determined to be 40.8 cm2/m2, resulting in 21 men (47.7%) being assigned to the sarcopenia group and 23 men (52.3%) to the non-sarcopenia group (Fig. 3A, B). Similarly, 27 female patients with an SMI cut-off of 37.9 cm2/m2 were assigned to 13 women (48.1%) in the sarcopenia group and 14 women (51.9%) in the non-sarcopenia group (Fig. 3D, E). The Kaplan–Meier curves for OS of both sexes between the two groups (male: p = 0.020; female: p = 0.004) are presented in Fig. 3C, F.
Treatment response
The median number of DEB-TACE treatments was 3.0 in sarcopenia group and 3.3 in non-sarcopenia group. Imaging assessment information with RECIST, version 1.1, response was conducted after the initial treatment are listed in Table 2. In the sarcopenia group, 4 out of 34 patients (11.8%) met the PR criteria, 13 patients (38.2%) achieved SD, 17 patients (50.0%) were PD, and there were no cases of CR. In the non-sarcopenia group, 12 patients (32.4%) met the PR criteria, 14 patients (37.9%) achieved SD, and 11 patients (29.7%) were PD. No cases of CR occurred in this group as well. The ORR of tumor response in the non-sarcopenia group was 32.4%, which was higher than the sarcopenia group’s ORR of 11.8% (p = 0.037). Although there was no statistically significant difference, the DCR of tumor response was higher in the non-sarcopenia group than sarcopenia group (70.3% versus 50.0%, p = 0.081).
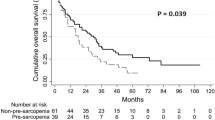
The median PFS was longer in the non-sarcopenia group (6.1 months, 95% confidence interval [CI]: 5.0, 7.2 months) compared with the sarcopenia group (4.3 months, 95% CI: 3.5, 5.1 months) (p = 0.012) (Fig. 4A). Similarly, the median OS was also significantly longer in the non-sarcopenia group than in the sarcopenia group (14.8 months [95% CI 11.1–18.5 months] versus 10.2 months [95% CI 8.9–11.5 months], p < 0.001) (Fig. 4B). The half-year OS in the non-sarcopenia group was 91.9%, which was higher than the 73.5% in the sarcopenia group (p = 0.039). Moreover, the one-year OS was also significantly higher in the non-sarcopenia group compared to the sarcopenia group (56.8% versus 26.5%, p = 0.010).
Sarcopenia associated with PFS and OS
At univariable analysis of PFS, the ECOG performance, location of liver metastases, extrahepatic metastases, neutrophil-to-lymphocyte ratio (NLR) > 5 and sarcopenia were considered as potential variables for the multivariable analysis in the CRLM patients. After adjustment by multivariant Cox regression model, the extrahepatic metastases (HR 2.069; 95% CI 1.221–3.506, p = 0.007), NLR > 5 (HR 1.884; 95% CI 1.094–3.245, p = 0.022) and sarcopenia (HR 1.900; 95% CI 1.141–3.163, p = 0.014) were independent risk factors in predicting PFS in CRLM patients (Table 3).
In the univariable analyses of OS in patients with CRLM, candidate variables included ECOG performance, synchronous metastatic status, extrahepatic metastases, NLR > 5, and sarcopenia. The final multivariable analysis revealed that extrahepatic metastases (HR 2.204; 95% CI 1.266–3.838, p = 0.005), NLR > 5 (HR 2.103; 95% CI 1.162–3.805, p = 0.014), and sarcopenia (HR 2.609; 95% CI 1.488–4.575, p < 0.001) were identified as independent risk factors for OS in CRLM patients (Table 4). In the subgroup analysis, Fig. 5 illustrates the HR of sarcopenia based on the patients’ clinical characteristics and survival. The analysis revealed that non-sarcopenia consistently showed beneficial effects on OS in all tested subgroups. Statistically significant differences were noted in all subgroups except for patients with right colon, ECOG = 0, unbilobar, non-extrahepatic metastases, and NLR ≤ 5.
Given that the study groups (sarcopenia and non-sarcopenia) employed SMI as the primary risk factor in a categorical form, we conducted a Cox regression analysis using SMI as a continuous variable to ensure the robustness and scientific rigor of our study’s conclusions. In the univariate analysis, SMI emerged as an independent influencing factor for both PFS and OS in patients with CRLM. Furthermore, in the multivariate regression models, SMI was identified as an independent risk factor for predicting PFS (HR 0.951; 95% CI 0.915–0.989, P = 0.012) and OS (HR 0.913; 95% CI 0.870–0.958, P < 0.001) in the CRLM patient population (Supplementary Table 1 and Supplementary Table 2). The findings indicate that utilizing SMI as a continuous variable yields conclusions consistent with those derived from treating SMI as a categorical variable, demonstrating that sarcopenia possesses significant predictive value for PFS and OS in unresectable CRLM patient. To further substantiate the validity of our conclusions, we generated receiver operating characteristic (ROC) curves for OS at various time points and calculated the corresponding area under the curve (AUC) value. Additionally, we constructed Kaplan–Meier survival curves for OS, utilizing the Youden index at 0.5-year and 1-year as optimal cutoff values (0.5-year: SMI = 37.8; 1-year: SMI = 43.6), which yielded results consistent with the result of using SMI as the categorical variable (Supplementary Fig. 1).
Adverse events
The adverse events (AEs) related to DEB-TACE treatments in both groups are presented in Table 5. No death events related to the treatment occurred in our study. Patients with sarcopenia were found to have a higher likelihood of experiencing vomiting compared to non-sarcopenic patients (73.8% vs 42.6%, p < 0.001). Additionally, the incidence of fatigue and abdominal pain was higher in the sarcopenia group compared to the non-sarcopenia group (43.7% versus 27.9%, p = 0.013; 41.7% versus 23.0%, p = 0.003). The probability of experiencing abdominal distension, fever, and hyperbilirubinemia did not significantly differ between the two groups (p > 0.05). No grade 4 or 5 toxicities related to DEB-TACE procedures were observed. In the sarcopenia group, three episodes of severe vomiting (grade 3) and four episodes of severe fever (grade 3) were observed. Similarly, the non-sarcopenia group experienced two episodes of severe vomiting (grade 3) and two episodes of severe fever (grade 3). The remaining adverse events were mostly mild to moderate, and all patients received successful symptomatic treatment, enabling timely administration of subsequent DEB-TACE operations.
Discussion
This study demonstrated that sarcopenia is an independent predictor of unresectable CRLM patients treated with DEB-TACE treatment following failed chemotherapy. Patients without sarcopenia had better treatment outcomes compared to those with sarcopenia. Moreover, we observed that patients with sarcopenia may experience a higher incidence of post-operation vomiting/nausea, fatigue, and abdominal pain when compared to non-sarcopenic patients. To our knowledge, this study is the first to investigate the association of sarcopenia with treatment outcomes in unresectable CRLM patients undergoing DEB-TACE.
Previous studies have shown that sarcopenia is associated the prognosis of treatment for tumor, such as head and neck cancer, stomach cancer, and pancreatic cancer23,24,25. Our study found that patients with non-sarcopenia had higher PFS and OS compared to the patients with sarcopenia who received DEB-TACE in patients with CRLM. Chen W et al. found that patients with sarcopenia after undergoing resection of liver metastases from rectal cancer had a poorer prognosis26, which aligns with our findings. In contrast, Thormann M et al. discovered that sarcopenia alone did not serve as an independent predictor of therapy outcomes in patients with CRLM who underwent interstitial brachytherapy27. The conflicting outcomes noted in these studies may be attributed to variations in cut-off values, discrepancies in the selection of clinical features, and differences in treatment modalities. The findings of our study indicate that sarcopenia serves as a prognostic factor in patients with CRLM. Clinically, this underscores the necessity of prioritizing the prevention of sarcopenia in this patient population. Prior research has demonstrated that enhanced nutritional intake and regular exercise can effectively prevent and ameliorate sarcopenia28,29, offering novel strategies for improving the prognosis of unresectable CRLM patients who underwent DEB-TACE treatments.
On CT images, sarcopenia is characterized by a reduction in skeletal muscle mass. Nevertheless, the underlying mechanisms that impact prognosis are still unclear and require further investigation. Sarcopenia can have an impact on physical activity and give rise to metabolic disorders, including heat regulation, amino acid metabolism, and insulin sensitivity28,30. The disorder of these metabolic activities will deal a severe blow to the body of the patients, adding to the already existing burden of the tumor and potentially affecting the prognosis of the tumor. Multiple studies have demonstrated that systemic inflammation, induced by inflammatory mediators generated from increased tumor metabolic activity in cancer patients, is linked to an elevated risk of sarcopenia31,32. Consequently, patients with sarcopenia are apt to exhibit higher levels of NLR compared to those without sarcopenia. Our findings align with this pattern, as we observed higher NLR values in patients with sarcopenia, although the difference was not statistically significant. The inflammatory response in patients with sarcopenia may contribute to the progression of liver metastases and reduce the efficacy of DEB-TACE therapy.
Cox regression analysis illustrated that sarcopenia was identified as an independent risk element on PFS and OS in patients with CRLM. Furthermore, our findings consistently demonstrated that non-sarcopenic individuals exhibited a favorable effect on OS across all tested subgroups. Notably, statistically significant differences were observed in all subgroups except for right colon, ECOG = 0, unbilobar, non-extrahepatic metastases, and NLR ≤ 5, thereby highlighting sarcopenia as an independent risk factor for long-term prognosis. Besides, our study revealed that NLR and extrahepatic metastases were also identified as independent risk factors for PFS and OS, consistent with findings from previous studies33. The presence of extrahepatic metastases may indicate a more aggressive tumor with a higher metastatic burden, posing greater challenges in controlling tumor growth34, which could affect PFS and OS in patients with unresectable CRLM. NLR, on the other hand, serves as an indicator of the inflammatory response within the systemic circulation. An elevated NLR suggests a more severe tumor-related inflammatory response, which in turn could affect the efficacy of tumor access therapy.
Previous research has noted the association between sarcopenia and complications following tumor treatment. Pecorelli N et al. discovered that in patients following pancreaticoduodenectomy for cancer, sarcopenia was associated with increased rates of adverse reactions and mortality, as well as longer hospital stays35. A meta-analysis revealed that sarcopenia is linked to a higher risk of complications following surgical resection of liver metastases in colorectal cancer36. In this study, we observed that CRLM patients with sarcopenia were more prone to experiencing AEs such as vomiting/nausea, fatigue, and abdominal pain. Although these symptoms are self-limited and can be controlled with symptomatic treatment, they also highlight the importance of conducting thorough preoperative conversations with patients who have sarcopenia, as they are more likely to experience concerns from postoperative AEs. Moreover, patients with sarcopenia should pay more attention to gastrointestinal protection and analgesic therapy after receiving DEB-TACE treatment to reduce postoperative adverse reactions. Additionally, it is imperative to provide augmented postoperative nutritional support therapy for patients with sarcopenia, which may subsequently reduce the incidence of associated complications. Notably, our study found no instances of grade 3 side effects, suggesting that DEB-TACE is a safe treatment option.
The limitations of the present study were as follows. Firstly, the cut-off value for sarcopenia is based on the association between SMI and survival date in our study. Nonetheless, using this cut-off value to predict survival rate may involve a recirculation of information to some extent. To address this issue, we also examined the association between SMI as a continuous variable and mortality in univariate and multivariate regression analyses utilizing the Cox proportional hazards model. Furthermore, the Youden index was applied to predict the population’s survival rate, and obtained results as with SMI based on cut-offs, which proves the scientific and reproducible of our study. Secondly, due to the retrospective character of our research, it introduced potential selection and time-dependent assessment biases. Thirdly, the limited population size of this study resulted in the restriction of its power. Therefore, further convincing prospective randomized controlled studies with larger scales are required to confirm our findings.
Conclusion
Sarcopenia demonstrated a strong predictive value for both PFS and OS in unresectable CRLM patients who underwent DEB-TACE treatments. Besides, NLR > 5 and extrahepatic metastases were addressed for independent risk factors linked to a poorer prognosis. Furthermore, patients with sarcopenia may face an increased likelihood of experiencing adverse events following DEB-TACE treatments.
Availability and requirements
-
Tomovision SliceOmatic version 4.3 software URL is https://www.tomovision.com/products/sliceomatic.html
-
X-tile version 3.6.1 software URL is https://x-tile.software.informer.com/3.6/
-
IBM SPSS version 25.0 software URL is https://www.ibm.com/cn-zh/spss
-
R version 4.1.1 software URL is https://www.r-project.org/
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- CRC:
-
Colorectal cancer
- CRLM:
-
Colorectal cancer liver metastases
- DEB-TACE:
-
Drug-eluting bead transarterial chemoembolization
- CT:
-
Computed tomography
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- ECOG:
-
Eastern Cooperative Oncology Group
- SMA:
-
Skeletal muscle are
- SMI:
-
Skeletal muscle index
- ORR:
-
Overall response rates
- DCR:
-
Disease control rate
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Progressive disease
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- NLR:
-
Neutrophil-to-lymphocyte ratio
- AEs:
-
Adverse events
References
Benson, A. B. et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. JNCCN 19, 329–359. https://doi.org/10.6004/jnccn.2021.0012 (2021).
Tsilimigras, D. I. et al. Liver metastases. Nat. Rev. Dis. Primers 7, 27. https://doi.org/10.1038/s41572-021-00261-6 (2021).
Kambakamba, P. et al. The evolution of surgery for colorectal liver metastases: A persistent challenge to improve survival. Surgery 170, 1732–1740. https://doi.org/10.1016/j.surg.2021.06.033 (2021).
Czauderna, C., Luley, K., von Bubnoff, N. & Marquardt, J. U. Tailored systemic therapy for colorectal cancer liver metastases. Int. J. Mol. Sci. 22, 11780. https://doi.org/10.3390/ijms222111780 (2021).
Raphael, M. J. & Karanicolas, P. J. Regional therapy for colorectal cancer liver metastases: Which modality and when?. J. Clin. Oncol. 40, 2806–2817. https://doi.org/10.1200/jco.21.02505 (2022).
Huppert, P., Wenzel, T. & Wietholtz, H. Transcatheter arterial chemoembolization (TACE) of colorectal cancer liver metastases by irinotecan-eluting microspheres in a salvage patient population. Cardiovasc. Intervent. Radiol. 37, 154–164. https://doi.org/10.1007/s00270-013-0632-0 (2014).
Iezzi, R. et al. Trans-arterial chemoembolization with irinotecan-loaded drug-eluting beads (DEBIRI) and capecitabine in refractory liver prevalent colorectal metastases: A phase II single-center study. Cardiovasc. Intervent. Radiol. 38, 1523–1531. https://doi.org/10.1007/s00270-015-1080-9 (2015).
Ren, Y. et al. Transarterial chemoembolization of unresectable systemic chemotherapy refractory liver metastases: A retrospective single-center analysis. Abdominal Radiol. (New York) 45, 2862–2870. https://doi.org/10.1007/s00261-020-02584-6 (2020).
Axelrod, C. L., Dantas, W. S. & Kirwan, J. P. Sarcopenic obesity: emerging mechanisms and therapeutic potential. Metab. Clin. Exp. 146, 155639. https://doi.org/10.1016/j.metabol.2023.155639 (2023).
Kamper, R. S. et al. Associations between inflammatory markers, body composition, and physical function: The Copenhagen Sarcopenia Study. J. Cachexia Sarcopenia Muscle 12, 1641–1652. https://doi.org/10.1002/jcsm.12832 (2021).
Can, B. et al. Serum markers of inflammation and oxidative stress in sarcopenia. Aging Clin. Exp. Res. 29, 745–752. https://doi.org/10.1007/s40520-016-0626-2 (2017).
Guo, B. et al. Associations of CBC-Derived inflammatory indicators with sarcopenia and mortality in adults: Evidence from Nhanes 1999∼2006. BMC Geriatr. 24, 432. https://doi.org/10.1186/s12877-024-05012-2 (2024).
Liu, J. et al. Sarcopenia in patients with cirrhosis after transjugular intrahepatic portosystemic shunt placement. Radiology 303, 711–719. https://doi.org/10.1148/radiol.211172 (2022).
Pessia, B. et al. The role of sarcopenia in the pancreatic adenocarcinoma. Eur. Rev. Med. Pharmacol. Sci. 25, 3670–3678. https://doi.org/10.26355/eurrev_202105_25933 (2021).
Kim, M. C., Kim, K. O. & Kang, M. K. Prevalence and associated risk of advanced colorectal neoplasia in adults with sarcopenia. Korean J. Internal Med. 37, 294–303. https://doi.org/10.3904/kjim.2020.569 (2022).
Ubachs, J. et al. Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. J. Cachexia. Sarcopenia Muscle 12, 2007–2021. https://doi.org/10.1002/jcsm.12804 (2021).
Sevgilioglu, Z. E. et al. Prognostic value of computed tomography associated body composition measurement changes in metastatic colorectal cancer patients. Acta Radiol. (Stockholm, Sweden: 1987) 64, 2849–2857. https://doi.org/10.1177/02841851231198345 (2023).
Yang, Y. R. et al. The impact of sarcopenia on overall survival in patients with pan-RAS wild-type colorectal liver metastasis receiving hepatectomy. Sci. Rep. 13, 6911. https://doi.org/10.1038/s41598-023-33439-x (2023).
Somasundaram, E., Castiglione, J. A., Brady, S. L. & Trout, A. T. Defining normal ranges of skeletal muscle area and skeletal muscle index in children on CT using an automated deep learning pipeline: Implications for sarcopenia diagnosis. AJR Am. J. Roentgenol. 219, 326–336. https://doi.org/10.2214/ajr.21.27239 (2022).
Camp, R. L., Dolled-Filhart, M. & Rimm, D. L. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 10, 7252–7259. https://doi.org/10.1158/1078-0432.Ccr-04-0713 (2004).
Schwartz, L. H. et al. RECIST 1.1-update and clarification: From the RECIST committee. Eur. J. Cancer (Oxford, England: 1990) 62, 132–137. https://doi.org/10.1016/j.ejca.2016.03.081 (2016).
Park, C. H. et al. Sex-specific associations between gut microbiota and skeletal muscle mass in a population-based study. J. Cachexia Sarcopenia Muscle 13, 2908–2919. https://doi.org/10.1002/jcsm.13096 (2022).
Gianotti, L. et al. Nutritional support and therapy in pancreatic surgery: A position paper of the International Study Group on Pancreatic Surgery (ISGPS). Surgery 164, 1035–1048. https://doi.org/10.1016/j.surg.2018.05.040 (2018).
Hua, X. et al. Sarcopenia is associated with higher toxicity and poor prognosis of nasopharyngeal carcinoma. Therapeut. Adv. Med. Oncol. 12, 1758835920947612. https://doi.org/10.1177/1758835920947612 (2020).
Şahin, M. E. H., Akbaş, F., Yardimci, A. H. & Şahin, E. The effect of sarcopenia and sarcopenic obesity on survival in gastric cancer. BMC Cancer 23, 911. https://doi.org/10.1186/s12885-023-11423-y (2023).
Chen, W. Z. et al. Prognostic value of myosteatosis and sarcopenia for elderly patients with colorectal cancer: A large-scale double-center study. Surgery 172, 1185–1193. https://doi.org/10.1016/j.surg.2022.05.031 (2022).
Thormann, M. et al. Sarcopenia does not limit overall survival in patients with colorectal liver metastases undergoing interstitial brachytherapy. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 195, 217–223. https://doi.org/10.1055/a-1936-2937 (2023).
Cruz-Jentoft, A. J. & Sayer, A. A. Sarcopenia. Lancet (London, England) 393, 2636–2646. https://doi.org/10.1016/s0140-6736(19)31138-9 (2019).
Arends, J. et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. (Edinburgh, Scotland) 36, 11–48. https://doi.org/10.1016/j.clnu.2016.07.015 (2017).
Chen, L. K. et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 21, 300-307.e302. https://doi.org/10.1016/j.jamda.2019.12.012 (2020).
Nardone, O. M. et al. Inflammatory bowel diseases and sarcopenia: The role of inflammation and gut microbiota in the development of muscle failure. Front. Immunol. 12, 694217. https://doi.org/10.3389/fimmu.2021.694217 (2021).
Khan, A. I. et al. Sarcopenia and systemic inflammation are associated with decreased survival after cytoreductive nephrectomy for metastatic renal cell carcinoma. Cancer 128, 2073–2084. https://doi.org/10.1002/cncr.34174 (2022).
Verter, E. et al. Neutrophil-to-lymphocyte ratio predicts recurrence pattern in patients with resectable colorectal liver metastases. Ann. Surg. Oncol. 28, 4320–4329. https://doi.org/10.1245/s10434-021-10000-6 (2021).
Klubien, J. et al. Ablation therapy for patients with colorectal liver metastases with and without extrahepatic metastases: Evaluation of long-term outcomes and prognostic factors. Ultrasonography (Seoul, Korea) 42, 410–420. https://doi.org/10.14366/usg.22208 (2023).
Pecorelli, N. et al. Impact of sarcopenic obesity on failure to rescue from major complications following pancreaticoduodenectomy for cancer: Results from a multicenter study. Ann. Surg. Oncol. 25, 308–317. https://doi.org/10.1245/s10434-017-6216-5 (2018).
Wagner, D. et al. Value of sarcopenia in the resection of colorectal liver metastases-a systematic review and meta-analysis. Front. Oncol. 13, 1241561. https://doi.org/10.3389/fonc.2023.1241561 (2023).
Acknowledgements
This work was financially supported by grants from the National Key Research and Development Program of China (Grant No. 2023YFC2413500) and National Natural Science Foundation of China (Grant No. 82102153).
Author information
Authors and Affiliations
Contributions
Fuquan Wang, Chuansheng Zheng, and Guofeng Zhou conceived and designed the study. Fuquan Wang, Bingxin Gong, lei Chen, and Yanyan Cao collected and assembled the data. Fuquan Wang analyzed the data and wrote the article. Bingxin Gong, Bin Chai and Jihua Wang helped to review the article critically. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical standards
The study was approved by the institutional review board of the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. The study was conducted in accordance with the standards of Good Clinical Practice and the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, F., Gong, B., Chen, L. et al. Prognostic value of sarcopenia in patients with unresectable colorectal liver metastases after drug-eluting beads transcatheter arterial chemoembolization: a single center retrospective study. Sci Rep 15, 11097 (2025). https://doi.org/10.1038/s41598-025-95782-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95782-5