Abstract
Infants and toddlers with mild traumatic brain injury (mTBI) and minor subdural hematoma (SDH) were found to have a higher risk of requiring neurosurgical intervention (NI). However, the ability to identify patients with mTBI and minor SDH who require NI remains limited. This study aims to develop a nomogram to predict NI in these patients. A nomogram predicting NI was established using demographic, clinical, radiographic, and laboratory data from patients with mTBI and minor SDH. The least absolute shrinkage and selection operator (LASSO) regression and best subsets regression (BSR) methods were employed to identify variables and select predictive factors. A nomogram was constructed using multivariable logistic regression. The model’s performance was evaluated using the area under the receiver operating characteristic curve, calibration curves, the Hosmer–Lemeshow test, and decision curve analysis. Immediate seizures, anemia, and subarachnoid space depth were identified as significant predictive factors by the BSR, leading to the development of a nomogram. The AUC for this nomogram, obtained through bootstrap validation (resampling = 500), was 0.893 (95% CI, 0.844–0.942). The model demonstrated good calibration, and decision curve analysis showed that when the threshold probability ranged from 7 to 83%, using the nomogram to predict NI provided a net benefit. A novel nomogram has been developed to accurately assess the risk of NI in children under 3 years of age with mTBI and minor SDH, potentially aiding in clinical decision-making.
Similar content being viewed by others
Introduction
Each year, approximately 69 million people worldwide sustain traumatic brain injury (TBI), with over 80% classified as mild TBI (mTBI)1,2.In children, the estimated incidence of TBI ranges from 47 to 280 per 100,000, with the highest rates occurring in those under 3 years of age2,3,4,5. Subdural hematoma (SDH) is the most common form of intracranial hemorrhage and one of the highest-risk types for neurosurgical intervention6. Over the past decade, there has been a notable increase in the number of patients presenting with mTBI and SDH7. Although most children with mTBI and minor SDH do not experience clinical deterioration during conservative observation, infants and toddlers have been found to be at higher risk of requiring neurosurgical intervention8,9,10,11.
However, due to the prevalence and often subtle symptoms, this group is particularly challenging to prioritize for heightened clinical attention. Additionally, families frequently resist repeat head CT scans, citing concerns about potential radiation-induced brain damage. These factors may delay the identification of disease progression, potentially resulting in poor outcomes.
Therefore, accurately identifying infants and toddlers with mTBI and minor SDH who are at risk for neurosurgical intervention could help clinicians increase vigilance and intervene promptly. However, no clear consensus guidelines exist for the neurosurgical management of patients with mTBI complicated by SDH12. Additionally, existing predictive models primarily focus on adult patients13,14,15. In this study, a nomogram was developed to predict the need for neurosurgical intervention in children aged ≤ 3 years with mTBI and minor SDH, utilizing demographic, clinical, radiographic, and laboratory data.
Materials and methods
Study design and population
This study retrospectively recruited patients diagnosed with SDH who were admitted to the Children’s Hospital of Soochow University between June 2015 and June 2024. All participant data were obtained from their electronic medical records. The inclusion criteria for the study were as follows: (1) traumatic brain injury; (2) minor subdural hematoma (with hematoma volume at admission not meeting surgical indications); and (3) age ≤ 3 years. Patients meeting any of the following exclusion criteria were removed from the study: (1) Child’s Glasgow Coma Scale (CGCS) score < 1316; (2) open fracture; (3) admission CT indicating brain herniation or diffuse brain edema; and (4) absence of a CT scan within 48 h post-injury or a follow-up CT during conservative observation.
This study received approval from the Ethics Committee of the Children’s Hospital of Soochow University in accordance with the Declaration of Helsinki (2024CS102). The requirement for informed patient consent was waived.
Data collection and definition
The authors collected the following data from the hospital’s electronic medical record system: (1) demographic information, including age, sex, weight, and gestational age at birth; (2) trauma history details, such as time from injury to hospital, mechanism of injury, vomiting, immediate seizures, and admission CGCS score; and (3) laboratory results obtained at admission, including prothrombin time (PT), activated partial thromboplastin time (APTT), international normalized ratio (INR), thrombin time (TT), and fibrinogen (FIB). Seizures occurring within 24 h post-injury were recorded as immediate seizures. Anemia was diagnosed based on hemoglobin levels at admission and the patient’s age17.
Head CT images were independently reviewed by two experienced neurosurgeons, and disagreements were settled through joint discussion. The following findings were systematically recorded: skull fractures, intracranial hematomas, subdural effusions, and cerebral contusions. Hematoma volume was calculated using the (ABC)/2 method on 2.5-mm slices of non-contrast brain CT. The depth of the subarachnoid space was measured as the maximum distance from the surface of the gyri to the nearest point on the inner skull cortex8,18,19,20. Subdural crescent-shaped fluid collections with CT attenuation values between those of a hematoma and cerebrospinal fluid were recorded as subdural effusion. The measurement was taken on axial CT scans at the level of the lateral ventricles, with the average of the left and right sides recorded.
Outcome
The neurosurgeon’s decision or intent to perform surgery was considered the gold standard. Since families may occasionally refuse neurosurgical intervention, patient communication records were reviewed to identify cases where surgery was recommended by the neurosurgeon but declined by the family. Clinical deterioration, along with repeat head CT showing a significant increase in hematoma volume or deepening of the subarachnoid space, were often considered indications for surgery. All NI decisions in our cohort were reviewed by at least two senior neurosurgeons, with discrepancies resolved through multidisciplinary discussions. The study’s outcome was defined as either the execution of a neurosurgical procedure or the neurosurgeon’s intent to perform one during the period of conservative observation.
Statistical analysis
Quantitative data following a normal distribution were reported as mean ± standard deviation, while data not adhering to a normal distribution were expressed as median (P25, P75). Categorical variables were presented as frequency and proportion. Independent sample t-tests were used for normally distributed quantitative data, and non-parametric tests were applied to data that did not follow a normal distribution. Chi-square tests were utilized for categorical variables.
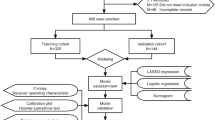
To prevent overfitting or underfitting of the model, the least absolute shrinkage and selection operator (LASSO) regression and best subsets regression (BSR) methods were employed for dimension reduction and predictor selection21,22,23. Variable selection for BSR was guided by the Bayesian information criterion (BIC)24. Restricted cubic splines (RCS) were used to assess potential nonlinear associations between continuous predictors and outcomes, with nonlinear predictors converted into categorical variables. Collinearity among predictors was evaluated using the variance inflation factor (VIF). A multivariable logistic regression analysis was performed to construct the predictive model and develop a nomogram for neurosurgical intervention. The model’s discriminatory ability was assessed by calculating the area under the curve (AUC), and internal validation was conducted using the bootstrapping method (resampling = 500). Model calibration was evaluated with the Hosmer–Lemeshow test, while its clinical utility was assessed through decision curve analysis (DCA).
R software (version 4.2.1; R Foundation for Statistical Computing; https://www.r-project.org/), the R survey package (version 4.1-1), and Free Statistics software (version 1.9.2; Beijing Free Clinical Medical Technology Co., Ltd.) were used for analyses. A two-sided p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
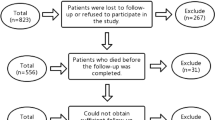
During the study period, 555 children aged ≤ 3 years with mTBI and minor SDH were admitted to the Children’s Hospital of Soochow University, with 133 patients excluded (Fig. 1). Among the remaining participants, 41 (9.72%) underwent neurosurgical intervention. The demographic and clinical characteristics of the study participants are presented in Table 1. The neurosurgical intervention group tended to be younger (95,13 vs. 116,22), anemia (24 [58.5%] vs. 51 [13.4%]), and more likely to develop subdural effusions (14 [34.1%] vs. 48 [12.6%]) and (13 [31.7%] vs. 29 [7.6%]). Additionally, they were more likely to have a lower level of PT (12.5 ± 1.5 vs. 12.9 ± 1.2), a higher level of FIB (18.1 ± 3.5 vs. 17.3 ± 2.2), and an enlarged subarachnoid space depth (4.4 [1.5, 5.0] vs. 1 [0, 3.5]).
Feature predictor selection
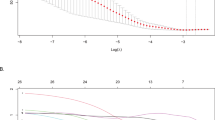
The optimal combination of variables was selected using the BSR method based on the minimum BIC, achieving the lowest BIC of -65.2 with three final variables (Fig. 2A and B). The selected variables were anemia, immediate seizures, and subarachnoid space depth, which were used to establish Model 1. Additionally, the authors applied LASSO regression to reduce the initial 23 variables to 5 significant predictors with non-zero coefficients (Fig. 2C and D). This process resulted in the creation of Model 2, which included anemia, subarachnoid space depth, immediate seizures, PT, and subdural effusion as the final predictors. None of these variables violated the VIF standards in the models. RCS analysis demonstrated a linear relationship between subarachnoid space depth, PT, and the risk of neurosurgical intervention (Supplementary Fig. 1).
Predictor selection was performed using LASSO regression analysis with tenfold cross-validation and BSR based on the minimum BIC. (A) The BSR method achieved the lowest BIC of -65.2. (B) The final number of selected variables was three. (C) The tuning parameter (lambda) selection in LASSO regression was based on the minimum criterion (left dashed line) and the 1-SE criterion (right dashed line). (D) A coefficient plot was generated for the log(lambda) sequence. In this study, the selection of predictive factors was guided by the minimum criterion (left dashed line), resulting in the identification of five non-zero coefficients. LASSO: least absolute shrinkage and selection operator; SE: standard error; BIC: Bayesian information criterion; BSR: best subsets regression.
Model development and performance
The models were internally validated using the bootstrap method (resampling = 500). The AUC for Model 1 was 0.893 (95% CI, 0.844–0.942), while Model 2 had an AUC of 0.901 (95% CI, 0.859–0.943) (Fig. 4A). The predictive performance of the two models was compared using DeLong’s test, which indicated no statistically significant difference. Consequently, Model 1, which contained fewer variables, was selected for further evaluation. The three predictors were ultimately integrated into the nomogram (Fig. 3).
The calibration curve comparing predicted and observed outcomes confirmed the model’s reliability in the validation set (Fig. 4B). Furthermore, the P-value obtained from the Hosmer–Lemeshow test was 0.793, indicating no statistically significant difference, which further supported the model’s calibration validity. Based on the nomogram developed in this study, DCA demonstrated that the threshold probability for neurosurgical intervention in children with mTBI and minor SDH ranged between 7 and 83% (Fig. 4C).
All results indicated that the nomogram prediction model, established using the included predictors, could accurately and consistently assess the probability of neurosurgical intervention in children aged ≤ 3 years with mTBI and minor SDH.
Website of nomogram model
The authors developed a web-based, user-friendly calculator that incorporates only three input variables (available at https://lyc-9876.shinyapps.io/dynnomapp/) to assist clinicians in utilizing the nomogram model more efficiently.
Discussion
Pediatric mTBI has long been a public health concern25. Symptoms following mTBI can persist for more than two weeks and, in some cases, may continue for over three months26. However, clear consensus guidelines for the neurosurgical treatment of patients with mTBI complicated by SDH are lacking12. Additionally, previous predictive models have primarily focused on adults13,14,15. Infants with mTBI exhibit distinct epidemiological characteristics, imaging features, physiological functions, and prognoses compared to adults. Therefore, it is essential to develop a predictive model that innovatively utilizes infant-specific characteristics as observational indicators to guide clinical decision-making.
This study developed and validated a web-based nomogram model to predict the risk of neurosurgical intervention in children under 3 years old with mTBI and minor SDH. The proposed model incorporated three easily obtainable predictive factors: anemia, immediate seizures, and subarachnoid space depth. The model demonstrated good calibration and discrimination. To the authors’ knowledge, this is the first nomogram model specifically designed to predict the risk of neurosurgical intervention during conservative observation in this population. The visualized model and web tool may assist healthcare professionals in conducting individualized risk assessments, thereby aiding in clinical decision-making and management.
Previous case series studies reported a potential association between subarachnoid space depth and the development of acute intracranial hypertension in children with mTBI and minor SDH8,9,10,11. Enlarged subarachnoid space (ESS) is a characteristic feature of benign external hydrocephalus, occurring in approximately 0.4 per 1,000 live births27. ESS typically resolves spontaneously before the age of three. However, Lee et al. suggested that children with ESS complicated by traumatic SDH are more likely to experience an “acute” stage10. In the study by Kumar et al., 35% (7/20) of children with ESS complicated by SDH required emergency surgical intervention11. In this study, subarachnoid space depth was identified as an important predictive factor for the need for neurosurgical intervention during conservative observation in children under 3 years old with mTBI and minor SDH.
Children with mTBI are estimated to experience immediate seizures in 5% to 21% of cases28, often triggered by ischemia and neuronal hypoxia29. Numerous studies have shown that patients with mTBI who develop immediate seizures tend to have longer hospital stays and a higher risk of clinical deterioration28,30,31. Similarly, this study indicated that this phenomenon also occurs in children under 3 years old with mTBI and minor SDH. However, whether immediate seizures necessitate surgical intervention remains controversial. On the one hand, some studies suggest that immediate seizures indicate a higher risk of intracranial hypertension or brain tissue injury, potentially warranting urgent surgical intervention to reduce mortality and improve prognosis32. On the other hand, other studies argue that immediate seizures are not always an independent predictor for surgery, and the effectiveness of surgical intervention may vary depending on the individual patient’s condition33,34.
In 2019, the World Health Organization estimated that 40% of children aged 6 to 59 months worldwide suffer from anemia17. Previous studies have indicated that anemia can significantly reduce venous oxygen saturation in patients with traumatic brain injury35. Furthermore, Hidenobu et al. reported a negative correlation between hemoglobin levels and the progression of traumatic intracranial hematomas36. These findings align with our results, underscoring the need for increased clinical attention for these patients.
The limitations of this study should be acknowledged. First, our single-center retrospective design and relatively small sample size may limit the generalizability of our findings. Although internal validation using bootstrap resampling (n = 500) demonstrated robust performance, external validation is necessary before clinical implementation. Additionally, continuous monitoring of certain parameter changes was not feasible. Second, although our model incorporates objective predictive factors, the outcome variable inherently reflects the subjective decisions of neurosurgeons at our institution. Variations in surgical thresholds across centers, influenced by local protocols, resource availability, and clinician experience, may limit the generalizability of our nomogram. Future studies should validate this tool in settings with standardized decision-making criteria. Finally, as children under the age of three often have difficulty remaining still during CT examinations, the imaging data are frequently skewed, making it challenging to measure the midline shift accurately. Consequently, this variable was not included in the model and should be further explored in future studies. Despite these limitations, this study represents the first effort to develop a nomogram for predicting the risk of neurosurgical intervention in children under 3 years of age with mTBI and minor SDH.
Conclusion
Our predictive model demonstrates the potential for estimating the risk of neurosurgical intervention in children under 3 years of age with mTBI and minor SDH. However, further studies are needed to validate its effectiveness and assess its broader applicability.
Data availability
The data are available from the corresponding author upon reasonable request.
Abbreviations
- mTBI:
-
Mild traumatic brain injury
- SDH:
-
Subdural hematoma
- CGCS:
-
Child’s Glasgow coma scale
- PT:
-
Prothrombin time
- APTT:
-
Activated partial thromboplastin time
- INR:
-
International normalized ratio
- TT:
-
Thrombin time
- FIB:
-
Fibrinogen
- LASSO:
-
Least absolute shrinkage and selection operator
- BSR:
-
Best subsets regression
- BIC:
-
Bayesian information criterion
- RCS:
-
Restricted cubic splines
- VIF:
-
Variance inflation factor
- AUC:
-
Area under the curve
- DCA:
-
Decision curve analysis
- ESS:
-
Enlarged subarachnoid space
References
Lumba-Brown, A. et al. Diagnosis and management of mild traumatic brain injury in children: A systematic review. JAMA Pediatr. 172(11), e182847 (2018).
Dewan, M. C. et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 130(4), 1080–1097 (2019).
Silverberg, N. D., Duhaime, A. C. & Iaccarino, M. A. Mild traumatic brain injury in 2019–2020. JAMA 323(2), 177–178 (2020).
Dewan, M. C., Mummareddy, N., Wellons, J. C. 3rd. & Bonfield, C. M. Epidemiology of global pediatric traumatic brain injury: Qualitative review. World Neurosurg. 91, 497-509.e491 (2016).
Lumba-Brown, A. et al. Centers for disease control and prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr. 172(11), e182853 (2018).
Orlando, A. et al. Epidemiology of mild traumatic brain injury with intracranial hemorrhage: Focusing predictive models for neurosurgical intervention. World Neurosurg. 107, 94–102 (2017).
Orlando, A. et al. Significant national declines in neurosurgical intervention for mild traumatic brain injury with intracranial hemorrhage: A 13-year review of the national trauma data bank. Neurotrauma Rep. 4(1), 137–148 (2023).
Hellbusch, L. C. Benign extracerebral fluid collections in infancy: clinical presentation and long-term follow-up. J. Neurosurg. 107(2 Suppl), 119–125 (2007).
Meybodi, K. T., Habibi, Z. & Nejat, F. Temporary exacerbation of benign external hydrocephalus following minor head trauma. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery 36(11), 2603–2604 (2020).
Lee, H. C. et al. Benign extracerebral fluid collection complicated by subdural hematoma and fluid collection: clinical characteristics and management. Child’s Nervous Syst. 34(2), 235–245 (2018).
Kumar, R., Singhal, N. & Mahapatra, A. K. Traumatic subdural effusions in children following minor head injury. Child’s Nervous Syst. 24(12), 1391–1396 (2008).
Barbosa, R. R. et al. Evaluation and management of mild traumatic brain injury: an Eastern Association for the Surgery of Trauma practice management guideline. J. Trauma Acute Care Surg. 73(5 Suppl 4), S307-314 (2012).
Lessard, J. et al. Can the “important brain injury criteria” predict neurosurgical intervention in mild traumatic brain injury? A validation study. Am. J. Emerg. Med. 38(3), 521–525 (2020).
Habibzadeh, A. et al. Machine learning-based models to predict the need for neurosurgical intervention after moderate traumatic brain injury. Health Sci. Rep. 6(11), e1666 (2023).
Noorbakhsh, S. et al. Key findings on computed tomography of the head that predict death or the need for neurosurgical intervention from traumatic brain injury. Am. Surg. 90(4), 616–623 (2024).
Kirkham, F. J., Newton, C. R. & Whitehouse, W. Paediatric coma scales. Dev. Med. Child Neurol. 50(4), 267–274 (2008).
Pasricha, S. R., Rogers, L., Branca, F. & Garcia-Casal, M. N. Measuring haemoglobin concentration to define anaemia: WHO guidelines. Lancet (London, England) 403(10440), 1963–1966 (2024).
Fingarson, A. K., Ryan, M. E., McLone, S. G., Bregman, C. & Flaherty, E. G. Enlarged subarachnoid spaces and intracranial hemorrhage in children with accidental head trauma. J. Neurosurg. Pediatr. 19(2), 254–258 (2017).
Zahl, S. M., Egge, A., Helseth, E. & Wester, K. Benign external hydrocephalus: a review, with emphasis on management. Neurosurg. Rev. 34(4), 417–432 (2011).
Tucker, J., Choudhary, A. K. & Piatt, J. Macrocephaly in infancy: benign enlargement of the subarachnoid spaces and subdural collections. J. Neurosurg. Pediatr. 18(1), 16–20 (2016).
Adams, S. T. & Leveson, S. H. Clinical prediction rules. BMJ (Clinical research ed) 344, d8312 (2012).
Collins, G. S., Reitsma, J. B., Altman, D. G. & Moons, K. G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement The TRIPOD Group. Circulation 131(2), 211–219 (2015).
Debray, T. P. et al. A guide to systematic review and meta-analysis of prediction model performance. BMJ (Clinical research ed) 356, i6460 (2017).
Li, W. & Nyholt, D. R. Marker selection by Akaike information criterion and Bayesian information criterion. Genet. Epidemiol. 21(Suppl 1), S272-277 (2001).
Guillaume, D. J. et al. Intra-arterial chemotherapy with osmotic blood-brain barrier disruption for aggressive oligodendroglial tumors: results of a phase I study. Neurosurgery 66(1), 48–58 (2010).
Babcock, L. et al. Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA Pediatr. 167(2), 156–161 (2013).
Zahl, S. M., Egge, A., Helseth, E. & Wester, K. Clinical, radiological, and demographic details of benign external hydrocephalus: A population-based study. Pediatr. Neurol. 96, 53–57 (2019).
Keret, A. et al. Posttraumatic epilepsy: Long-term follow-up of children with mild traumatic brain injury. J. Neurosurg. Pediatr. 20(1), 64–70 (2017).
Algattas, H. & Huang, J. H. Traumatic Brain Injury pathophysiology and treatments: early, intermediate, and late phases post-injury. Int. J. Mol. Sci. 15(1), 309–341 (2013).
Lin, S. et al. Establishment and validation of PTE prediction model in patients with cerebral contusion. Sci. Rep. 12(1), 20574 (2022).
Lee, S. T., Liu, T. N., Wong, C. W., Yeh, Y. S. & Tzaan, W. C. Relative risk of deterioration after mild closed head injury. Acta Neurochir. 135(3–4), 136–140 (1995).
Gao, X. et al. A systematic review and meta-analysis of surgeries performed for cerebral cavernous malformation-related epilepsy in pediatric patients. Front. Pediatr. 10, 892456 (2022).
Chao, M. et al. The influence of serious extracranial injury on in-hospital mortality in children with severe traumatic brain injury. J. Pers. Med. 12(7), 1075 (2022).
Binder, H. et al. Management and outcome of traumatic intracerebral hemorrhage in 79 infants and children from a single level 1 trauma center. Children Basel Switzerland 8(10), 854 (2021).
Robertson, C. S. et al. SjvO2 monitoring in head-injured patients. J. Neurotrauma 12(5), 891–896 (1995).
Ochiai, H. et al. Factors associated with the progression of traumatic intracranial hematoma during interventional radiology to establish hemostasis of extracranial hemorrhagic injury in severe multiple trauma patients. Acute Med. Surg. 7(1), e580 (2020).
Acknowledgements
We thank Dr. Liu Jie (People’s Liberation Army of China General Hospital, Beijing, China) for helping in this revision. We also gratefully thank the patients who participated in this retrospective study and the attending team for their hard work and support.
Funding
The authors are grateful for the financial support provided by the Medical New Technology Program of Suzhou City (SKY2021050).
Author information
Authors and Affiliations
Contributions
Yuchen Liu and Houxin Fu conducted data collection, statistical analysis, visualization, and original draft writing. All authors contributed to the data curation, conceptualization, and supervision. All authors have approved the submitted version and agreed both to be personally accountable for the author’s contributions and to ensure that questions related to the accuracy or integrity of any part of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Research Ethics Committee of the Children’s Hospital of Soochow University (2024CS102). Due to the retrospective nature of this study, the need for informed consent was waived by the Research Ethics Committee of the Children’s Hospital of Soochow University.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Fu, H., Sun, J. et al. A nomogram for neurosurgical intervention in children with mild traumatic brain injury and minor subdural hematoma under 3 years. Sci Rep 15, 11230 (2025). https://doi.org/10.1038/s41598-025-95784-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95784-3