Abstract
To analyze the changes in ocular biometry readings in patients with dry eye (DE) and non-dry eye (NDE) after instilling artificial tear drop (ATD). A prospective study was conducted on 98 eyes of 98 patients (DE group, n = 54 eyes; NDE group, n = 44 eyes) who underwent ocular biometry with four consecutive measurements using the IOL Master 700. The baseline flat meridian of the anterior corneal surface (K1), steep meridian of the anterior corneal surface (K2), corneal curvature radius(R1, R2), axial length (AL), anterior chamber depth (ACD), central corneal thickness (CCT), white-to-white corneal diameter (WTW), pupil diameter, non-invasive tear break-up time (NITBUT) and tear meniscus height, (TMH) was measured at baseline, 30-s, 2-min, and 5-min intervals after instilling one drop of ATD. Variability in biometry measurements was assessed and compared. The mean age of the NDE group and DE group was 27.09 ± 10.21 years (30 females, 68.18%) and 29.96 ± 12.08 years (38 females, 70.37%), respectively. Following the application of ATD, intergroup comparisons revealed significant changes in K1 at 2 min and 5 min (p = 0.043, p = 0.043, and p = 0.038, respectively), and significant changes in R1 and R2 at 2 min. Intragroup comparisons showed that AL exhibited significant changes at 30 s (p < 0.001), while CCT demonstrated significant alterations across all time points (p < 0.001). Changes in ACD were observed at 30 s and 2 min in the dry eye group, and pupil diameter showed significant changes at 2 min in both the DE and NDE groups. No significant changes were detected in WTW at any point (all p > 0.05). Artificial tears significantly affect the readings of AL and CCT during ocular biometry. It is recommended to wait for at least 5 min after the installation of eye drops before performing ocular biometry measurements.
Similar content being viewed by others
Introduction
Dry eye disease (DED) is a multifactorial disorder affecting the ocular surface, marked by an imbalance in tear film homeostasis and accompanied by ocular symptoms. Key contributing factors include tear film instability, hyperosmolarity, inflammation and damage to the ocular surface, as well as neurosensory abnormalities1. The prevalence of DED is estimated to vary depending on the operational definition of dry eye and the characteristics of the studied population2. Over the past few decades, the incidence of DED has increased significantly, affecting an estimated 5–50% of the global population in studies involving symptoms with or without clinical signs and DED is also frequently observed in patients visiting routine cataract clinics2,3,4,5,6. Key players in the pathophysiology of DED include instability and hyperosmolarity of the tear film and inflammation of the ocular surface and the lacrimal glands7,8,9. Localized damage to the tear film, known as tear film breakup, is exacerbated by unstable tear films, leading to further damage to the ocular surface10. Changes in tear film dynamics and ocular surface damage can result in optical measurement errors, such as aberrations11 or unpredictable corneal curvature measurements12, which can affect the outcomes of procedures like cataract surgery that heavily rely on these assessments12,13,14.
Cataract surgery is widely recognized as a highly cost-effective treatment and is frequently performed in numerous nations15. Over the past two decades, the prevalence of cataracts has decreased, largely due to an increase in the rate of cataract surgeries—defined as the number of surgeries per million population per year—driven by advancements in surgical techniques and proactive surgical initiatives16. In modern cataract and refractive surgery, it is essential to improve visual acuity and address existing issues affecting visual quality. Therefore, accurately estimating the biometric parameters used in IOL formulas is crucial for ensuring optimal vision and refractive outcomes17,18,19. Ocular biometry is utilized to calculating intraocular lens (IOL) power to achieve the desired preoperative outcomes. Key parameters such as axial length (AL), anterior chamber depth (ACD), white-to-white corneal diameter (WTW), and corneal curvature (K-values) must be considered during measurement. Additionally, selecting an appropriate IOL calculation formula and IOL constants is essential for optimal results. Among various technologies for ocular biometric measurement, optical biometry has been shown to be more accurate and safer than ultrasonic biometry20,21. The IOL Master700 (Carl Zeiss Meditec AG, Jena, Germany), the first biometric device based on swept-source optical coherence tomography (SS-OCT) technology, has been widely used in clinical ophthalmologic practice22. Due to the instability of the tear film, the biometry often needs to be done multiple times in a clinical environment23. The high variability of both short-term and long-term repeatability in keratometry of DE is well-known12. Due to the tear film’s instability, the quality of the refractive surface is unpredictable and often changes dramatically between blinks24. Artificial tears are commonly employed to optimize ocular surface conditions, thereby enhancing the reliability of ocular measurements. Sodium hyaluronate (HA), known for its excellent biocompatibility and viscoelastic properties, is widely used and is the most commonly used formulation for artificial tears23,25,26. Consequently, enhancing the condition of the eye surface in individuals with dry eye will result in improved precision when choosing the power of IOL27,28,29. Given the above, it is crucial to thoroughly understand the factors that affect biometric measurements and surgical outcomes in patients with DED. This study aimed to analyze the changes of ocular biometry readings in patients with and without dry eye after instilling artificial tear drop (ATD).
Methods
Study design and participants
This study was approved by the Institutional Review Board of He Eye Specialist Hospital in Shenyang, China, and adhered to the Declaration of Helsinki’s precepts. Consecutive volunteers were recruited from the outpatient department of He Eye Specialist Hospital in Shenyang. All participants provided informed consent after a thorough description of the study’s purpose and potential outcomes. He Eye Specialist Hospital ethics committee approved the study protocol (IRB (2024) K009.01) and registered with ClinicalTrials.gov (NCT06656403). Patients were recruited at He Eye Specialist Hospital, China between November 1, 2024 and November 20, 2024. Ninety-eight eyes of 98 patients (68 females, 30 male) who met the inclusion criteria were evaluated. The mean patient age was 28.67 ± 11.31 years.
Inclusion criteria comprised the following: (1) age ≥ 18 years; (2) able and willing to comply with the treatment schedule.
Exclusion criteria were: (1) The eyes cannot fixate on the fixation lamp (such as children, nystagmus diseases, severe low vision, inattention, etc.) and cannot act according to the user’s instructions and sit in the front of the equipment (the forehead or lower song is injured so that it cannot be supported on the forehead / lower song bracket); (2) The eyes are cloudy with optical media (such as corneal opacity, central corneal scar, mature cataract, posterior chamber bag opacity, vitreous hemorrhage, etc.); (3) The eyelid is completely closed or too small (drooping, relaxation) resulting in complete or partial occlusion of the cornea; (4) Just after contact measurement or examination, use a corneal local anesthesia solution. (IOL Master 700 should avoid using local anesthetic before all contact examinations); (5) Tear film deformation (lack of specular reflection of cornea during corneal curvature measurement), severe dry eye, and whether eye drops were used 24 h before examination; (6) Any corneal lesions (corneal irregularity, corneal scar or corrosive burning, severe irregular astigmatism of cornea); (7) Fundus lesions (changes in the anatomical morphology of retinal macular fovea during axial length measurement, such as retinal detachment, edema, ulcer, etc.); (8) Recent ocular surgery or trauma, such as photodynamic therapy (PDT), were excluded.; (9) Systemic diseases affecting the ocular surface (e.g., autoimmune disorders); (10) Medications influencing tear production (e.g., antihistamines, antidepressants); (11) Participants allergic to using 0.1% Sodium hyaluronate eye drops.
Clinical assessments
DE diagnostic criteria
All participants were diagnosed according to the Tear Film and Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS) II Diagnostic criteria30: (1) Subjective symptoms assessed using a validated DED questionnaire (Ocular surface disease index (OSDI) ≥ 13 scores); (2) presence of either qualitative or quantitative disturbance of the tear film (Non-invasive tear break-up time (NITBUT) < 10 s); The presence of all criteria was needed to establish a positive DED diagnosis.
NITBUT and tear meniscus height (TMH)
NITBUT and TMH were assessed using the Keratograph 5 M (OCULUS, Germany) topographer. Three sequential readings were captured, and the median value was included in the final analysis31,32.
Tear film lipid layer (TFLL)
Non-invasive assessment was performed using the DR-1 tear film interferometer (Kowa, Nagoya, Japan)33. The thickness and stability of the TFLL were evaluated based on observed changes in the color and structure of the tear film.
OSDI (Allergan, Inc., Irvine, CA) is a frequently used instrument to assess DE and provides a quantifiable assessment of DE symptom frequency and the impact of these symptoms on vision-related functioning. It contains 12 items (items 1–5 refer to ocular pain and visual difficulties; items 6–9 are about visual functionality; and items 10–12 assess environmental factors) and the score can range from 0 (no symptoms) to 100 (severe symptoms) points34.
Biometry parameter
Each eye included in this study was measured and evaluated on the same day by the same ophthalmologist (YC) under consistent low-light conditions (0.63 lx) in a standardized darkroom environment using the IOL Master 700. The IOL Master 700 uses a refractive index of 1.3375 for biometric parameters and combines SS-OCT technology with a tunable laser centered at 1055 nm (ranging from 1035 to 1095 nm) and a multidot keratometer. It employs 1050 nm infrared light for scanning and performs optical B-scans to measure AL, ACD, central corneal thickness (CCT) and pupil, providing two-dimensional OCT images of axial anatomical structures and ensuring precise alignment through foveal visualization. Additionally, K-values are measured using a six-point telecentric technique, and WTW is assessed with an LED light source35,36. Pupil size, as described in the IOL Master 700 user manual was measured under an LED light source with a wavelength of 660 nm and an output power of less than 1 µW (4.17 × 10−5 lux).
Measurement time points
The selection of measurement time slots was based on the following considerations: (1) Immediate Effects of ATD: The primary aim of our study was to evaluate the immediate effects of ATD on biometric measurements, as this aligns with common clinical practices where measurements are often taken shortly after the application of eye drops. The 5-min time point was chosen to capture the initial stabilization of the tear film and its impact on biometric parameters. (2) Related study23 indicate that significant changes in tear film thickness (TFT) and stability occur within the first few minutes after the application of ATD. This time frame is also practical in a clinical setting, as it minimizes patient waiting time and enhances workflow efficiency.
Experimental design
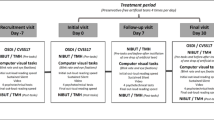
AL, corneal curvature radius (R1, R2), baseline flat meridian of the anterior corneal surface (K1), steep meridian of the anterior corneal surface (K2), ACD, CCT, WTW and pupil diameter values were measured at baseline using the IOL Master 700. After the baseline measurement, artificial tears (0.1% sodium hyaluronate, preservative-free) were dropped into the inferior conjunctival vault. After 30 s, 2 min and 5 min of ATD, the above measurements for IOL Master 700 were repeated to obtain AL, R1, R2, K1, K2, ACD, CCT, WTW and pupil diameter values (Fig. 1).
Statistical analysis
Statistical analysis was performed using SPSS software ver. 26.0 (SPSS Inc., Chicago, IL, USA). All continuous variables are presented as mean ± standard deviation (SD), while categorical variables are expressed as absolute and relative frequencies. The Kolmogorov–Smirnov test was used to assess the normality of the data. For normally distributed data, paired t-tests were conducted to compare mean differences. Analysis of variance (ANOVA) was applied to analyze ordinal variables and those with non-normal distributions. Repeated measures analysis allowed for comparisons across time periods, while paired analyses allowed for comparisons of pre- and post- data at specific time periods. A p-value of < 0.05 was considered statistically significant.
Sample size calculation
The G*Power 3.1 (Heinrich Heine Universität, Düsseldorf, Germany) program was utilized in this investigation to determine Repeated Measure ANOVA sample size. The probability level of significance was adjusted according to the post hoc Bonferroni procedure to maintain an overall type I error equal to 0.05. The power (1 − β) was 0. 95 at the level of a = 0.05, with an effect size of 0.15, confirming that the sample size was sufficient.
Results
Demographics
Ninety-eight individuals were included in the final analysis. Demographic data are shown in Table 1. The mean standard deviation (SD) age of participants in the NDE and DE group was 27.05 ± 10.25 years; 68.18% of participants were female and was 29.96 ± 12.08 years, 70.37% of participants were female, respectively.
The classification was performed using TMH and TFLL assessment1. Among the dry eye patients, 20.37% were classified as having aqueous-deficient dry eye (ADDE), 35.19% as having evaporative dry eye (EDE), and 44.44% as having mixed-type dry eye (a combination of EDE and ADDE).
Axial length
Table 2 shows the comparative ocular biometry readings at Different Time Points Between NDE and DE groups. The AL of DE group was not significant different with NDE group (p > 0.05); The AL of NDE group was improved to 24.74 ± 1.32 (p < 0.001) at 30 s, and the AL of DE group also reported a statistically improvement to 24.82 ± 1.53 (p < 0.001) at 30 s.
Anterior corneal surface
The K1 of DE group was significant different with NDE group in baseline, 2 min and 5 min (p = 0.043, p = 0.043, p = 0.038, respectively).
Corneal curvature radius
The R2 of DE group was significant different with NDE group in baseline (p = 0.042) and 2 min (p = 0.039); Compared with baseline, the R1 of NDE group was a significant difference at 5 min after instilling one drop of ATD (p = 0.010).
Anterior chamber depth
Compared with baseline measurements, the ACD of NDE group was a statistically significant difference in the NDE group at 30 s (p = 0.043), and the ACD of DE group was a statistically significant difference in the DE group in 30 s (p < 0.001) and 2 min (p = 0.001).
Central corneal thickness
Comparison between baseline, 30 s, 2 min and 5 min within NDE and DE group and did differ significantly (p < 0.001) (Table 3).
White-to-white corneal diameter
The mean difference in WTW measurements (both NDE and DE group) was not significantly different from zero (p > 0.05). Comparison between baseline, 30 s, 2 min and 5 min within NDE and DE group and did not differ significantly (p = 1.000) (Table 3).
Pupil
The Pupil of DE group was significant different with NDE group in 5 min (4.88 ± 0.90 mm vs. 4.45 ± 0.73 mm, p = 0.011); Compared with baseline, both the NDE group and the DE group had significant differences at 2 min after installing of eye drops (p = 0.015, p = 0.010, respectively).
Discussion
With IOL implantation established as the standard of care for cataract surgery, biometry has become a critical step in modern practice, as precise estimation of parameters used in IOL power prediction algorithms is essential for achieving good visual and refractive outcomes, and improved patient-reported satisfaction17,29,37,38. However, the stability of the tear film and the ocular surface environment can affect the repeatability of data measurements. The IOL Master 700 (Carl Zeiss Meditec AG, Jena, Germany) employs SS-OCT, a modern Fourier-domain OCT technology that measures all light echoes simultaneously through Fourier transformation, offering dependability, and superior reproducibility and accuracy in measuring biological parameters such as AL, K1, and K2 compared to other devices22,35,39,40,41. This study primarily aimed to use the IOL Master 700 to analyze the changes of ocular biometry readings in patients with and without dry eye after instilling ATD.
Regarding the measurement repeatability of AL, no significant difference was observed between the DE and NDE groups. These results are in agreement with those of Takahiro et al.42. However, AL increased within 30 s after 0.1% sodium hyaluronate eye drops were applied to the eye regardless of dry eye. The results are predictable, as the device used in this study measures the AL as the distance from the tear film to the retinal pigment epithelium along the visual axis. AL plays a critical role in IOL calculation, with approximately 36% of refractive errors attributed to AL measurement inaccuracies. It has been reported that a 0.1 mm error in AL measurement can lead to a 0.3 diopter (D) refractive error43. Little is known about the ocular residence time of artificial tears on the ocular surface. Most previous studies employed radioactive labeling techniques to detect artificial tears post-administration, with quantitative gamma scintigraphy used to obtain results. Mean ocular surface residence times of 23.5 min and 11.1 min were reported for 0.3% hyaluronic acid (HA) and 0.2% HA, respectively. In the study conducted by Schmidl et al.44, which utilized HA, repeated measurements of tear film thickness at 10 min, 20 min, 40 min, 60 min, 120 min, and 240 min post-instillation. The results demonstrated that the topical application of artificial tears increases tear film thickness (TFT), with sodium hyaluronate eye drops sustaining an increase in TFT for up to 40 min. However, we did not measure tear film thickness in the study, it remains unclear whether certain influencing factors were present. Therefore, future studies should consider allowing a more extended waiting period after the application of artificial tears before conducting biometric and tear film measurements. The discrepancies between the current study and previous research may be attributed to differences in the techniques employed, potential variations in viscosity (which are hypothesized to influence the ocular surface residence time of the product), and differences in patient characteristics, among other factors45.
The change of CCT was also caused by the thickening of tear film after applying artificial tears. Artificial tear therapy increases CCT in dry eye patients by thickening the tear film, according to multiple studies44,46,47. Data from animal studies indicate that hyaluronic acid has a prolonged ocular residence time. Furthermore, another experiment showed that the ocular retention time of topically applied hyaluronic acid is influenced by its concentration, most likely depending on the length of the polymer chains48. The International Workshop on Meibomian Gland Dysfunction concluded that the duration of action of topical lubricants depends on their residence time on the ocular surface as well as patient characteristics45.
In contrast, there was no significant difference in the consistency of measurements for the K2. These results contrast with those reported by Takahiro et al., who found no significant difference in variability between the flat and steep meridians42. Shajari et al. reported repeatability coefficients of 0.093 for the flat meridian and 0.084 for the steep meridian, suggesting slightly greater variability in the flat meridian49. The observed results may be attributed to potential sources of error, and the sample size of 44/54 individuals is insufficient to confirm these findings with certainty.
Although our study did not assess the correlation between tear film stability and corneal curvature parameters due to the limitation of only measuring baseline NITBUT, recent findings by Ahn et al.50 have highlighted a significant association between these factors. This study demonstrated that a shorter TBUT and a higher corneal staining score (CSS) was associated with greater variability in K-values, suggesting that tear film instability and ocular surface diseases, such as DE, may lead to irregular refractive surfaces, potentially affecting the accuracy of ocular biometry, particularly in cataract surgery patients. The correlation between tear film stability and K-value variability underscores the importance of preoperative management of ocular surface diseases to reduce the risk of postoperative refractive errors.
Current corneal refractive surgeries and intraocular lens implantations can lead to visual disturbances, glare, and other undesirable symptoms, with pupil diameter being a limiting factor for optimal outcomes following corneal and refractive lens surgeries. Therefore, preoperative measurement of pupil size, especially under scotopic light conditions, is crucial51,52,53,54,55. Regarding the pupil assess in this study, one issue related to pupil measurements is that the pupil is never completely still but experiences slight, ongoing fluctuations referred to as Hippus56. Our findings on pupil dynamics align with those reported by H. Bouma et al., who demonstrated that during the Hippus phase, the pupil exhibits oscillations and a reduction in average diameter. This phenomenon is hypothesized to result from periodic pulse trains occurring approximately every 5 s within the parasympathetic innervation of the pupil. Each pulse train triggers a transient contraction, but due to the inherently slow dilation of the pupil, the diameter does not fully return to its baseline value before the next pulse train arrives56. Consequently, a solitary “snapshot” measurement of pupil size cannot be considered a dependable indicator of its actual average size. Differences in pupil size measurements can also be attributed to different adaptation time, patient compliance and ocular surface condition.
The limitations of this study are as follows: (1) In this preliminary experiment, only a relatively younger population was recruited, and older patients were not included in the study cohort. (2) Biological parameters were assessed only within 5 min after eye drop administration, leaving the longer-term effects unexplored. (3) Although ocular biological parameters were measured, their potential impact on intraocular lens calculation outcomes was not evaluated.
Conclusion
Artificial tears are widely employed in clinical treatment for patients with DE. This treatment effectively alleviates the bothersome symptoms of DE, enhances the smoothness of the corneal surface, and improves visual performance. Artificial tears significantly affect the readings of AL and CCT during ocular biometry. It is recommended to wait for at least 5 min after the installation of eye drops before performing ocular biometry measurements.
Data availability
Anonymized datasets generated and analyzed during the current study will be made available on reasonable request by the corresponding author (Guanghao Qin, qinguanghao2020@163.com).
References
Craig, J. P. et al. TFOS DEWS II definition and classification report. Ocul. Surf. 15, 276–283. https://doi.org/10.1016/j.jtos.2017.05.008 (2017).
Stapleton, F. et al. TFOS DEWS II epidemiology report. Ocul Surf. 15, 334–365. https://doi.org/10.1016/j.jtos.2017.05.003 (2017).
Gupta, P. K., Drinkwater, O. J., VanDusen, K. W., Brissette, A. R. & Starr, C. E. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J. Cataract Refract. Surg. 44, 1090–1096 (2018).
Paulsen, A. J. et al. Dry eye in the beaver dam offspring study: Prevalence, risk factors, and health-related quality of life. Am. J. Ophthalmol. 157, 799–806 (2014).
Titiyal, J. S., Falera, R. C., Kaur, M., Sharma, V. & Sharma, N. Prevalence and risk factors of dry eye disease in North India: Ocular surface disease index-based cross-sectional hospital study. Indian J. Ophthalmol. 66, 207–211 (2018).
Donthineni, P. R. et al. Incidence, demographics, types and risk factors of dry eye disease in India: Electronic medical records driven big data analytics report I. Ocul. Surf. 17, 250–256 (2019).
Sullivan, B. D. et al. An objective approach to dry eye disease severity. Investig. Ophthalmol. Vis. Sci. 51, 6125–6130 (2010).
Mantelli, F., Massaro-Giordano, M., Macchi, I., Lambiase, A. & Bonini, S. The cellular mechanisms of dry eye: From pathogenesis to treatment. J. Cell. Physiol. 228, 2253–2256. https://doi.org/10.1002/jcp.24398 (2013).
Messmer, E. M. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Arztebl Int. https://doi.org/10.3238/arztebl.2015.0071 (2015).
Nibandhe, A. S. & Donthineni, P. R. Understanding and optimizing ocular biometry for cataract surgery in dry eye disease: A review. Semin. Ophthalmol. 38, 24–30. https://doi.org/10.1080/08820538.2022.2112699 (2023).
Baudouin, C. et al. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: Proceedings of the ocean group meeting. Ocul. Surf. 11, 246–258. https://doi.org/10.1016/j.jtos.2013.07.003 (2013).
Epitropoulos, A. T., Matossian, C., Berdy, G. J., Malhotra, R. P. & Potvin, R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J. Cataract Refract. Surg. 41, 1672–1677 (2015).
Koh, S. Irregular astigmatism and higher-order aberrations in eyes with dry eye disease. Investig. Ophthalmol. Vis. Sci. 59, DES36–DES40 (2018).
Koh, S., Tung, C. I., Inoue, Y. & Jhanji, V. Effects of tear film dynamics on quality of vision. Br. J. Ophthalmol. 102, 1615–1620 (2018).
Jaycock, P. et al. The cataract National dataset electronic multi-centre audit of 55 567 operations: Updating benchmark standards of care in the united Kingdom and internationally. Eye 23, 38–49 (2009).
Lansingh, V. C., Carter, M. J. & Martens, M. Global cost-effectiveness of cataract surgery. Ophthalmology 114, 1670–1678 (2007).
McAlinden, C. et al. A head-to-head comparison of 16 cataract surgery outcome questionnaires. Ophthalmology 118, 2374–2381 (2011).
Skiadaresi, E. et al. Subjective quality of vision before and after cataract surgery. Arch. Ophthalmol. 130 (2012).
McAlinden, C. & Moore, J. E. Multifocal intraocular lens with a surface-embedded near section: Short-term clinical outcomes. J. Cataract Refract. Surg. 37, 441–445 (2011).
Savini, G., Hoffer, K. J. & Schiano-Lomoriello, D. Agreement between lens thickness measurements by ultrasound immersion biometry and optical biometry. J. Cataract Refract. Surg. 44, 1463–1468 (2018).
Guimarã, E. et al. Z. Updates in Biometry. http://www.internat-ophthalmology.com (2017).
Akman, A., Asena, L. & Güngör, S. G. Evaluation and comparison of the new swept source OCT-based iolmaster 700 with the iolmaster 500. Br. J. Ophthalmol. 100, 1201–1205 (2016).
Röggla, V. et al. Influence of artificial tears on keratometric measurements in cataract patients. Am. J. Ophthalmol. 221, 1–8 (2021).
Montés-Micó, R., Cáliz, A. & Alió, J. L. Changes in ocular aberrations after instillation of artificial tears in dry-eye patients. J. Cataract Refract. Surg. 30, 1649–1652 (2004).
Rah, M. J. A review of hyaluronan and its ophthalmic applications. Optometry 82, 38–43 (2011).
Lee, J. H., Ahn, H. S., Kim, E. K. & Kim, T. I. Efficacy of Sodium Hyaluronate and Carboxymethylcellulose in Treating Mild to Moderate Dry Eye Disease. http://www.corneajrnl.com
Sheard, R. Optimising biometry for best outcomes in cataract surgery. Eye (Basingstoke). 28, 118–125 (2014).
Hovanesian, J., Epitropoulos, A., Donnenfeld, E. D. & Holladay, J. T. The effect of Lifitegrast on refractive accuracy and symptoms in dry eye patients undergoing cataract surgery. Clin. Ophthalmol. 14, 2709–2716 (2020).
Sahin, A. & Hamrah, P. Clinically relevant biometry. Curr. Opin. Ophthalmol. 23, 47–53. https://doi.org/10.1097/ICU.0b013e32834cd63e (2012).
Wolffsohn, J. S. et al. TFOS DEWS II diagnostic methodology report. Ocul. Surf. 15, 539–574. https://doi.org/10.1016/j.jtos.2017.05.001 (2017).
Best, N., Drury, L. & Wolffsohn, J. S. Clinical evaluation of the oculus keratograph. Contact Lens Anterior Eye 35, 171–174 (2012).
Tian, L., Qu, J. H., Zhang, X. Y. & Sun, X. G. Repeatability and reproducibility of noninvasive keratograph 5 m measurements in patients with dry eye disease. J. Ophthalmol. 2016 (2016).
Yokoi, N., Takehisa, Y. & Kinoshita, S. Correlation of tear lipid layer interference patterns with the diagnosis and severity of dry eye. Am. J. Ophthalmol. 122, 818–824 (1996).
Zhang, X. M. et al. Reliability of Chinese web-based ocular surface disease index questionnaire in dry eye patients: A randomized, crossover study. Int. J. Ophthalmol. 14, 834–843 (2021).
Liao, X., Peng, Y., Liu, B., Tan, Q. Q. & Lan, C. J. Agreement of ocular biometric measurements in young healthy eyes between iolmaster 700 and OA-2000. Sci. Rep. 10, 3134 (2020).
Yang, C. M., Lim, D. H., Kim, H. J. & Chung, T. Y. Comparison of two swept-source optical coherence tomography biometers and a partial coherence interferometer. PLoS One 14 (2019).
McAlinden, C. et al. Agreement of anterior ocular biometric measurements with a new optical biometer and a Scheimpflug Tomographer. J. Cataract Refract. Surg. 42, 679–684 (2016).
Koh, S. et al. Effect of instillation of Eyedrops for dry eye on optical quality. Investig. Ophthalmol. Vis. Sci. 54, 4927–4933 (2013).
Arriola-Villalobos, P. et al. Agreement and clinical comparison between a new swept-source optical coherence tomography-based optical biometer and an optical low-coherence reflectometry biometer. Eye (Basingstoke). 31, 437–442 (2017).
Kurian, M. et al. Biometry with a new swept-source optical coherence tomography biometer: Repeatability and agreement with an optical low-coherence reflectometry device. J. Cataract Refract. Surg. 42, 577–581 (2016).
de Boer, J. F., Leitgeb, R. & Wojtkowski, M. Twenty-five years of optical coherence tomography: The paradigm shift in sensitivity and speed provided by Fourier domain OCT [Invited]. Biomed. Opt. Express. 8, 3248 (2017).
Hiraoka, T. et al. Influence of dry eye disease on the measurement repeatability of corneal curvature radius and axial length in patients with cataract. J. Clin. Med. 11 (2022).
Olsen, T. Calculation of intraocular lens power: A review. Acta Ophthalmol. Scand. 85, 472–485 (2007).
Schmidl, D. et al. Tear Film Thickness After Treatment With Artificial Tears in Patients With Moderate Dry Eye Disease. http://www.corneajrnl.com (2015).
Geerling, G. et al. The international workshop on meibomian gland dysfunction: Report of the subcommittee on management and treatment of meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 52, 2050–2064 (2011).
Wozniak, P. A. et al. Effect of different lubricant eye gels on tear film thickness as measured with ultrahigh-resolution optical coherence tomography. Acta Ophthalmol. 95, e307–e313 (2017).
Çakır, B. et al. Effects of artificial tear treatment on corneal epithelial thickness and corneal topography findings in dry eye patients. J. Fr. Ophtalmol. 41, 407–411 (2018).
Zheng, X., Goto, T. & Ohashi, Y. Comparison of in vivo efficacy of different ocular lubricants in dry eye animal models. Investig. Ophthalmol. Vis. Sci. 55, 3454–3460 (2014).
Shajari, M. et al. Comparison of axial length, corneal curvature, and anterior chamber depth measurements of 2 recently introduced devices to a known biometer. Am. J. Ophthalmol. 178, 58–64 (2017).
Ahn, S., Eom, Y., Song, J. S. & Kim, D. H. Short-term variability in ocular biometry and the impact of preoperative dry eye. Sci. Rep. 14, 26762 (2024).
S O, D. P., Corbett, M. C., Lohmann, C. P., Muir, K. & Marshall, J. M. G. The Effects of Ablation Diameter on the Outcome of Excimer Laser Photorefractive Keratectomy A Prospective, Randomized, Double-Blind Study. http://archopht.jamanetwork.com/
Holladay, J. T. et al. The Relationship of Visual Acuity, Refractive Error, and Pupil Size After Radial Keratotomy. http://archopht.jamanetwork.com/
The importance. of pupil size in optical quality measurements following radial keratotomy.
Predicting visual performance following excimer photorefractive keratectomy.
Roberts, C. W. & Koesterf, C. J. Optical Zone Diameters for Photorefractive Corneal Surgery.
Bouma, H. & Baghuis, L. C. J. Hippus of the pupil: Periods of slow oscillations of unknown origin. Vis. Res. II (1971).
Acknowledgements
We appreciate their participation in this research. This research was funded by the He Eye Specialty Hospital in Shenyang, China. The authors have no proprietary interest in any of the products discussed.
Author information
Authors and Affiliations
Contributions
Conception and design of the research: YC, EEP; Analysis and interpretation of the data: YC; Writing original draft preparation: YC; Data collection: YC, ML, JC, JZ; Critical revision of the manuscript: GQ, EEP; Supervision: XH, GQ. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Ethics Committee of He Eye Specialist Hospital (IRB (2024) K009.01) and conducted by the Department of Ophthalmology.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Y., Li, M., Chen, J. et al. To evaluate the effects of artificial tears on ocular biological parameters in dry eye and non-dry eye patients. Sci Rep 15, 12392 (2025). https://doi.org/10.1038/s41598-025-95801-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95801-5
Keywords
This article is cited by
-
Effect of Low-Level Light Therapy on Ocular Surface Parameters in Patients Undergoing Cataract Surgery: A Prospective Double-Masked Randomized Controlled Clinical Trial
Ophthalmology and Therapy (2025)
-
A Practical Approach for Optimizing Ocular Surface Status Before Cataract Surgery to Improve Visual Outcomes and Reduce the Risk of Postoperative Dry Eye
Ophthalmology and Therapy (2025)