Abstract
Infections are serious postoperative complications, and strongly affects the mortality and prognosis of patients. Body mass index (BMI) and lipids are factors in postoperative infection, but a causal relationship has not been know. In this Mendelian randomization (MR) study, we utilized genome-wide association study (GWAS) data from the Global Lipids Genetics Consortium and FinnGen database, treating lipids and BMI as exposures. Postoperative infection GWAS data from the UK Biobank served as the outcome. We utilized linkage disequilibrium score regression (LDSC) analysis to evaluate the genetic correlations between lipids, BMI, and postoperative infections. We employed univariate and reverse MR analyses to explore the causal relationships between exposure and outcome factors. The analysis primarily utilized the inverse variance weighted method, supplemented by MR-Egger and weighted median methods. The MR-PRESSO method was used to detect horizontal pleiotropy and potential outliers. Additionally, stepwise mediation MR analysis was employed to investigate indirect factors potentially influencing the relationships between lipids, BMI, and postoperative infections. The genetic covariance analysis indicates that there is no sample overlap among all the GWAS conducted. In the LDSC analysis, genetic correlations (GC) were found between BMI(GC = 0.430, P < 0.05), HDL-C(GC = − 0.414, P < 0.05), nonHDL-C(GC = 0.137, P < 0.05), TG(GC = 0.417, P < 0.05), and postoperative infection. HDL-C showing a negative genetic association with postoperative infection, while other phenotypes showed positive associations. In MR analysis, causal relationships were identified between BMI and postoperative infection (OR = 1.36, 95% CI = 1.16–1.60, P < 0.05) and HDL-C and postoperative infection (OR = 0.87, 95% CI = 0.78–0.96, P < 0.05), with BMI showing a positive causal association and HDL-C showing a negative causal association with postoperative infection. These findings are consistent with the LDSC results. In the reverse MR analysis, there was no significant causal relationship identified between postoperative infection and both BMI and lipids. Stepwise mediation MR analysis excluded the impact of potential mediating factors between exposure and outcomes. In this study, through LDSC and MR analyses, we identified genetic correlations and causal links between BMI, HDL, and postoperative infection. It was found that BMI might increase the risk of postoperative infection, whereas HDL could potentially lower the risk of developing postoperative infection.
Similar content being viewed by others
Introduction
Postoperative infection is one of the most common complications after surgeries, which significant increase postoperative mortality and prolong ICU-stay. A cohort study discovered patients with 30-day postoperative infection had a 3.2-fold higher risk of 1-year infection and a 1.9-fold higher risk of mortality compared with those who had no 30-day infection1.Different source of postoperative infection, which may be caused by either diverse bacteria or fungal pathogens2. The types of postoperative infection are complex and postoperative patient’s immunosuppressed state leads to a heightened risk of infection3, making it challenging to rapidly and accurately diagnose and administer appropriate treatment. It is significantly important to recognize the risk of infection and the likelihood of severe disease after surgeries, even before surgeries.
Many studies have indicated a definitive link between obesity, lipids (high density lipoprotein cholesterol, low density lipoprotein cholesterol, non-high density lipoprotein cholesterol, total cholesterol and triglyceride), and postoperative infections4,5,6. There is a close relationship between obesity, lipid levels, and postoperative infections, which is often negative. However, HDL stands out in many studies as a distinctive protective factor7,8. Properly understanding the roles of obesity and blood lipids in postoperative infections is beneficial for perioperative preparation and patient prognosis.
As is knows, Low HDL, a hallmark of dyslipidemia, signifies unhealthy lifestyle habits, disrupted metabolism, and heightened cardiovascular risk. Recent research suggests that low HDL is also associated with increased risk of inflammatory disorders, malignant tumors, diabetes, and other infected diseases9. A prospective study10 explored that association between low HDL level and postoperative infection was increased. HDL levels may be used as predictive parameter for postoperative infections. Preoperative screening for low-HDL may be beneficial in controlling the risk for postoperative complications such as postoperative infection among surgical patients. Clinically, postoperative infection patients accompanied with significantly decreased HDL, and multiple studies have shown that HDL plays an important role in infected patients11,12.
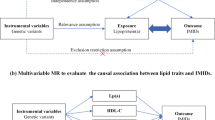
Mendelian randomization (MR) is a method that uses genetic variations closely linked to modifiable exposures to generate more reliable evidence about which interventions should provide health benefits13. MR essence lies in using genetic data as a bridge to explore the causal connection between a specific exposure and outcome. Unlike observational studies, Mendelian randomization (MR) leverages genetic variants as instrumental variables, which are randomly assigned at conception, thus mitigating biases from confounding factors and reverse causation. This approach provides stronger evidence for causality compared to prior epidemiological studies. No existing comprehensive MR studies have focused on BMI, lipids and postoperative infection. The present study explored the genetic relationship and causal relationships between BMI, lipids and postoperative infection with genetic statistics (Fig. 1). We searched the FinnGen Biobank (Round 9) bring into BMI, and Using summary data from Global Lipids Genetics Consortium GWAS across multiple lipid traits, including HDL-C, LDL-C, nonHDL-C, TC and TG. We searched the UK Biobank for postoperative infection according to ICD-10 (Version: 2016). This study used genetic statistical methods to conduct MR and genetic association analysis on lipid and BMI GWAS and postoperative infections GWAS. Exploring Genetic Correlations and Causal Associations of BMI and lipids with postoperative infection and providing genetic statistical guidance for the role of BMI and lipids in postoperative infection in clinical practice.
Materials and methods
GWAS summary data source
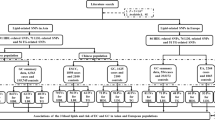
We included five lipid-related traits (HDL-C, LDL-C, nonHDL-C, TC and TG) and BMI as exposures in this study. The postoperative infection was chosen as the outcome factor. The GWAS summary data of lipid-related traits were derived from the published research14. The Global Lipids Genetics Consortium aggregated GWAS results from 1,654,960 individuals. To keep all patients in this study were of European, our study used European group including 1,320,016 individuals. The GWAS summary data of BMI and postoperative infection used in this study were obtained from the FinnGen R9 release and UK Biobank, including 266,130 and 406,832 individuals (Table 1). Based on literature related to BMI, lipid and infection15,16,17,18, we found that research on lipids often focuses on the HDL in modulating cytokines and inflammatory factors during infections. On infections, tend to concentrate on the mechanisms of sepsis development, which also primarily involves the expression of blood cells, cytokines and immunoglobulins. Therefore, we selected 21 types of mediators (Additional file 1), including counts of basophils, eosinophils, lymphocytes, monocytes, neutrophils, white blood cells, IgA, IgM, IgG, IgE, IL-1α, IL-6, IL-12, IL-4, IL-10, IL-13, TGF-β1, TGF-β2, TGF-β3, TNF-α, IFN-γ and Had major operations. These mediators may lie in the pathway from BMI and lipids to postoperative infection, and thus are considered as mediating factors in MR analysis. Since all analyses in this article are based on publicly available summary data, this study does not require ethical approval from the institutional review board. This study is reported in line with the STROBE-MR guidance (Additional file 2), with the checklist available in the Supporting information19,20.
Data processing
Sample overlapping of GWAS summary data
This study included The Global Lipid Genetics Consortium’s five lipid-related GWAS, FinnGen’s BMI GWAS and the UK Biobank’s postoperative infection GWAS. These GWAS are derived from different databases. In MR analyses, sample overlap can lead to biased estimates of causal effects due to overfitting and the inclusion of non-independent samples. To further confirm the overlap between exposure and outcome datasets, Genetic Covariance analysis was utilized in the GWAS, calculated between the exposures and the outcome21. Genetic Covariance refers to the degree of shared genetic variation between two or more traits. It describes the interrelationship of genetic variations among different traits, reflecting whether they are influenced by similar or related genetic factors.
GWAS quality control
All GWAS data could be used for genetic analysis after stringent quality control filtering. Firstly, the 1000 Genome Project (1KGP) Phase 3 European population was used as the reference panel, reserving all the gene with minor allele frequency (MAF) ≥ 0.01, and restricted to biallelic allelicity. Secondly, when matched, single nucleotide polymorphisms (SNPs) without a rsID or repeating rsID were discarded. Thirdly, all the SNPs need to matched the chromosomal position with hg19 as the human reference genome coordinate.
Linkage disequilibrium score regression
LDSC estimates the heritability of individual traits or the genetic correlation between pairs of traits by constructing a regression relationship between LD score and GWAS test statistics. We used univariate linkage disequilibrium (LD) score regression (LDSC) to estimate the GWAS heritability of each trait22. To explore the shared genetic components between LDL-C, HDL-C, TG, TC, nonHDL-C, and BMI with postoperative infection, we further conducted global genetic correlation analysis using bivariate LDSC. The LD score was calculated using European ancestry reference data from 1000 Genomes, limited to 1.2 million well-controlled HapMap3 SNPs, excluding SNPs in the major histocompatibility complex (MHC) region due to the complex LD relations affecting the estimation of genetic correlations. Due to unknown sample overlap, in LDSC analysis, we did not constrain the intercept, using it instead to assess potential population stratification in individual trait GWAS or potential sample overlap between pairs of GWAS. A significance threshold (q < 0.05) was set post false discovery rate (FDR) correction (BH method) for P < 0.05.
Mendelian randomization analysis
For MR analysis, the following three assumptions must be met and align with instrumental variables (IVs). First, IVs that the SNPs were screened from the exposure must be closely related to exposures; Second, IVs are independent of confounding factors; Third, IVs should only influence the outcome through their impact on exposure and not directly or through other pathways.
In this study, SNPs closely related to exposures were used as IVs to investigate the causal relationship between exposure and outcomes. We looked for IVs closely related to exposures with linkage disequilibrium of r2 < 0.001, genome-wide significance of p < 5 × 10−8, and a clumping window of 10,000 kb. The F statistic (F > 10) is used to ensure a strong correlation between the IVs and exposure. After this, we conducted bidirectional two-sample Mendelian randomization23 to investigate causal links between lipid profiles (LDL-C, HDL-C, TG, TC, nonHDL-C) and BMI with postoperative infection. Cochrane’s Q value is used to assess heterogeneity. Considering the heterogeneity introduced by multiple instrumental variables, we opted for a random effects IVW (inverse variance weighted) model instead of a fixed-effect model for our outcome measures. The primary method, IVW was used for a meta-analysis of SNP Wald ratios, yielding a more accurate overall effect, even amid instrumental variable heterogeneity. We also used MR-Egger24 and weighted median methods25 as supplements to IVW. Using the scatter plot visualizes the relationship between the genetic associations with the exposure (on one axis) and the genetic associations with the outcome (on the other axis). Each point on the scatter plot represents a different genetic variant (or SNP single nucleotide polymorphism). The slope of the line fitted through these points can indicate the magnitude of the causal effect of the exposure on the outcome, assuming no pleiotropy or other biases are affecting the analysis. To ensure the accuracy of MR, further supplementary analyses were conducted: (1) MR-Egger intercept test for horizontal pleiotropy. (2) MR-PRESSO test single SNP pleiotropy; (3) leave-one-out analysis to assess if a single SNP drives the causal effect; (4) MR-Steiger directionality test to confirm the correct direction of causal estimation. (5) Stepwise mediation MR analysis is utilized to eliminate the potential influence of mediating factors. Finally, for data requiring multiple hypothesis correction, the Benjamini–Hochberg (BH) method is used to adjust the threshold of p-values, thereby reducing the likelihood of accidentally discovering significant results. FDR correction (BH method) post-analysis with a P < 0.05 as the significance threshold (q < 0.05). All statistical analyses were conducted using the Mendelian Randomization (0.4.2), TwoSampleMR (0.5.7), MR-PRESSO (1.0) packages in R, version 4.2.2 (https://www.r-project.org/).
Result
GWAS overlapping
Potential sample overlap was assessed for all GWAS datasets used in this study. The LDSC intercept values did not indicate significant sample overlap or population stratification, suggesting that the genetic correlation estimates are not biased due to overlapping samples. Specifically, no significant evidence of sample overlap was found between the GWAS datasets for Postoperative Infection and BMI (P > 0.1), Postoperative Infection and HDL-C (P > 0.1), Postoperative Infection and LDL-C (P > 0.1), Postoperative Infection and nonHDL-C (P > 0.1), Postoperative Infection and TG (P > 0.1), or Postoperative Infection and TC (P > 0.1). Based on these findings, we conclude that there is no substantial overlap between the exposure and outcome GWAS datasets, and thus all data can be reliably used for further Mendelian randomization (MR) and genetic correlation analyses.
Genetic correlation between BMI, lipids and postoperative infection
Initially, we estimated the heritability of individual traits for both exposure and outcome using univariate LDSC. The results showed that all traits exhibited significant heritability (P < 0.05), confirming their polygenic nature. We then applied bivariate LDSC to assess the genetic correlations between BMI, lipid traits, and postoperative infection. After false discovery rate (FDR) correction (Q < 0.05), significant genetic correlations were observed between BMI, HDL-C, nonHDL-C, TG, and postoperative infection (P < 0.05). Specifically, HDL-C showed a negative genetic correlation with postoperative infection (r = − 0.414), indicating that a higher HDL-C level is associated with a lower genetic predisposition to postoperative infection. In contrast, BMI, nonHDL-C, and TG showed positive genetic correlations with postoperative infection, with correlation coefficients of r = 0.430, r = 0.137, and r = 0.417, respectively, suggesting shared genetic influences predisposing individuals to both higher BMI/lipid levels and increased postoperative infection risk. LDL-C and TC did not exhibit statistically significant genetic correlations with postoperative infection (P > 0.05), indicating no strong shared genetic basis between these traits (Table 2).
Causality between BMI, lipids and postoperative infection
Selection of instrumental variables
By identifying SNPs closely associated with exposure in BMI, HDL-C, LDL-C, nonHDL-C, TC, and TG with linkage disequilibrium r2 < 0.001 and filtering for genome-wide significance P < 5 × 10−8 and a clumping window of 10,000 kb, we obtained instrumental variables for six exposure factors. The F statistic was used to ensure strong correlation between the instrumental variables and exposures. An F statistic greater than 10 is generally considered indicative of strong correlation. (All instrumental variables are provided in Additional file 3.)
Mendelian randomization analysis
We performed bidirectional Mendelian randomization analyses on six pairs of exposure-outcome traits. Using the random effects IVW method, after Benjamini–Hochberg correction, two pairs of exposure-outcome traits were found to have causal associations (q < 0.05). Specifically, HDL-C showed a strong negative correlation with postoperative infection (OR = 0.87), and BMI exhibited a significant positive correlation (OR = 1.36). TG also showed a positive correlation with postoperative infection (OR = 1.14, q-value = 0.082). However, no apparent causal relationships were found between LDL-C, nonHDL-C, TC, and postoperative infection (q > 0.1) (Fig. 2). Through the scatter plot (Fig. 3), we could clearly see that BMI has a significant positive correlation with postoperative infection, while HDL-C shows a strong negative correlation with postoperative infection.
The F statistics for instrumental variables (IVs) and the estimated power for all analyses are presented in Additional file 3. The F statistics for these IVs all exceed the threshold of 10. Mild heterogeneity was detected in the analysis of certain exposures (Additional file 4), therefore, we further utilized MR-PRESSO to identify for heterogeneity biases in the MR analyses of BMI, HDL-C, and postoperative infection. There was no significant outlier SNPs were detected upon individual SNP testing (Additional file 5). In the analysis of all exposures and outcomes through MR-Egger intercept test, no pleiotropy was detected (Additional file 6). The MR-Egger method for the pairs of BMI, HDL, and TG with postoperative infection provided significant estimates consistent with those obtained through IVW, indicating that causal associations still exist in these directions even after considering horizontal pleiotropy (Additional file 7). Leave-one-out analysis was used to analyze the six pairs of exposure-outcome traits. Only BMI-postoperative infection and HDL-postoperative infection, there were no instances of a single SNP driving the overall causal effect (Additional file 8). Using the MR-Steiger directionality test to confirm the correct direction of causal estimation, the results showed that the directionality was consistent across all six pairs of exposure-outcome traits. (Additional file 9). To explore the potential for BMI and lipids to impact postoperative infection risk through intermediary factors, we included mediators related to lipids and infection such as Cytokines, immunoglobulins, and blood cells. In the stepwise mediation MR analysis, we observed significant associations between exposure factors and several mediating factors, as well as between certain mediating factors and postoperative infection outcomes. However, no mediating factors showed a significant association with both exposure and outcome simultaneously. This suggests that these mediators do not serve as confounding variables in the causal pathway between exposure and postoperative infection. Therefore, the observed relationships between BMI, lipids, and postoperative infection remain robust without being driven by indirect mediating effects (Fig. 4 presents the main results, with detailed outcomes available in Additional file 10). The use of stepwise mediation MR analysis further enhanced the reliability of the MR analysis results26.
Reverse Mendelian randomization analysis
We conducted reverse MR analysis, treating postoperative infection as the exposure factor. Due to the absence of SNPs with P < 5 × 10−8 in the GWAS for postoperative infection, the selection criteria were adjusted to P < 5 × 10−6, with other filtering criteria remaining the same as in the MR analysis. The analysis revealed that when postoperative infection is considered as an exposure factor, it does not have a significant causal relationship with outcome factors (P > 0.05). (Additional file 11 and Additional file 12).
Discussion
In this study, through LDSC analysis, we explored the genetic correlation between BMI, lipid, and postoperative infection, revealing strong genetic correlations between BMI, HDL-C, nonHDL-C, TG, and postoperative infection. MR analysis explored causal associations between BMI, HDL, and postoperative infections, avoiding biases from confounders and reverse causality often present in observational studies. MR sits between intervention epidemiology and observational epidemiology in terms of evidence level27. Notably, BMI showed a significant positive causal relationship with postoperative infection, HDL-C demonstrated a significant negative causal relationship, and TG was identified as a potential risk factor for increased infection risk. These findings are consistent with the LDSC results, demonstrating genetic causal relationships between BMI, HDL, and postoperative infection. Through mediator factor analysis, we did not find any potential associations between cytokines, immunoglobulins, blood cells, and the relationships among BMI, lipids, and postoperative infection. These factors do not mediate the causal relationship between BMI, lipids, and postoperative infection. While prior observational studies10,28 have identified associations between BMI, HDL-C, and postoperative infection, they could not establish causality due to confounding and reverse causation. Our MR study addresses this gap by using genetic instrumental variables, providing stronger evidence that BMI increases risk while HDL-C serves as a protective factor.
The World Health Organization defines obesity as conditions characterized by excessive or abnormal fat accumulation that poses a risk to health. The prevalence of obesity is continually increasing worldwide. We discussed the impact of obesity on postoperative infection using BMI as a proxy. Obese patients have a significantly increased risk of hospital-acquired infections29, like catheter infections in ICU patients30. Recent studies on COVID-19 also mentioned that surgical weight reduction can significantly improve COVID-19 outcomes31. The many pro-inflammatory mediators and adipokines in the adipose tissue of obese patients are associated with chronic low-grade inflammation, which might enhance the characteristic COVID-19 cytokine storm32. Our study focuses on postoperative infection patients. Traditional views suggest that surgery-induced endogenous danger signals triggering a nonspecific inflammatory response are beneficial to patients, but they reduce resistance to infection33. Using genetic statistics, we explored the connections between lipids, obesity, and postoperative infections, seeking reasons for the increased risk of infection in postoperative patients. Zhai et al.6 showed a significant increase in the risk of surgical site infections post-colorectal cancer surgery in obese patients, and Chakaroun et al.4 indicated that bariatric surgery could reduce the risk of postoperative complications and infections, which aligns with our findings of a positive correlation between BMI and postoperative infection.
HDL has traditionally been considered protective against cardiovascular diseases34. Increasing evidence now indicates other roles of high-density lipoprotein, including its promotion of cholesterol efflux, and its apolipoprotein composition’s anti-inflammatory, antioxidant, and anti-diabetic properties35. Research has shown that lipids are involved in the development of infections, with low lipid and lipoprotein concentrations linked to poor prognosis. Serum lipoproteins decrease in quantity during the acute phase of infection, marking them as significant negative reactants5. HDL is considered a transporter of lipopolysaccharides in plasma, binding with lipopolysaccharides entering the bloodstream and transporting them to target sites for clearance. A decrease in HDL levels may increase the risk of postoperative infection10. A prospective observational study on cardiac surgery with cardiopulmonary bypass showed that low cholesterol levels pre-surgery might be a simple biomarker for early identification of patients at high risk of sepsis36. Trinder M and colleagues demonstrated that inhibiting cholesterol ester transfer protein preserves HDL cholesterol and improves survival rates in sepsis patients37. These findings are consistent with our study’s result of a significant negative correlation between HDL-C and postoperative infection. The study by Feng et al.38 indicated that LDL-C levels are not directly associated with the risk of sepsis or adverse outcomes in hospitalized patients with infections. This aligns with the results of our study, where no causal link was found between LDL-C and postoperative infection.
Currently, there are few MR studies on the relationship between obesity, lipids, and postoperative infections. This paper is the first to explore the genetic and causal connections between them, clarifying that obesity and HDL have genetic associations with postoperative infections, with obesity positively correlated and HDL negatively correlated. It provides statistical genetic guidance for perioperative treatment of surgical patients and for predicting outcomes using obesity and lipid markers in postoperative patients. Our findings extend existing epidemiological evidence by confirming a genetic causal association between BMI, Lipid, and postoperative infections. The MR approach overcomes biases inherent in observational research, reinforcing the importance of BMI and lipid management in perioperative care. There are some limitations in this study. The research population consisted solely of European individuals, which may not adequately represent other ethnic groups; the study also did not distinguish between different genders and age groups. Further validation with GWAS from more diverse populations is needed. Many studies have shown a U-shaped association between obesity and certain diseases39, but MR analysis does not demonstrate such a U-shaped relationship between obesity and some diseases, making it challenging to identify a similar association with postoperative infections. Although HDL-C is commonly used clinically to represent HDL concentration, many of HDL’s functions are exerted by the entire particle or components other than cholesterol17, meaning HDL-C does not fully describe all functions of HDL.
Conclusion
In this study, through LDSC and MR analyses, we identified genetic correlations and causal links between BMI, HDL, and postoperative infection. It was found that BMI might increase the risk of postoperative infection, whereas HDL could potentially lower the risk of developing postoperative infection.
Data availability
The datasets analyzed in this study are publicly available summary statistics. GWAS Catalog (https://www.ebi.ac.uk/gwas/), The Global Lipid Genetics Consortium (http://lipidgenetics.org/#), FinnGen database (https://www.finngen.fi/fi), and Neale Lab UKB GWAS (http://www.nealelab.is/uk-biobank/) provided access to genome-wide association study data. We thank all research participants who provided DNA samples for these studies. Data used can be obtained upon a reasonable request to the corresponding author.
References
O’Brien, W. J., Gupta, K. & Itani, K. M. F. Association of postoperative infection with risk of long-term infection and mortality. JAMA Surg. 155, 61–68. https://doi.org/10.1001/jamasurg.2019.4539 (2020).
Bassetti, M., Eckmann, C., Giacobbe, D. R., Sartelli, M. & Montravers, P. Post-operative abdominal infections: Epidemiology, operational definitions, and outcomes. Intensive Care Med. 46, 163–172. https://doi.org/10.1007/s00134-019-05841-5 (2020).
Angele, M. K. & Faist, E. Clinical review: Immunodepression in the surgical patient and increased susceptibility to infection. Crit. Care 6, 298–305. https://doi.org/10.1186/cc1514 (2002).
Chakaroun, R. M. et al. Circulating bacterial signature is linked to metabolic disease and shifts with metabolic alleviation after bariatric surgery. Genome Med. 13, 105. https://doi.org/10.1186/s13073-021-00919-6 (2021).
Gordon, B. R. et al. Relationship of hypolipidemia to cytokine concentrations and outcomes in critically ill surgical patients. Crit. Care Med. 29, 1563–1568. https://doi.org/10.1097/00003246-200108000-00011 (2001).
Zhai, W. et al. Impact of visceral obesity on infectious complications after resection for colorectal cancer: A retrospective cohort study. Lipids Health Dis. 22, 139. https://doi.org/10.1186/s12944-023-01890-4 (2023).
Vekic, J., Zeljkovic, A., Stefanovic, A., Jelic-Ivanovic, Z. & Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 92, 71–81. https://doi.org/10.1016/j.metabol.2018.11.005 (2019).
Kjeldsen, E. W., Nordestgaard, L. T. & Frikke-Schmidt, R. HDL cholesterol and non-cardiovascular disease: A narrative review. Int. J. Mol. Sci. 22, 4547. https://doi.org/10.3390/ijms22094547 (2021).
Rohatgi, A., Westerterp, M., von Eckardstein, A., Remaley, A. & Rye, K. A. HDL in the 21st Century: A multifunctional roadmap for future HDL research. Circulation 143, 2293–2309. https://doi.org/10.1161/circulationaha.120.044221 (2021).
Canturk, N. Z., Canturk, Z., Okay, E., Yirmibesoglu, O. & Eraldemir, B. Risk of nosocomial infections and effects of total cholesterol, HDL cholesterol in surgical patients. Clin. Nutr. 21, 431–436. https://doi.org/10.1054/clnu.2002.0575 (2002).
De Geest, B. & Mishra, M. Impact of high-density lipoproteins on sepsis. Int. J. Mol. Sci. 23, 12965. https://doi.org/10.3390/ijms232112965 (2022).
Trinder, M. et al. Cholesteryl ester transfer protein influences high-density lipoprotein levels and survival in sepsis. Am. J. Respir. Crit. Care Med. 199, 854–862. https://doi.org/10.1164/rccm.201806-1157OC (2019).
DaveySmith, G. & Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23, R89-98. https://doi.org/10.1093/hmg/ddu328 (2014).
Graham, S. E. et al. The power of genetic diversity in genome-wide association studies of lipids. Nature 600, 675–679. https://doi.org/10.1038/s41586-021-04064-3 (2021).
Tanaka, S. et al. High-density lipoproteins during sepsis: From bench to bedside. Crit. Care 24, 134. https://doi.org/10.1186/s13054-020-02860-3 (2020).
Stasi, A. et al. Beneficial effects of recombinant CER-001 high-density lipoprotein infusion in sepsis: Results from a bench to bedside translational research project. BMC Med. 21, 392. https://doi.org/10.1186/s12916-023-03057-5 (2023).
von Eckardstein, A., Nordestgaard, B. G., Remaley, A. T. & Catapano, A. L. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur. Heart J. 44, 1394–1407. https://doi.org/10.1093/eurheartj/ehac605 (2023).
Li, H. et al. Association of autoimmune diseases with the occurrence and 28-day mortality of sepsis: An observational and Mendelian randomization study. Crit. Care 27, 476. https://doi.org/10.1186/s13054-023-04763-5 (2023).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomisation (STROBE-MR): Explanation and elaboration. BMJ 375, n2233. https://doi.org/10.1136/bmj.n2233 (2021).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: The STROBE-MR statement. JAMA 326, 1614–1621. https://doi.org/10.1001/jama.2021.18236 (2021).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241. https://doi.org/10.1038/ng.3406 (2015).
Bulik-Sullivan, B. K. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295. https://doi.org/10.1038/ng.3211 (2015).
Hemani, G., Tilling, K. & Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 13, e1007081. https://doi.org/10.1371/journal.pgen.1007081 (2017).
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525. https://doi.org/10.1093/ije/dyv080 (2015).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314. https://doi.org/10.1002/gepi.21965 (2016).
Carter, A. R. et al. Mendelian randomisation for mediation analysis: Current methods and challenges for implementation. Eur. J. Epidemiol. 36, 465–478. https://doi.org/10.1007/s10654-021-00757-1 (2021).
Zuccolo, L. & Holmes, M. V. Commentary: Mendelian randomization-inspired causal inference in the absence of genetic data. Int. J. Epidemiol. 46, 962–965. https://doi.org/10.1093/ije/dyw327 (2017).
Meijs, A. P. et al. The effect of body mass index on the risk of surgical site infection. Infect, Control Hosp, Epidemiol, 40, 991–996. https://doi.org/10.1017/ice.2019.165 (2019).
Falagas, M. E. & Kompoti, M. Obesity and infection. Lancet Infect. Dis. 6, 438–446. https://doi.org/10.1016/s1473-3099(06)70523-0 (2006).
Buetti, N. et al. Obesity and risk of catheter-related infections in the ICU. A post hoc analysis of four large randomized controlled trials. Intensive Care Med. 47, 435–443. https://doi.org/10.1007/s00134-020-06336-4 (2021).
Aminian, A. et al. Association of weight loss achieved through metabolic surgery with risk and severity of COVID-19 infection. JAMA Surg. 157, 221–230. https://doi.org/10.1001/jamasurg.2021.6496 (2022).
Landecho, M. F., Marin-Oto, M., Recalde-Zamacona, B., Bilbao, I. & Frühbeck, G. Obesity as an adipose tissue dysfunction disease and a risk factor for infections—Covid-19 as a case study. Eur. J. Intern. Med. 91, 3–9. https://doi.org/10.1016/j.ejim.2021.03.031 (2021).
Cui, P. & Fang, X. Pathogenesis of infection in surgical patients. Curr. Opin. Crit. Care 21, 343–350. https://doi.org/10.1097/mcc.0000000000000227 (2015).
Soppert, J., Lehrke, M., Marx, N., Jankowski, J. & Noels, H. Lipoproteins and lipids in cardiovascular disease: From mechanistic insights to therapeutic targeting. Adv. Drug Deliv. Rev. 159, 4–33. https://doi.org/10.1016/j.addr.2020.07.019 (2020).
Mehta, N. et al. The evolving role of cholesteryl ester transfer protein inhibition beyond cardiovascular disease. Pharmacol. Res. 197, 106972. https://doi.org/10.1016/j.phrs.2023.106972 (2023).
Lagrost, L. et al. Low preoperative cholesterol level is a risk factor of sepsis and poor clinical outcome in patients undergoing cardiac surgery with cardiopulmonary bypass. Crit. Care Med. 42, 1065–1073. https://doi.org/10.1097/ccm.0000000000000165 (2014).
Trinder, M. et al. Inhibition of cholesteryl ester transfer protein preserves high-density lipoprotein cholesterol and improves survival in sepsis. Circulation 143, 921–934. https://doi.org/10.1161/circulationaha.120.048568 (2021).
Feng, Q. et al. Association between low-density lipoprotein cholesterol levels and risk for sepsis among patients admitted to the hospital with infection. JAMA Netw. Open 2, e187223. https://doi.org/10.1001/jamanetworkopen.2018.7223 (2019).
Dobner, J. & Kaser, S. Body mass index and the risk of infection—from underweight to obesity. Clin. Microbiol. Infect. 24, 24–28. https://doi.org/10.1016/j.cmi.2017.02.013 (2018).
Funding
GuangDong Basic and Applied Basic Research Foundation(No. 2021A1515111050).
Author information
Authors and Affiliations
Contributions
TY, ZC, and DC were responsible for writing the main manuscript and data analysis. XS and RS ensured the scientific rigor and precision of the article. WS, JJ, and XM created the figures and schematics. ZM assisted with language editing, while JW and XG performed the final review and corrections of the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
Ethical approval was not required because of the public characteristics of the data of GWAS.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, T., Chen, Z., Cao, D. et al. Genetic correlations and causal associations between BMI, HDL-C, and postoperative infections: a two-sample Mendelian randomization study. Sci Rep 15, 11834 (2025). https://doi.org/10.1038/s41598-025-95812-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95812-2