Abstract
The nematode Haemonchus contortus causes severe anemia in sheep and goats. Drug-resistant isolates are common, prompting a need for parasite control measures beyond chemotherapeutics. Vaccination is one promising approach for mitigation of clinical signs associated with haemonchosis. One challenge for H. contortus vaccine efforts is the need to administer repeated boosting doses at regular intervals. In this study, we evaluated a vaccine platform for extended antigen release (VPEAR) designed to initiate and maintain long-term immunity following a single immunization event in sheep. We compared a soluble vaccine depot with montanide adjuvant to the VPEAR platform with two different adjuvant combinations. Vaccination with VPEAR adjuvanted with DEAE-dextran induced antibody titers in 5 out of 6 vaccinated sheep up to 47 weeks post-vaccination. Challenge experiments revealed a 73% decrease in adult worm burden in this vaccine group compared to adjuvant alone and serum antibodies from these animals bound the luminal surface of the parasite intestine. Overall, the VPEAR platform was effective for long-term vaccination with no indication of immune tolerance to the parasite upon challenge.
Similar content being viewed by others
Introduction
Haemonchus contortus is a strongyle nematode parasite of the ruminant abomasum. Blood feeding by the worms cause anemia, hypoproteinemia, and subsequent edema due to loss of vascular oncotic pressure. Alarmingly, multidrug resistant isolates of H. contortus are common1,2. The failure of anthelmintics has prompted a call for alternative methods of control such as vaccination.
Animals typically develop an immune response against H. contortus that decreases the number of mature worms but does not eliminate them entirely. This both mitigates the consequences of the primary infection and provides protection against subsequent infections with H. contortus3,4. Persistence of relatively low parasite numbers facilitates the phenomenon of premunition, wherein a chronic low parasite load provides immune protection against subsequent infection by the same or similar parasite, often referred to as concomitant immunity5. Measurable, effective, immunity against H. contortus has led to the discovery and development of vaccination strategies using H. contortus antigens that result in decreased number of parasites, egg shedding, and clinical pathology6. One vaccine strategy is based on parasite intestine “hidden antigens” that are accessed by host antibodies during parasite blood feeding but are not routinely captured and processed by the host immune response7,8,9,10,11,12. These antigens make effective vaccine targets and a commercial vaccine composed of select hidden antigens is available in several parts of the world13. However, since such antigens remain hidden from the immune response, humoral immunity is short-lived, thereby requiring multiple doses of vaccine during a single grazing season14. Therefore, creating a platform for extended vaccine release would maximize vaccine benefits and ideally mimic premunition while minimizing producer inputs.
Polyanhydride(PyAn)-based biomaterials have been studied extensively for their use in vaccines due to their surface erosion which can be tuned by changing polymer composition15,16,17,18,19,20,21,22. PyAn was initially deployed in a nanoparticle format where the particles are functionalized to target certain tissues or elicit immune response using molecular patterns of pathogens17,18,19. More recently, the PyAn has been used in an implantable pellet/tablet that enables researchers to recover the material for research purposes or putatively to remove it from the carcass of a food animal prior to slaughter23,24,25,26.
Using these PyAn biomaterials, we developed a 3-part vaccine strategy for (1) priming, (2) boosting, and (3) immune maintenance. Part 1 is a traditional soluble vaccine to prime the immune response. Part 2 is a depot based on a fully-exposed PyAn polymer rod (free rod) loaded with antigen and adjuvant administered subcutaneously that will degrade over several months for boost effect27,28. Part 3 is a more sophisticated implant designed to maintain the immune response over a period of years and is called the vaccine platform for extended antigen release (VPEAR). VPEAR is a polyethylene implant that contains antigen-loaded PyAn rod (implant rod) that is overlaid by a collagen diffusion barrier. The implant is capped with a cytoexclusive porous PVDF membrane. The VPEAR design limits diffusion of antigen from the device by creating an opportunity for antigen-antibody complexes to form in the collagen diffusion matrix. We hypothesize that antibody levels influence antigen release, and the formation of immune complexes enhances antigen uptake by antigen presenting cells. The VPEAR and its diffusion barrier limit surface erosion, thereby extending antigen release over a period of years27,28. The three parts of this vaccine strategy (soluble, free rod, VPEAR) can be administered together at a single timepoint.
This study was an investigation of our 3-part strategy using PyAn and VPEAR technology applied to vaccination against H. contortus in sheep using a whole-killed H. contortus extract, encompassing the entire repertoire of pathogen antigens. Specifically, we hypothesized that combining traditional soluble vaccine, biodegradable free rod, and VPEAR at a single administration would provide a long-term immune response against H. contortus antigens compared to the same quantity of antigens administered in a soluble preparation.
Materials and methods
Antigens and polymers
Whole adult male and female live H. contortus parasites were obtained opportunistically from naturally infected animals in Iowa, USA and initially frozen in PBS. A whole-killed vaccine was prepared by homogenizing adult worms in a pyrex tissue grinder with cold PBS. The protein concentration of the homogenate was determined by bicinchoninic acid assay and stored at − 20 °C in 1 mL aliquots. This concentration was used to normalize the antigen load throughout the vaccine compounding process which included both soluble and insoluble total whole-killed antigen and referred to as total H. contortus antigen.
Polyanhydride (PyAn) copolymers were based on a molar composition of 20% 1,8-bis(p-carboxyphenoxy)-3,6-dioxaoctane (CPTEG) and 80% 1,6-bis(p-carboxyphenoxy)hexane (CPH) (20:80 CPTEG: CPH). 20:80 CPTEG: CPH copolymers were synthesized using melt polycondensation as described previously16. The number-average molecular weight and molar composition was quantified using1H NMR (DXR 500), specifically by end group analysis. The molecular weights and molar compositions of the various batches of 20:80 CPTEG: CPH copolymers were consistent with previous reports, with molecular weights between 5000 and 10,000 Daltons and molar composition within 5% of the desired 20:80 molar ratio.
Vaccine formulations
The soluble vaccine antigen/adjuvant formulation was made by emulsifying 300 µg of total H. contortus antigen in 0.5 mL phosphate buffered saline (PBS) with 0.5 mL Montanide ISA 61 VG Water and Oil adjuvant (Seppic) designed for long-term immunity with a total soluble vaccine volume of 1 mL/dose. The group receiving only the soluble antigen/adjuvant vaccine (soluble group) were given 900 μg of H. contortus antigen emulsified in 1 mL total volume of Montanide and PBS. This controlled for total antigen load, since the other experimental groups also received 900 μg antigen but distributed into soluble, PyAn, or VPEAR systems described below.
The PyAn (free) rods were formed and pressed as described previously27,28. Each rod consisted of 210 mg PyAn, 300 µg parasite antigen, and 75 mg of DEAE-dextran adjuvant. Parasite antigen, PyAn and adjuvant were solubilized in 1 mL methylene chloride, mixed, air-dried and then pressed into rods under 0.5 tons-on-ram for 5 s.
The VPEAR polyethylene implant was similarly loaded with 140 mg PyAn, 300 µg parasite antigen, and adjuvant. One experimental group adjuvant had 25 mg of DEAE-dextran (DD) while the other experimental group had 500 µg Quil-A, (DQ). Loaded PyAn was placed into the polyethylene implant and overlayed with 100 µl 5% bovine collagen (EZ-gel, Advanced BioMatrix) and cross-linked as described previously27,28. The 0.45 μm porous PVDF membrane cap was adhered with n-butyl cyanoacrylate and stored at 4 °C in PBS prior to administration.
The adjuvant-only group consisted of the soluble vaccine of 0.5 mL PBS emulsified with 0.5 mL Montanide adjuvant, a PyAn (free) rod with DEAE-dextran adjuvant and no parasite antigen, and a VPEAR with DEAE-dextran adjuvant and no parasite antigen. To maximize the numbers of animals per treatment group only the DEAE-dextran (DD) control was chosen for comparison given that it contained the highest amount of adjuvant by weight.
Experimental groups
A summary of animal groups is shown in Table 1. The four experimental groups were (1) “soluble” (900 µg of H. contortus antigen in Montanide ISA 61), (2) “DD” (combination of 300 µg soluble antigen in montanide, a free rod with 300 µg antigen with DEAE-dextran, and a VPEAR implant with 300 µg antigen and with DEAE–dextran), (3) “DQ” combination of 300 µg soluble antigen in montanide, PyAn with 300 µg antigen with DEAE-dextran, and VPEAR with 300 µg antigen with quil A, and (4) “adjuvant only” (same as group 2, but with no H. contortus antigens in any of the 3 vaccine components). In summary, 3 groups all received 900 µg of antigen but distributed in different forms while 1 group received adjuvant only.
Animals
All experiments were conducted in accordance with applicable laws, were in accordance with ARRIVE guidelines, and were approved by the university institutional animal care and use committee under protocol 19–109. Mixed breed white-faced lambs (25–30 kg) were randomly assigned to one of the four treatment groups (n = 6/group). Lambs were sourced from the university sheep teaching farm. Lambs were born in indoor sheds with a dirt floor, and were never allowed to be on pasture. Sheep were maintained under trichostrongyle-free conditions for the entire study which was confirmed by centrifugal fecal flotation at the time of purchase, prior to vaccination, and prior to challenge.
Soluble vaccine was administered in the neck via 18-gauge needle into subcutaneous tissue. Local lidocaine anesthesia was administered and a stab incision facilitated the insertion of a commercial implant needle (Compudose, Elanco) into the neck which was used to administer the free rod and VPEAR. A single interrupted suture was placed to ensure tissue healing. Jugular venipuncture was used to obtain blood for monitoring serum antibodies during the duration of the study. Sheep were vaccinated during a single event at 3 months of age and then maintained under trichostrongyle-free conditions for the entire study period. Animals were not shedding trichostrongyle eggs as determined by centrifugal fecal flotation until the time of challenge with a field isolate of H. contortus.
47 weeks post-vaccination, sheep were challenged with H. contortus. Larva from the same source were stored at 4 °C in PBS and the number of live larvae were determined by microscopy. Animals were challenged with approximately 7500 L3 in a single event using a drenching syringe and blunt feeding needle. While grid counting of larvae is error-prone, volumetric aliquoting was used to ensure equitable distribution among each group of sheep. Severity of infection was assessed by fecal egg counts (McMasters), FAMACHA scoring via an investigator blinded to the treatment groups, and packed cell volume.
Animals were euthanized 50–60 days post-challenge using penetrating captive bolt in accordance with AVMA guidelines. Abomasum, spleen, lymph nodes, implants, and implant-associated lymphoid tissues were obtained for histopathology. The abomasal contents and mucosa were washed and the total number of adult parasites was enumerated. Total abomasal content was weighed and diluted and worms were removed and counted from three aliquots equivalent to 10% of total digesta. In addition, the mucosal surface was washed and all worms were counted. The total digesta count and the mucosa count were combined to achieve a total parasite count. When counting, immature adults or sex of worms was not differentiated.
H. contortus ELISA
Serum antibodies binding H. contortus antigens were measured by indirect ELISA. High-binding ELISA plates (Thermo Scientific) were coated overnight at 4 °C with vaccine antigen in PBS using 0.5 µg antigen/well, followed by washing in 5% non-fat dried milk blocking buffer. Duplicate dilutions of sheep serum were incubated for 1 h at room temperature and washed 3 times in PBS-T. Plates were then incubated with rabbit anti-sheep IgG-HRP (1:10,000) for 1 h in blocking buffer at room temperature. Plates were washed 3 times in PBS-T, developed with 1-Step™ Ultra TMB-ELISA Substrate Solution according to the manufacturer’s instructions, and stopped with 1 N hydrochloric acid. The absorbance of each well was read at 450 nm using a SpectraMax M2 microplate reader. Serum was diluted via 1:2 dilutions from 1:125 to 1:32,000 and the titer was determined as the last dilution with an absorbance greater than the same animal’s pre-bleed serum + 2 standard deviations at the same dilution. In addition, ELISA absorbance at a serum dilution of 1:500 was measured independently of titers.
Immunohistochemistry
Immunolocalization of antibody binding was determined on 5 μm thick paraffin sections of adult worms from the adjuvant group. Paraffin embedded slides were deparaffinized in xylene, and rehydrated with graded ethanol and distilled water. Endogenous peroxidase was blocked with 0.3% hydrogen peroxide, and sections were then washed twice with PBS. Slides were boiled in 0.01 M citrate buffer (pH 9.0) for 2 min, placed in a steamer for 20 min and then kept at room temperature for 30 min. Non-specific binding sites were blocked with 5% non-fat dried milk for 30 min. Sections were incubated overnight with sheep serum pooled from all animals in each experimental group from the final blood sample, diluted 1:500 in blocking buffer, and washed 1× in PBS. Biotinylated rabbit anti-sheep antibody (Vectastain) was placed on the sections at a 1:500 dilution in blocking buffer for 30 min and washed with PBS. Sections were incubated with Vectastain® ABC Reagent diluted in 10 mL of PBS for 30 min and washed 3× with PBS. A compatible NovaRED® peroxidase substrate was added for 10 min and sections were washed in deionized-double distilled water. Slides were counterstained with hematoxylin, dehydrated through graded ethanol with a final wash in xylene, and mounted.
Statistical analysis
Statistical analysis was performed using Graphpad Prism or JMP. For each time point Dunnett’s multiple comparison was used to determine if the means of treatment groups were significantly different (P ≤ 0.05) from the values of the adjuvant-only group control (JMP Pro 14). Titer values were log2 transformed for the analysis.
Results
Long-term implantation of VPEAR in sheep
Over a 3-week post-vaccination period, 2 animals in the DD group developed mild front leg lameness with no visible or palpable signs of swelling or abscessation. NSAIDs (flunixin meglumine, meloxicam) were administered at 2 weeks post-implantation and the lameness resolved without further incident. In contrast, all animals in the adjuvant-only group developed abscesses. The abscesses varied in size and resolved over time with or without ceftiofur treatment. Other than the adjuvant-only group, the 3-component vaccine approach with the subcutaneous VPEAR implants containing H. contortus antigen were well-tolerated over the duration of the study.
Immune response to parasite antigens
Serum was obtained pre-vaccination, 4 weeks and 16 weeks post-vaccination, prior to challenge (up to 47 weeks post-vaccination) and at necropsy (8 weeks post-challenge). ELISA assay at 1:500 serum dilution demonstrated that H. contortus specific antibodies were increased in the DD and DQ groups compared to the adjuvant only control group at 4 weeks post-vaccination (p < 0.05, Fig. 1). Only the DD group maintained higher absorbance values at 16 weeks post-vaccination and at necropsy (p < 0.05, Fig. 1). In terms of titers, all vaccine groups had significantly increased titers compared to adjuvant controls (Fig. 2) at 4 weeks post-vaccination. At 16 weeks the DD group remained significantly higher than the adjuvant control but at pre-challenge (37 weeks post-vaccination) one animal was a non-responder and this remained consistent at the time of necropsy. Overall, vaccinated animals had higher titers than the adjuvant control group, as did the soluble only vaccine group, but these differences were not statistically significant due to non-responders (Fig. 2).
Antigen-specific antibodies in sheep vaccinated with VPEAR following H. contortus challenge. Values represent absorbance for indirect ELISA measured in serum diluted 1:500 of the 4 treatment groups at indicated time points post-vaccination. Dots indicate individual animals with bars indicating the group mean ± SEM. Asterisks indicate significant differences compared to the adjuvant only group (Dunnett’s multiple comparison) for each given time point, p < 0.05.
Endpoint serum titers for H. contortus specific antibodies of the 4 treatment groups at indicated time points post-vaccination. Dots represent individual animals and bars represent geometric means for each group. Asterisks indicate significant differences compared to the adjuvant only group (Dunnett’s multiple comparison after log2 transformation) for each given time point, p < 0.05.
Impact of vaccination on clinical signs following challenge
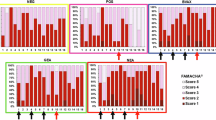
The impact of vaccination on clinical haemonchosis was measured following infectious challenge using PCV, FAMACHA, and fecal egg counts. The DQ group had a higher PCV (Fig. 3, p < 0.05), indicating some relief from the anemia typically observed with H. contortus infection. However, no group had a mean PCV of less than 27 following infection. FAMACHA scores were similar in all groups, and are graphically represented in Fig. 4. Means are not calculated for FAMACHA values as they are more accurately treated as non-linear categorical values29. Fecal egg counts appeared higher for the adjuvant group compared to all vaccine groups (Fig. 5) although this was not statistically significant.
Qualitative distribution of FAMACHA scores following challenge with H. contortus. Each chart represents a vaccine group (adjuvant, DD, DQ, soluble). Numbers on the left-hand side of the charts indicate number of days post infection. Each colored square represents an individual sheep bearing a FAMACHA score of 1–4. Note that in all groups the distributions shift to the right over time, indicating increased pallor in the conjunctival membrane.
Vaccinated animals have fewer adult parasites post-challenge
At 8 weeks post-challenge, the animals were necropsied and total worm burden was determined. Although the mean total worm burdens of all the vaccinated groups were lower than the adjuvant only control group, only the DD group had worm counts that were statistically different from the adjuvant only group, with a mean of 475 vs. 1752 worms, respectively (p < 0.02, Fig. 6).
Antibodies from vaccinated animals bind nematode antigens
As the DD vaccination decreased parasite load we hypothesized that antibodies generated by this vaccination would bind cells of the intestinal lumen of the parasite. Sections of adult H. contortus were immunohistochemically probed with pooled serum collected from sheep prior to vaccination and at necropsy. Figure 7 depicts representative images from the DD group and the adjuvant only treatment groups. The DD group demonstrated parasite-specific antibodies following VPEAR vaccination that bound the body wall, coelomic surfaces of intestine and reproductive organs as well as the luminal surface of intestinal cells. This was consistent with the fact that our antigen was a crude whole worm homogenate.
Immunohistochemistry of adult H. contortus probed using pooled sheep sera (1:500 dilution). Individual slides were probed with sera from unvaccinated animals (Pre-vaccination) or sera obtained at the time of necropsy. Positive peroxidase staining is indicated in red. Parasite-specific antibodies bound throughout the parasite including in the parasite intestine (arrowhead) and body wall (arrow) for both VPEAR and soluble vaccine preparations while minimal antibody binding is seen in the adjuvant-only group (Magnification = 100×).
Discussion
Vaccination against H. contortus has been successful with irradiated larvae30,31 and a variety of preparations from parasite collagens, excretory/secretory products32,33, and more refined antigens from the parasite intestine such as contortin34, and its components H-11/H-gal-GP antigens35,36,37, culminating in a commercial product6. However, the hidden antigens of H. contortus intestine do not naturally boost immunity post-vaccination and continued vaccinations are required. Therefore, in the commercial product, sheep receive 3 priming doses followed by a booster dose every 4–6 weeks. In addition, the production of these specific antigens is laborious, requiring biochemical separation of the antigens from adult worms. Thus, the aim of our study was to determine if extended release of soluble and insoluble whole-killed H. contortus, encompassing the entire spectrum of adult worm antigens, could mimic premunition and provide protection against parasite challenge thereby developing a method for delivering H. contortus antigens and optimize a long-term immune response.
Protection from new infection afforded by an ongoing low-level infection with the same or similar pathogen is known as premunition or concomitant immunity and is associated with several protozoan and metazoan parasites including H. contortus5,38. This phenomenon indicates that the host immune response can be manipulated to provide effective protection against helminths and other pathogens. Although the immune mechanisms of premunition are not completely defined and presumably differ with different pathogens the phenomenon of protective immunity being maintained by persistent antigen (infection) is a common theme. We propose that techniques leading to vaccine-induced premunition will advance the development of vaccines targeting parasitic diseases. The vaccination strategy employed in this study was designed to recapitulate premunition by initiating, boosting and maintaining immunity to vaccine antigens while minimizing tolerance to chronic antigen exposure.
Antigens from adult H. contortus are known to provide protective immunity by inducing an antibody response7,39,40. In our study, VPEAR was effective at inducing an antibody response following a single vaccination event. At nearly 9 months post-vaccination animals in the DD group had higher absorbance values and higher titers than those in the adjuvant only group, although a non-responder prevented statistical significance. Parasite-specific antibodies in the DD group at 1:500 dilution did significantly increase after challenge compared to the adjuvant only group, indicating stimulation of antigen-specific immunity instigated by our vaccine platform.
Clinical signs associated with H. contortus infection are primarily associated with anemia which can be reflected in FAMACHA scores and quantitated by PCV. FAMACHA scoring did not identify any differences among groups, but scores tended to increase with time following challenge infection. DQ had higher PCV values, however, no group had particularly low PCV following challenge. Overall, we attributed the lack of clinical disease in these sheep partially due to a high quality of nutrition and husbandry maintained during the study, and possibly the genetic background of the sheep.
Parasite burdens were reduced by vaccination. All vaccine groups had lower fecal egg counts compared to adjuvant controls. However, this was not statistically significant due to wide variation of egg shedding among individuals which is typical of helminth infections in livestock. If egg counts from all time points are aggregated to enumerate the total reduction in egg counts, the DQ group had a statistically significant reduction of eggs (data not shown). There was an apparent discrepancy between a high number of worms and relatively low egg counts; it is possible that vaccination had an inhibitory effect on egg release since this has been seen with other H. contortus vaccines9,10,11,12. Differences in the adult worm burden were more dramatic with a significant reduction in the numbers of adult worms observed in the DD group compared to the adjuvant only group. In addition, several individuals in the DQ group had very low counts compared to both the soluble only or adjuvant only vaccine groups.
Parasite gut membrane proteins have been developed as vaccine antigens and have been an emphasis of H. contortus hidden antigen vaccine strategies6,14. The protection afforded by the VPEAR approach demonstrated characteristic immunoreactivity of the vaccinate serum to the luminal surface of the parasite intestine, which is consistent with this expected mechanism of protection. The antibodies also bound a variety of other parasite antigens, which is expected due to the nature of the immunogen, which was a homogenate of H. contortus adults.
There are limitations to the present study which we aim to address in future studies. Importantly, the VPEAR approach should be deployed with more refined antigens, such as those in commercial preparations41. In our study, we used whole killed adult parasites as the immunogen which led us to have a relatively high amount of protein present in each vaccine dose. Our rationale was that supplying this amount of total lysate was likely to deliver micrograms levels of H. contortus gut antigen that is known to be protective.
A long-term vaccine implant approach will balance maintaining dynamic antigen release to generate an effective immune response while minimizing tissue reactions or biofouling of the implant. Thus, we hypothesized that adjuvant choice would have a significant impact on the response to vaccination with this vaccine strategy. Both DEAE-dextran and Quil A are commonly used individually or in combination in ruminant vaccines both experimentally and commercially6. Both adjuvants performed well in this study, but were not optimized. The vaccine platform will have to balance reactogenicity and antibody responses in the short-term and long-term with a minimal tissue response. Fine tuning of polymers and adjuvants is likely to further improve the level of inflammation needed to maintain premunition. The amount of inflammation-promoting adjuvant should be reduced over time as the memory B cells require less stimulation to become activated. Every animal in the adjuvant only group developed an abscess. These results suggest that the balance between the vaccines inflammatory signals and antigen load is important. In the adjuvant only group an absence of lymphocytes responding to vaccine antigen could restrict a required antigen-specific T and/or B cell regulatory response that would normally modulate adjuvant-induced inflammation often defined by antigen-specific production of IL-10 or TGF-beta. Other than the adjuvant group, only two individuals in vaccine groups required minor treatment to improve comfort following vaccine-associated pain. Thus, overall, the VPEAR with antigen is relatively well-tolerated. The majority of implants could be recovered at necropsy with only a small amount of granulation tissue except for the adjuvant group that had more dramatic granulomatous and purulent tissue reaction. In an animal production system optimal dosing to maximize protection with an acceptable amount of inflammation and appropriate tissue site for implantation will need to be explored, such as the base of the ear.
The aim of our VPEAR vaccination strategy is to mimic premunition and generate a long-lived antibody response following a single vaccination event. Our results demonstrate that bioerodible vaccine depots and VPEAR can be developed for protection against H. contortus. We predict this strategy will be useful in developing vaccination strategies for numerous other parasites that demonstrate the phenomenon of concomitant immunity. The customized immune response needed for vaccine-induced concomitant immunity would involve tempering innate inflammation through a T and/or B cell immunoregulatory response although this aspect of the long-term vaccine platform was not explored in this study. Further investigation of refined antigens and materials that instigate specific immune cells and effectors at the implant site will enhance and prolong anti-H. contortus immunity.
Data availability
All data is included in the manuscript.
References
Whittaker, J. H., Carlson, S. A., Jones, D. E. & Brewer, M. T. Molecular mechanisms for anthelmintic resistance in strongyle nematode parasites of veterinary importance. J. Vet. Pharmacol. Ther. 40 (2), 105–115. https://doi.org/10.1111/jvp.12330 (2017).
Kotze, A. C. & Prichard, R. K. Anthelmintic resistance in Haemonchus contortus: History, mechanisms and diagnosis. Adv. Parasitol. 93, 397–428. https://doi.org/10.1016/bs.apar.2016.02.012 (2016).
Barger, I. A. Resistance of young lambs to Haemonchus contortus infection, and its loss following anthelmintic treatment. Int. J. Parasitol. 18 (8), 1107–1109. https://doi.org/10.1016/0020-7519(88)90082-3 (1988).
Donald, A. D., Dineen, J. K. & Adams, D. B. The dynamics of the host-parasite relationship. VII. The effect of discontinuity of infection onesistance to Haemonchus contortus in sheep. Parasitology 59 (3), 497–503 (1969).
Brown, S. P. & Grenfell, B. T. An unlikely partnership: Parasites, concomitant immunity and host defence. Proc. Biol. Sci. 268 (1485), 2543–2549. https://doi.org/10.1098/rspb.2001.1821 (2001).
Bassetto, C. C. & Amarante, A. F. Vaccination of sheep and cattle against haemonchosis. J. Helminthol. 89 (5), 517–525. https://doi.org/10.1017/S0022149X15000279 (2015).
Smith, W. D. & Smith, S. K. Evaluation of aspects of the protection afforded to sheep immunised with a gut membrane protein of Haemonchus contortus. Res. Vet. Sci. 55 (1), 1–9. https://doi.org/10.1016/0034-5288(93)90025-b (1993).
Smith, W. D., Smith, S. K. & Murray, J. M. Protection studies with integral membrane fractions of Haemonchus contortus. Parasite Immunol. 16 (5), 231–241. https://doi.org/10.1111/j.1365-3024.1994.tb00345.x (1994).
Benavides, M. V., Souza, C. J. H., Smith, W. D. & Moraes, J. C. F. Evaluation of protection in grazing lambs immunised with different doses of Haemonchus contortus gut membrane glycoproteins in Southern Brazil. Vet. Parasitol. 290, 109360. https://doi.org/10.1016/j.vetpar.2021.109360 (2021).
Jasmer, D. P., Perryman, L. E., Conder, G. A., Crow, S. & McGuire, T. Protective immunity to Haemonchus contortus induced by immunoaffinity isolated antigens that share a phylogenetically conserved carbohydrate gut surface epitope. J. Immunol. 151 (10), 5450–5460 (1993).
Piedrafita, D. P. et al. Field vaccination of sheep with a larval-specific antigen of the Gastrointestinal nematode, Haemonchus contortus, confers significant protection against an experimental challenge infection. Vaccine 30 (50), 7199–7204. https://doi.org/10.1016/j.vaccine.2012.10.019 (2012).
Smith, W. D. & Zarlenga, D. S. Developments and hurdles in generating vaccines for controlling helminth parasites of grazing ruminants. Vet. Parasitol. 139 (4), 347–359. https://doi.org/10.1016/j.vetpar.2006.04.024 (2006).
Bassetto, C. C. et al. Trials with the Haemonchus vaccine, Barbervax((R)), in Ewes and lambs in a tropical environment: Nutrient supplementation improves protection in periparturient Ewes. Vet. Parasitol. 264, 52–57. https://doi.org/10.1016/j.vetpar.2018.11.006 (2018).
Nisbet, A. J., Meeusen, E. N., Gonzalez, J. F. & Piedrafita, D. M. Immunity to Haemonchus contortus and vaccine development. Adv. Parasitol. 93, 353–396. https://doi.org/10.1016/bs.apar.2016.02.011 (2016).
Huntimer, L. et al. Evaluation of biocompatibility and administration site reactogenicity of polyanhydride-particle-based platform for vaccine delivery. Adv. Healthc. Mater. 2 (2), 369–378. https://doi.org/10.1002/adhm.201200181 (2013).
Torres, M. P., Vogel, B. M., Narasimhan, B. & Mallapragada, S. K. Synthesis and characterization of novel polyanhydrides with tailored erosion mechanisms. J. Biomed. Mater. Res. A. 76 (1), 102–110. https://doi.org/10.1002/jbm.a.30510 (2006).
Haughney, S. L., Ross, K. A., Boggiatto, P. M., Wannemuehler, M. J. & Narasimhan, B. Effect of nanovaccine chemistry on humoral immune response kinetics and maturation. Nanoscale 6 (22), 13770–13778. https://doi.org/10.1039/c4nr03724c (2014).
Huntimer, L. et al. Single immunization with a suboptimal antigen dose encapsulated into polyanhydride microparticles promotes high titer and avid antibody responses. J. Biomed. Mater. Res. B. 101b (1), 91–98. https://doi.org/10.1002/jbm.b.32820 (2013).
Phanse, Y. et al. Functionalization of polyanhydride microparticles with di-mannose influences uptake by and intracellular fate within dendritic cells. Acta Biomater. 9 (11), 8902–8909. https://doi.org/10.1016/j.actbio.2013.06.024 (2013).
Maina, T. W. et al. Immunization with a mucosal, post-fusion F/G protein-based polyanhydride nanovaccine protects neonatal calves against BRSV infection. Front. Immunol. 14, 1186184. https://doi.org/10.3389/fimmu.2023.1186184 (2023).
Lopez, C. E. et al. Polyanhydride nanovaccine against H3N2 influenza A virus generates mucosal resident and systemic immunity promoting protection. NPJ Vaccines. 9 (1), 96. https://doi.org/10.1038/s41541-024-00883-3 (2024).
Grego, E., Kelly, S. M., McGill, J. L., Wannemuehler, M. & Narasimhan, B. Bovine respiratory syncytial virus nanovaccine induces long-lasting humoral immunity in mice. ACS Pharmacol. Transl Sci. 7 (10), 3205–3215. https://doi.org/10.1021/acsptsci.4c00375 (2024).
Boggiatto, P. M. et al. Sustained antigen release polyanhydride-based vaccine platform for immunization against bovine brucellosis. Heliyon 5 (8), e02370. https://doi.org/10.1016/j.heliyon.2019.e02370 (2019).
Curtis, A. K. et al. Delivering an immunocastration vaccine via a novel subcutaneous implant. Animals (Basel) 12 (19). https://doi.org/10.3390/ani12192698 (2022).
Curtis, A. K. et al. Development of a subcutaneous ear implant to deliver an anaplasmosis vaccine to dairy steers. J. Anim. Sci. 98 (6). https://doi.org/10.1093/jas/skz392 (2020).
Wilson-Welder, J. H. et al. Bovine immune response to leptospira antigen in different novel adjuvants and vaccine delivery platforms. Vaccine 38 (18), 3464–3473. https://doi.org/10.1016/j.vaccine.2020.02.086 (2020).
Schaut, R. G. et al. A single dose polyanhydride-based vaccine platform promotes and maintains anti-GnRH antibody titers. Vaccine 36 (7), 1016–1023. https://doi.org/10.1016/j.vaccine.2017.12.050 (2018).
Schaut, R. G. et al. A polyanhydride-based implantable single dose vaccine platform for long-term immunity. Vaccine 36 (7), 1024–1025. https://doi.org/10.1016/j.vaccine.2017.11.067 (2018).
Mahieu, M. Famacha(c) scores should not be handled as numerical data. Vet. Parasitol. 247, 7–9. https://doi.org/10.1016/j.vetpar.2017.09.014 (2017).
Smith, W. D. & Christie, M. G. Haemonchus-contortus—Local and serum antibodies in sheep immunized with irradiated larvae. Int. J. Parasitol. 8 (3), 219–223. https://doi.org/10.1016/0020-7519(78)90082-6 (1978).
Smith, W. D. & Angus, K. W. Haemonchus-contortus—Attempts to immunize lambs with irradiated larvae. Res. Vet. Sci. 29 (1), 45–50. https://doi.org/10.1016/S0034-5288(18)32684-5 (1980).
Vervelde, L. et al. Vaccination-induced protection of lambs against the parasitic nematode Haemonchus contortus correlates with high IgG antibody responses to the LDNF glycan antigen. Glycobiology 13 (11), 795–804. https://doi.org/10.1093/glycob/cwg107 (2003).
Bakker, N. et al. Vaccination against the nematode with a thiol-binding fraction from the excretory/secretory products (ES). Vaccine 22 (5–6), 618–628. https://doi.org/10.1016/j.vaccine.2003.08.025 (2004).
Munn, E. A. A helical, polymeric extracellular protein associated with the luminal surface of Haemonchus contortus intestinal cells. Tissue Cell. 9 (1), 23–34. https://doi.org/10.1016/0040-8166(77)90046-5 (1977).
Smith, S. K. & Smith, W. D. Immunisation of sheep with an integral membrane glycoprotein complex of Haemonchus contortus and with its major polypeptide components. Res. Vet. Sci. 60 (1), 1–6. https://doi.org/10.1016/S0034-5288(96)90121-6 (1996).
Nisbet, A. J., Meeusen, E. N., González, J. F. & Piedrafita, D. M. Immunity to and vaccine development. Haemonchus contortus and haemonchosis—Past, present and future trends. Adv. Parasitol. 93, 353–396. https://doi.org/10.1016/bs.apar.2016.02.011 (2016).
Roberts, B. et al. Novel expression of vaccine candidate aminopeptidase H11 using the free-living nematode. Vet. Res. 44. https://doi.org/10.1186/1297-9716-44-111 (2013).
King, I. L. & Li, Y. Host-parasite interactions promote disease tolerance to intestinal helminth infection. Front. Immunol. 9, 2128. https://doi.org/10.3389/fimmu.2018.02128 (2018).
Jasmer, D. P. & McGuire, T. C. Protective immunity to a blood-feeding nematode (Haemonchus contortus) induced by parasite gut antigens. Infect. Immun. 59 (12), 4412–4417 (1991).
Smith, W. D., Pettit, D. & Smith, S. K. Cross-protection studies with gut membrane glycoprotein antigens from Haemonchus contortus and Teladorsagia circumcincta. Parasite Immunol. 23 (4), 203–211. https://doi.org/10.1046/j.1365-3024.2001.00375.x (2001).
Kebeta, M. M., Hine, B. C., Walkden-Brown, S. W., Kahn, L. P. & Doyle, E. K. Evaluation of Barbervax(R) vaccination for lambing Merino Ewes. Vet. Parasitol. 283, 109187. https://doi.org/10.1016/j.vetpar.2020.109187 (2020).
Funding
This work was supported by USDA-NIFA 2017-04619 provided to MTB and USDA/APHIS cooperative agreement AP17VSSPRS00G0022 provided to RWG.
Author information
Authors and Affiliations
Contributions
MTB and DEJ conceived the experiments, designed, and conducted the study. MM, AC, JJC, KAM, KCV, RWG, and DEJ conducted experiments and collected data. RWG and MTB obtained funding. MTB and DEJ drafted the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Brewer, M.T., Mertens, M., Colina-Iturralde, A. et al. Implantation of a vaccine platform for extended antigen release (VPEAR) induces long-term immunity against Haemonchus contortus in sheep. Sci Rep 15, 12168 (2025). https://doi.org/10.1038/s41598-025-95929-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95929-4