Abstract
This study aims to deliver a systematic review and meta-analysis to scrutinize the tolerability of different doses of oliceridine in acute pain patients. A comprehensive search was carried out in essential databases (PubMed, Embase, Cochrane Library, and Web of Science) for relevant studies up to the most recent available date.We included Randomized Controlled Trials (RCTs) that compared oliceridine with other interventions in acute pain management. Patients received an equi-analgesic dose of oliceridine relative to morphine, ensuring comparable Sum of Pain Intensity Differences (SPID-48 or SPID-24) or mean pain score reductions across groups. The initial loading dose was 1.5 mg for oliceridine and 4 mg for morphine, followed by demand doses via patient-controlled analgesia (PCA). For oliceridine, the demand doses were 0.1, 0.35, or 0.5 mg, while for morphine, it was 1 mg.Utilizing the Review Manager 5.4, data on nausea, vomiting, sedation, dizziness, pruritus, and hypoxemia were assembled and evaluated.We conducted sensitivity analyses to confirm the robustness of our findings. The preliminary search discovered 710 potential studies. Having gone through a careful screening process, a total of 7 RCTs met our inclusion benchmarks. Five distinct publications analyzed postoperative nausea and vomiting (PONV). According to our meta-analysis findings, patients assigned to the oliceridine group experienced a notably lower rate of postoperative nausea (PON) and postoperative vomiting (POV) compared to the morphine group (PON: RR = 0.55, 95% CI 0.41–0.74, P < 0.001; POV: RR = 0.36, 95% CI 0.28–0.47, P < 0.001). Data from 4 documents examined sedation and dizziness. Our findings demonstrate that oliceridine recipients had a significant decline in the incidence of both sedation and dizziness (sedation: RR = 0.64, 95% CI 0.45–0.91, P = 0.01; dizziness: RR = 0.71, 95% CI 0.57–0.88, P = 0.002).Moreover, the oliceridine group recorded a lower incident of hypoxemia showcasing a favorable safety profile (RR = 0.52, 95% CI 0.41–0.65, P < 0.001).Sensitivity analyses confirmed the robustness of these findings. The systematic review and meta-analysis reveal that oliceridine is a well-tolerated and safe intravenous analgesic for acute pain patients, often reducing the incidence of adverse events in comparison to morphine.
Similar content being viewed by others
Introduction
The management of acute pain is a crucial facet in patient care, underlining the ongoing need for effective and safe analgesics1. In particular, postoperative contexts demand medical practitioners to constantly explore treatments that can offer optimal pain alleviation, simultaneously reducing potential side effects2,3. Morphine—a widely utilized mu-opioid receptor agonist— often acts as the first line of treatment. Regardless of its powerful analgesic properties, the drug is notorious for fostering a host of adverse effects including nausea, vomiting, sedation, dizziness, pruritus, and hypoxemia4,5. Due to these drawbacks, researchers seek alternative analgesics with better safety and tolerability 6,7.
A promising contender is oliceridine, a peculiar G-protein path-selective mu-opioid receptor agonist, which is being hailed as a potential alternative8. Oliceridine is a novel opioid developed for the management of moderate to severe acute pain. It’s a biased agonist, meaning it’s designed to selectively activate certain intracellular signals of the opioid receptor to produce analgesic effects, while avoiding the activation of those signals responsible for adverse reactions and side effects9. Despite these promising advancements and potential benefits of oliceridine, robust, reliable, and comparative data outcomes are required to substantiate its clinical utility10.
Several studies have investigated the safety and effectiveness of oliceridine, but the findings remain inconsistent11,12.Some studies laud oliceridine for its significant pain relief attributes , while others highlight the emergence of adverse effects such as nausea, vomiting, sedation, dizziness, pruritus, and hypoxemia13,14.This divergence in the literature underscores the imperative nature of conducting a comprehensive systematic review and meta-analysis to address the ongoing debate concerning the efficacy and safety of oliceridine.
To ensure comparability across studies, we included only those RCTs where oliceridine was administered at equi-analgesic doses relative to morphine, ensuring equivalent Sum of Pain Intensity Differences (SPID-48 or SPID-24) or mean pain score reductions. This approach allows for a fair comparison of the analgesic efficacy and side effect profile between oliceridine and morphine.
This study aims to evaluate the safety and tolerability of oliceridine in the management of acute pain. To achieve this, we conducted a systematic review and meta-analysis of available Randomized Controlled Trials (RCTs) concerning the safety and tolerability of oliceridine. Our meta-analysis pivots its focus on a host of specific adverse effects including nausea, vomiting, sedation, dizziness, pruritus, and hypoxemia15. It is our hope that by amalgamating the available evidence, we can offer a clearer perspective on the safety and tolerability profile of oliceridine, potentially paving a fresh course for acute pain management.
Methods
Search strategy and selection criteria
Our research methodology entailed a systematic exploration of four pertinent databases, namely: PubMed, Embase, Cochrane Library and Web of Science. We aimed to uncover randomized controlled trials (RCTs) that evaluated the tolerability and safety of oliceridine in acute pain management.Our search strategy incorporated the following terms:Subject headings (MeSH and Emtree): “Oliceridine”, “Tolerability”, “Safety”, “Randomized Controlled Trial”, “Acute Pain”, “Nausea”, “Vomiting”, “Sedation”, “Dizziness”, “Pruritus”, “Hypoxemia”. Free-text terms: “oliceridine OR TRV130 OR oliceridine trihydrochloride OR Olinvyk”, “tolerable OR tolerability”, “safe OR safety”, “RCT OR randomized controlled trial”, “acute pain OR postoperative pain”, “nausea OR emesis”, “vomiting”, “sedation”, “dizziness”, “pruritus”, “hypoxemia OR hypoxia”.
Our search parameters were unrestricted by language or the year of publication.
The inclusion criteria
We included randomized controlled trials (RCTs) that made a comparative study of the efficacy of oliceridine and morphine in the management of acute pain. Further, the control group must have received morphine as the standard treatment for acute pain management. Studies had to provide data on potential side effects including nausea, vomiting, sedation, dizziness, pruritus, or hypoxemia.
Definitions of Side Effects:
Nausea: A subjective feeling of unease and discomfort localized in the upper abdomen, often accompanied by an involuntary urge to vomit.
Vomiting: The forceful expulsion of stomach contents through the mouth.
Sedation: A state of reduced alertness or drowsiness, which may impair cognitive function and motor skills.
Dizziness: A sensation of unsteadiness or lightheadedness, which may be associated with vertigo or imbalance.
Pruritus: An uncomfortable sensation leading to the desire to scratch, often due to irritation or inflammation of the skin.
Hypoxemia: A condition where there is an abnormally low level of oxygen in the blood, particularly in the arteries.
We also limited our focus to studies conducted on adult human subjects (18 years and above). Special populations, such as elderly patients and those with comorbidities, were included if the studies provided separate data for these subgroups.Each study must have ensured that comparisons between oliceridine and morphine were made under conditions where the Sum of Pain Intensity Differences (SPID-48 or SPID-24) or mean pain score reductions were equivalent.
Exclusion criteria
Exclusion criteria saw the removal of case reports, observational studies, reviews, animal studies, or investigations involving pediatric or adolescent subjects from our consideration. Mixed-age studies were excluded unless they provided separate data for adults (18 years and older). Studies with multiple research designs were included only if they contained a randomized controlled trial component.Studies not specifying the equi-analgesic dosing regimen for oliceridine relative to morphine were also excluded.
Study selection and data collection process
The data curation process was carried out by two researchers who worked independently, sifting through search results, reviewing titles, abstracts, and disregarding duplicates. The two reviewers independently screened the titles and abstracts, and any discrepancies were resolved by consensus.In cases where consensus could not be reached, a third researcher was consulted to mediate and resolve the disagreement. Following an in-depth review of full-text potential studies, we compiled data utilizing a standardized data extraction format. This encompassed author details, year of publication, study design, sample size, patient characteristics, details regarding the dosage of oliceridine and morphine administered (including the initial loading dose and subsequent demand doses via PCA), duration of treatment, and outcomes central to tolerability and safety, specifically addressing side effects like nausea, vomiting, sedation, dizziness, pruritus, and hypoxemia. Additionally, we performed a methodological quality assessment of the included studies using the Cochrane Risk of Bias tool to evaluate the risk of bias in each study.For each study, we extracted information on the specific equi-analgesic dose of oliceridine used relative to morphine and whether the morphine dose varied across studies.
Risk of bias assessment
Two researchers independently evaluated potential bias risks within the RCTs by employing the Cochrane Collaboration’s tool. To ensure consistency in the risk of bias assessment, we calculated the inter-rater reliability using Cohen’s Kappa coefficient, which indicated substantial agreement between the two researchers. Disagreements in the assessment were resolved through discussion until consensus was reached, and when necessary, a third researcher was consulted to mediate and resolve any remaining disagreements.We scrutinized diverse bias domains including selection bias, performance bias, detection bias, attrition bias, as well as reporting bias and other possible sources of bias. Each bias domain was subsequently categorized as either being of high, unclear, or low bias.
Statistical analysis
Quantitative analysis of the data was carried out utilizing the Review Manager 5.4, URL link: https://community.cochrane.org/tools/review-production-tools/revman-5. Relative risk (RR) assessment alongside 95% confidence intervals (CI) was employed to investigate dichotomous data. We determined potential heterogeneity in the studies by quantifying the with the I2 statistic. Should the I2 statistic exceed 50%, we construed this as substantial heterogeneity. In such instances, we applied a random-effects model, or else, we utilized a fixed-effects model. To evaluate the robustness of our findings, a sensitivity analysis was performed using the leave-one-out method in RevMan software. This involved sequentially excluding each study to assess its impact on the overall results.To address the issue of publication bias, we assessed it using funnel plots . Statistical significance was attributed to a p-value of less than 0.05.
This work has been reported in line with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)28 and AMSTAR (Assessing the methodological quality of systematic reviews) Guidelines29. The methods have been pre-registered on Prospero, and the study was registered on May 30, 2024.
Ethical statement
This study is a meta-analysis and did not involve human or animal experiments; therefore, it did not require ethical approval.
Results
Literature screening
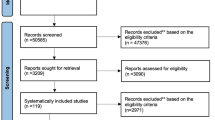
Initially, a total of 710 studies were identified from PubMed, Embase, Cochrane Library and Web of Science databases. After the removal of duplicates, 231 records underwent title and abstract screening according to the predefined eligibility criteria, which resulted in 179 studies being potentially eligible. The two reviewers independently screened the titles and abstracts, and any discrepancies were resolved by consensus.Subsequent full-text review narrowed the final inclusion to 7 RCTs for this meta-analysis (Fig. 1).
Basic characteristics of included studies
The final ensemble of 7 RCTs involved a total of 2104 patients, wherein 1508 and 596 patients received oliceridine and morphine, respectively. The oliceridine dosage varied from 0.1 mg to 0.35 mg to 0.5 mg, and all patients received an initial loading dose of 1.5 mg oliceridine or 4 mg morphine, followed by demand doses via PCA. Morphine was the comparison group in all studies. The basic characteristics of the included RCTs are depicted in Table 1.
Quality evaluation of included studies
Upon risk of biases assessment via the Cochrane Collaboration’s tool, the quality of the included randomized controlled trials was deemed medium to high as they generally adhered to the classified randomization process, allocation concealment, and blinding methods. A detailed graphic and narrative representation of the risk of bias assessment is provided (Fig. 2).
Results of meta analysis
Analysis of postoperative nausea
Postoperative nausea was the topic of discussion in 6 distinct studies. The nausea in comparison between oliceridine and morphine demonstrated significant variability within the study (P < 0.001, I2 = 67%), necessitating the implementation of the random effects model for the statistical analysis. Meta-analysis revealed a noticeably reduced incidence of nausea in patients administered oliceridine as opposed to those given morphine (RR = 0.55, 95%CI [0.41–0.74], P < 0.001). To assess the robustness of this finding, we conducted a sensitivity analysis by sequentially removing each study and recalculating the pooled estimate. The results remained consistent, indicating that the overall conclusion is robust. We executed a subgroup analysis on the basis of oliceridine dosage in contrast with morphine: 0.1 mg group (RR = 0.34, 95%CI [0.19,0.61], P = 0.001, I2 = 76%); 0.35 mg group (RR = 0.55, 95%CI [0.38,0.80], P = 0.002, I2 = 43%); 0.5 mg group (RR = 0.95, 95%CI [0.71,1.26], P = 0.71, I2 = 0%)(Fig. 3).
Vomiting comparison: oliceridine versus morphine
Similarly, a noteworthy reduction in postoperative vomiting was observed in the oliceridine group compared to the morphine group (RR = 0.36, 95% CI [0.28–0.47], P < 0.001). The comprehensive study exhibited homogeneity (P = 0.002, I2 = 56%). A sensitivity analysis confirmed the robustness of this result, with exclusion of any single study not altering the direction or magnitude of the effect.This comparison was based on 6 studies. When compared to morphine, significant differences were noted in the oliceridine 0.1 mg group (RR = 0.22, 95% CI [0.16–0.30], P < 0.001, I2 = 0%), 0.35 mg group (RR = 0.37, 95% CI [0.25–0.55], P < 0.001, I2 = 48%), and 0.5 mg group (RR = 0.61, 95% CI [0.46–0.81], P = 0.001, I2 = 0%) (Fig. 4).
Sedation comparison: oliceridine versus morphine
The frequency of postoperative sedation was evaluated collectively in 5 different literature sources. Our meta-analysis did not identify any clinically noteworthy differences in the occurrence of postoperative sedation among patients in the 0.35 mg and 0.5 mg oliceridine groups compared to the morphine group. The amalgamated studies offered statistically significant evidence (RR = 0.64, 95% CI [0.45–0.91], P = 0.01) which demonstrated noteworthy heterogeneity (P = 0.01, I2 = 52%) . Sensitivity analysis did not reveal any significant influence of individual studies on the overall effect size, suggesting the results are stable (Fig. 5).
Dizziness comparison: oliceridine versus morphine
The incidence of dizziness, as studied across 5 research pieces, was compared between oliceridine and morphine-treated groups. The findings underlined insignificant heterogeneity, as suggested by the Cochrane standard test (P = 0.35, I2 = 9%), thereby prompting the use of the fixed-effect model for statistical analysis. Compared to morphine, oliceridine-treated patients exhibited a notably lower occurrence of dizziness (RR = 0.71, 95% CI [0.57–0.88], P = 0.002).Sensitivity analysis confirmed the stability of this finding, with no single study significantly influencing the overall effect size. Subgroup analyses revealed the following: The oliceridine 0.1 mg group notably diverged from the morphine-treated group (RR = 0.60, 95% CI [0.41,0.88], P = 0.009, I2 = 43%). However, the 0.35 mg group (RR = 0.78, 95%CI [0.53,1.12], P = 0.18, I2 = 0%) and 0.5 mg group (RR = 0.76, 95%CI [0.53,1.10], P = 0.15, I2 = 19%) did not (Fig. 6).
Pruritus comparison: oliceridine versus morphine
From the analysis, it is discernible that patients under oliceridine therapy have registered a lower incidence of pruritus, indicating superior tolerance characteristics of oliceridine (RR = 0.38, 95%CI [0.25–0.57], P < 0.001). This comparison was based on 4 studies. Nevertheless, it should be noted that there’s a certain level of heterogeneity in the studies included (P = 0.003; I2 = 60%), hence a random-effects model was employed for statistical analysis.Sensitivity analysis confirmed the robustness of this finding, with the exclusion of any single study not altering the overall effect. In comparison to morphine, all three different dosage groups of oliceridine exhibited reduced rates of pruritus occurrence. Specifically, in the group using 0.1 mg of oliceridine (RR = 0.21, 95%CI [0.06,0.68], P = 0.001, I2 = 79%); the 0.35 mg group (RR = 0.55, 95%CI [0.38,0.81], P = 0.002, I2 = 0%); and 0.5 mg group (RR = 0.34, 95%CI [0.15,0.77], P = 0.009, I2 = 65%), the use of oliceridine showed a higher propensity to reduce the incidence of pruritus than morphine (Fig. 7).
Hypoxia comparison: oliceridine versus morphine
The incidence of hypoxia following treatment was reviewed across a compilation of 5 pieces of literature. It was found that Oliceridine showcased a better safety profile with a less frequent occurrence of hypoxia among patients as compared to the traditional opioid painkiller, morphine, (RR = 0.52, 95% CI[ 0.41–0.65], P < 0.001). The variations in results weren’t of notable significance (P = 0.13; I2 = 30%), thus we applied a fixed effect model and conducted a subgroup analysis to reconcile the inconsistency of outcomes. Sensitivity analysis confirmed the robustness of this finding, with the exclusion of any single study not altering the overall effect size.When comparing Oliceridine to morphine, the results of subgroup analyses were as follows: 0.1 mg group (RR = 0.20, 95%CI [0.12,0.34], P < 0.001, I2 = 0%); 0.35 mg group (RR = 0.66, 95%CI [0.45,0.96], p = 0.03, I2 = 0%); 0.5 mg group (RR = 0.78, 95%CI [0.52,1.16], P = 0.21, I2 = 0%). Remarkably, the 0.1 mg and 0.35 mg groups demonstrated significant statistical significance (Fig. 8).
Publication bias assessment
To address the issue of publication bias, we assessed it using funnel plots for the primary outcomes of postoperative nausea and vomiting. The funnel plot for postoperative nausea (Fig. 9) did not indicate significant asymmetry, suggesting a low risk of publication bias. Similarly, the funnel plot for postoperative vomiting (Fig. 10) also did not show significant asymmetry. For other secondary outcomes, including sedation, dizziness, pruritus, and hypoxemia, we performed similar assessments, and no significant publication bias was detected.
Discussion
This meta-analysis offers a comprehensive review of seven randomized controlled trials that emphatically compare the relative tolerability and safety of oliceridine and morphine in the administration of acute pain16. The side effects evaluated in this analysis encompass postoperative nausea and vomiting, sedation, dizziness, pruritus, and hypoxemia.
In examining the impacts of oliceridine, it is crucial to clarify what constitutes an equi-analgesic dose compared to morphine. The included studies ensured comparisons were made under conditions of equivalent pain control, using either the Sum of Pain Intensity Differences (SPID-48 or SPID-24) or average pain score reductions over 24 h. Six of the studies used SPID-48/24 to compare side effect profiles, ensuring comparable analgesic efficacy between oliceridine and morphine regimens. One study reported that patients receiving oliceridine experienced a time-weighted average decrease in pain scores by 2.3 points over 24 h, similar to the 2.1-point reduction observed in those receiving morphine. Across the studies, morphine was administered at a fixed dose of 4 mg as a loading dose, followed by demand doses of 1 mg via patient-controlled analgesia (PCA). However, it should be acknowledged that the specific dosing protocols varied among studies, with some adjusting morphine doses to achieve equivalent analgesia to oliceridine.egarding the incremental dosing of oliceridine, each step-up in oliceridine dosage (0.1 mg, 0.35 mg, 0.5 mg) was not necessarily matched with a corresponding increase in morphine dosage. Instead, morphine dosing strategies aimed to provide equivalent analgesic effectiveness across different oliceridine regimens.
A highlight in our study was its significantly reduced incidence of postoperative nausea and vomiting (PONV) compared to morphine, a routinely employed drug for pain management. This underscores oliceridine’s potential superiority in postoperative pain management17,18. The ability to deter such postoperative complications is a vital aspect, as these symptoms often pose grave distress to patients. The grim ramifications of PONV have been extensively chronicled in preceding scientific literature. Meta-analyses of diverse studies have consistently brought to light the pervasiveness and incapacitating effects of postoperative nausea, painting it as a common, debilitating residue of surgical procedures that patients frequently grapple with19. Far from being a mere mild discomfort, it holds the potential to significantly erode the patient’s quality of life post-surgery. In severe scenarios, it may disrupt the patient’s recovery course, trigger additional health issues, and protract the patient’s hospital stay. Thus, the effective management of PONV emerges as an integral component of postoperative care. The importance of this current analysis is emphasized by its clear-cut outcomes, especially when seen within the context of this enduring postoperative complication20. The findings corroborate that oliceridine could serve as an effective instrument in combating PONV, a conclusion that could notably influence future medical protocols and practices.To address the variability in definitions of adverse effects, we conducted a sensitivity analysis using the leave-one-out method to sequentially remove each study and recalculate the pooled estimates. This analysis confirmed the robustness of our results, indicating that varying definitions did not significantly alter the overall conclusions regarding the incidence of PONV.
In coherence with preceding discoveries regarding post-surgical nausea and vomiting, the research noticeably revealed a significantly diminished incidence of dizziness amongst patients administered with oliceridine in comparison to those receiving morphine treatment21. The reduction in these side effects holds substantial weight, primarily because it can immensely boost the postoperative recovery phase. These findings hold high relevance considering patient safety, as dizziness may contribute to potential risks such as falls eventually causing additional setbacks in the patient’s recuperation journey22. The decreased frequency of these adverse reactions signifies a significant enhancement in oliceridine’s overall safety profile when pitched against morphine. The noticed reduction in side effects gains significant attention, particularly during ambulatory and outpatient procedures where the discharge readiness is contingent on requisite post-surgery evaluations. These patient reviews post-surgery are of supreme importance and necessitate patients to be alert, responsive, and well-oriented. However, the meta-analysis did not significantly manifest an increased occurrence of excessive sedation amongst patients in the 0.35 mg, 0.5 mg oliceridine group juxtaposed with the morphine group, thus indicating no statistical significance. This insinuates that oliceridine therapy does not inherently amplify sedation levels, a highly crucial factor in hastening patients’ postoperative recovery duration. Therefore, the advantage of fewer dizziness-associated side effects accentuates oliceridine’s potential. As a novel opioid, it has the potential to significantly contribute to augmented patient safety, the enhancement of clinical outcomes, and the improvement of healthcare deliverance efficiency.
Our analysis further verifies that Oliceridine, a novel opioid analgesic, showcases a superior safety profile in comparison to its traditional counterpart, Morphine. This conclusion is reinforced by the drastically decreased prevalence of two frequent, yet distressing side effects—pruritus (itching) and hypoxemia (low blood oxygen levels).
Morphine, regarded as a powerful analgesic, has long been linked with adverse side effects like pruritus and hypoxemia23. Pruritus, an unpleasant urge to scratch, is especially prevalent among patients employing Morphine for pain management. This seemingly harmless, yet troublesome effect can significantly hinder patient comfort and overall satisfaction regarding pain management. Even worse, it might result in discontinuation of opioid therapy, and consequently, exposing patients to intolerable pain. Hence, Oliceridine’s ability to mitigate the occurrence of pruritus undeniably grants it a substantial advantage.
Moreover, Oliceridine’s lower incident rate of hypoxemia, a condition characterized by reduced blood oxygen levels, further accentuates its benefits. Hypoxemia can lead to dire consequences and is a significant clinical concern, principally among vulnerable groups such as the elderly and those with preexisting respiratory conditions24. Reduced oxygen levels can trigger a wide range of symptoms and complications, from shortness of breath and increased heart rate, escalating eventually to the weakening of the immune system, heart damage, and other organ failures due to limited oxygen supply25. Thus, Oliceridine’s capability to lessen the risks of hypoxemia induction marks yet another major advantage over Morphine in pain management.
To ensure transparency and consistency in reporting side effects, we reviewed the definitions used by each included RCT. Specifically:
Nausea: Defined based on patient self-reporting in some studies, while others required medical intervention. Vomiting: Recorded when it occurred, regardless of severity, in some studies, whereas others documented only episodes leading to treatment discontinuation. Sedation: Measured using standardized scales in most studies, but some relied on clinical judgment. Dizziness: Defined as any self-reported feeling of lightheadedness or unsteadiness. Pruritus (Itching): Reported based on patient complaints, with varying thresholds for severity across studies. Hypoxemia: Defined as oxygen saturation levels below a specified threshold, which varied among studies.The differences in the definitions of side effects across studies can influence the interpretation of results. For instance, regarding nausea and vomiting, some studies included mild symptoms, while others focused only on more severe cases. Such variations in definition may lead to underestimation or overestimation of the incidence rates of side effects, thereby impacting the overall comparative outcomes. We emphasize that despite these differences, the overall trend in the side effect profiles remains consistent, indicating the robustness of our study results. Additionally, the sensitivity analysis confirmed that the overall conclusions were not significantly affected by the exclusion of any single study, reinforcing the reliability of our findings.
However, it is important to acknowledge several limitations of this study. The inclusion of only seven RCTs in the meta-analysis presents certain limitations on the study quality. The sample sizes in the included trials varied, which may impact the generalizability of our findings. Additionally, differences in study designs, such as variations in patient populations and surgical procedures, may affect the interpretation of the results. Future studies with larger sample sizes and standardized methodologies would help to address these limitations.
Furthermore, while oliceridine shows a reduced incidence of many typical opioid side effects, it is known to cause QT interval prolongation, which can be a significant concern, especially at higher doses26. This side effect requires careful monitoring and dose adjustment to ensure patient safety.Clinicians should be aware of this potential side effect and consider the following recommendations when prescribing oliceridine:Conduct thorough pre-screening for patients with pre-existing cardiac conditions or those receiving other medications that may also affect the QT interval27.Implement regular electrocardiogram (ECG) monitoring during treatment, particularly in high-risk patients. This will help detect any changes in the QT interval early and allow for timely intervention.Adjust the dosage based on patient-specific factors such as age, renal function, and concomitant medications. Lower initial doses and gradual titration can help minimize the risk of QT interval prolongation. Educate patients about the signs and symptoms of QT interval prolongation, such as palpitations, dizziness, and fainting, and instruct them to seek medical attention if they experience these symptoms.Avoid prescribing oliceridine concurrently with other drugs that are known to prolong the QT interval unless absolutely necessary. If co-administration is unavoidable, closely monitor the patient’s ECG and adjust dosages accordingly. Additionally, more research is needed to determine the optimal dosing strategies for oliceridine to minimize the risk of QT interval prolongation while maintaining effective pain control.
To further validate and extend the findings of this study, we recommend conducting long-term follow-up studies and studies involving larger patient populations. Specifically, future work should highlight investigations into the long-term safety and tolerability of oliceridine. Comprehensive understanding of long-term outcomes and cumulative risks associated with oliceridine use is crucial. This includes evaluating its impact on cardiovascular and endocrine systems, kidney function, and neurocognitive outcomes over extended periods.
Generally, this meta-analysis reinforces the evidence supporting oliceridine’s superior safety and tolerance profile relative to morphine for acute pain management. To further validate and extend the findings of this study, we recommend conducting long-term follow-up studies, dose–response evaluations to refine oliceridine dosing routines, and exploring other potential side effects related to the use of oliceridine, including cardiovascular and endocrine system impacts, kidney function, and neurocognitive outcomes.
Future investigations should probe into other potential side effects related to the use of oliceridine, including cardiovascular and endocrine system impacts, kidney function, and neurocognitive outcomes. Comprehensive understanding of oliceridine’s stability and efficacy across a wide range of surgical and pain management contexts is crucial.
Finally, this meta-analysis underscores the significance of disseminating outcome data from further top-tier clinical trials to reinforce the observed improved safety and efficacy of oliceridine in the examined studies. This could ideally lead to broad acceptance and usage of oliceridine, thereby positing it as a significant addition to the pain-relieving arsenal and boosting patient safety and comfort in acute pain management.
In conclusion, this meta-analysis validates the tolerability and safety of oliceridine in managing acute pain, and accentuates its potential as a credible alternative to morphine for analgesia. The study further advocates for more extensive research and larger Randomized Controlled Trials (RCTs) for substantiating this evidence, with particular emphasis on long-term outcomes and cumulative risks.
Data availability
The datasets generated and/or analysed during the current study are included in this published article.
References
Amaechi, O., Huffman, M. M. & Featherstone, K. Pharmacologic therapy for acute pain. Am. Fam. Physician. 104(1), 63–72 (2021).
Hammer, G. B. et al. Oliceridine exhibits improved tolerability compared to morphine at equianalgesic conditions: Exploratory analysis from two phase 3 randomized placebo and active controlled trials. Pain Ther. 10(2), 1343–1353. https://doi.org/10.1007/s40122-021-00299-0 (2021).
Qureshi, I. et al. Comparison of intravenous paracetamol (acetaminophen) to intravenously or intramuscularly administered non-steroidal anti-inflammatory drugs (NSAIDs) or opioids for patients presenting with moderate to severe acute pain conditions to the ED: Systematic review and meta-analysis. Emerg. Med. J. 40(7), 499–508 (2023).
Beard, T. L. et al. Oliceridine is associated with reduced risk of vomiting and need for rescue antiemetics compared to morphine: Exploratory analysis from two phase 3 randomized placebo and active controlled trials. Pain Ther. 10(1), 401–413 (2021).
Noufal, Y., Kringel, D., Toennes, S. W., Dudziak, R. & Lötsch, J. Pharmacological data science perspective on fatal incidents of morphine treatment. Pharmacol. Ther. 241, 108312 (2023).
Liu, Y., Hu, Q. & Yang, J. Oliceridine for the management of acute postoperative pain. Ann. Pharmacother. 55(10), 1283–1289 (2021).
Markham, A. Oliceridine: First approval [published correction appears in drugs. Drugs 80(16), 1739–1744 (2020).
Viscusi, E. R. et al. A randomized, phase 2 study investigating TRV130, a biased ligand of the μ-opioid receptor, for the intravenous treatment of acute pain. Pain 157(1), 264–272 (2016).
Singla, N. et al. A randomized, Phase IIb study investigating oliceridine (TRV130), a novel µ-receptor G-protein pathway selective (μ-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J. Pain Res. 10, 2413–2424 (2017).
Wolf, A., Unterberg, M., Witowski, A., Adamzik, M. & Wolf, A. Efficacy, safety, and side effects of oliceridine in acute postoperative pain, a protocol for a systematic review and meta-analysis. PLoS ONE 19(2), e0299320 (2024).
Tan, H. S. & Habib, A. S. Safety evaluation of oliceridine for the management of postoperative moderate-to-severe acute pain. Expert Opin. Drug Saf. 20(11), 1291–1298 (2021).
Viscusi, E. R. et al. APOLLO-1: a randomized placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the µ-opioid receptor, for management of moderate-to-severe acute pain following bunionectomy. J. Pain Res. 12, 927–943 (2019).
Singla, N. K. et al. APOLLO-2: A randomized, placebo and active-controlled phase iii study investigating oliceridine (TRV130), a G protein-biased ligand at the μ-opioid receptor, for management of moderate to severe acute pain following abdominoplasty. Pain Pract. 19(7), 715–731 (2019).
Daksla, N., Wang, A., Jin, Z., Gupta, A. & Bergese, S. D. Oliceridine for the management of moderate to severe acute postoperative pain: A narrative review. Drug Des. Devel. Ther. 17, 875–886 (2023).
Biskupiak, J., Oderda, G., Brixner, D. & Wandstrat, T. L. Gastrointestinal adverse effects associated with the use of intravenous oliceridine compared with intravenous hydromorphone or fentanyl in acute pain management utilizing adjusted indirect treatment comparison methods. J. Comp. Eff. Res. 13(5), e230041 (2024).
Kaye, A. D. et al. Pharmacological advances in opioid therapy: A review of the role of oliceridine in pain management. Pain Ther. 10(2), 1003–1012 (2021).
Jin, Z. et al. Evaluating oliceridine as a treatment option for moderate to severe acute post-operative pain in adults. Expert Opin. Pharmacother. 23(1), 9–17 (2022).
Wang, A. et al. Novel opioids in the setting of acute postoperative pain: A narrative review. Pharmaceuticals 17(1), 29 (2023).
Rajan, N. & Joshi, G. P. Management of postoperative nausea and vomiting in adults: Current controversies. Curr. Opin. Anaesthesiol. 34(6), 695–702 (2021).
Schlesinger, T., Meybohm, P. & Kranke, P. Postoperative nausea and vomiting: risk factors, prediction tools, and algorithms. Curr. Opin. Anaesthesiol. 36(1), 117–123 (2023).
Soergel, D. G. et al. Biased agonism of the μ-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: A randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain 155(9), 1829–1835 (2014).
Fiore, J. F. Jr. et al. Opioid versus opioid-free analgesia after surgical discharge: A systematic review and meta-analysis of randomised trials. Lancet 399(10343), 2280–2293 (2022).
Viscusi, E. R. et al. A comparison of opioid-related adverse events with fentanyl iontophoretic transdermal system versus morphine intravenous patient-controlled analgesia in acute postoperative pain. Pain Manag. 6(1), 19–24 (2016).
Song, Q., Pan, Y., Kanazawa, T. & Morimatsu, H. Relationship of intraoperative SpO2 and ETCO2 values with postoperative hypoxemia in elderly patients after non-cardiac surgery. Acta Med. Okayama. 77(5), 537–543 (2023).
Lee, M., Lee, C., Choi, G. J. & Kang, H. Remimazolam for procedural sedation in older patients: A systematic review and meta-analysis with trial sequential analysis. J Pers Med. 14(3), 276 (2024).
Burashnikov, A. et al. Intracellular uptake of agents that block the hERG channel can confound the assessment of QT interval prolongation and arrhythmic risk. Heart Rhythm 18(12), 2177–2186. https://doi.org/10.1016/j.hrthm.2021.08.028 (2021).
El Sherbini, A. et al. Opioids-induced inhibition of HERG ion channels and sudden cardiac death, a systematic review of current literature. Trends Cardiovasc. Med. 34(5), 279–285. https://doi.org/10.1016/j.tcm.2023.03.006 (2024).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 88, 105906 (2021).
Shea, B. J. et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Br. Med. J. 358, j4008 (2017).
Author information
Authors and Affiliations
Contributions
Yulin Liu served as the first author. He contributed to the research and data collection, data analysis, and interpretation. He also took primary responsibility for drafting the initial manuscript and revising the content based on feedback. Ying Zhu was the co-first author who significantly contributed to the literature review, data extraction, methodological validity assessment of the included studies, and assisted in the data interpretation. Zhu also contributed equally to manuscript writing and revisions. Hong Fu, as the corresponding author, was instrumental in conceptualizing the research study, guiding the research process, and overseeing the data analysis. He also provided significant input in the final manuscript preparation, ensuring the coherence and relevance of the findings.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Zhu, Y. & Fu, H. Tolerability of different doses of oliceridine versus traditional opioids in acute pain management: a systematic review and meta-analysis. Sci Rep 15, 11470 (2025). https://doi.org/10.1038/s41598-025-95978-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95978-9