Abstract
While the association between COVID-19 and acute kidney injury (AKI) is well documented, the impact of COVID-19 on the development of advanced chronic kidney disease (CKD) remains unclear, particularly in patients without initial AKI. Using the TriNetX healthcare database, we conducted a matched cohort study comparing 141,587 COVID-19 and 141,587 influenza patients. We excluded patients with AKI within one month of infection and matched groups on demographics, comorbidities, and baseline laboratory values. The primary outcome was the incidence of advanced CKD (stages 3–5) at the 12-month follow-up. COVID-19 patients showed higher 12-month risks of advanced CKD (hazard ratio [HR]:2.02, 95% confidence interval [CI]:1.69–2.42, p < 0.0001), AKI (HR 3.04, 95%CI:2.61–3.55, p < 0.0001), and estimated glomerular filtration rate < 60 mL/min/1.73 m2 (HR:3.01, 95%CI:2.74–3.30, p < 0.0001) compared to influenza patients. Subgroup analyses showed consistently elevated risks across sexes and in patients over 45 years, while younger patients did not demonstrate an increased risk of advanced CKD at the 12-month follow-up. Diabetes mellitus and hypertension have emerged as the strongest predictors of advanced CKD development. In conclusion, COVID-19 is associated with an increased risk of long-term renal dysfunction compared with influenza, suggesting the need for extended monitoring of kidney function in high-risk populations.
Similar content being viewed by others
Introduction
The emergence of SARS-CoV-2 has created unprecedented challenges for healthcare systems worldwide, with effects extending far beyond the acute phase of infection1.
Although COVID-19 was initially recognized for its respiratory manifestations, growing evidence indicates that SARS-CoV-2 infection can lead to long-term complications affecting multiple organ systems2,3,4. The impact of the virus on kidney function has emerged as a particular concern, as research suggests potential associations between COVID-19 infection and the development of chronic kidney disease (CKD)5,6,7,8. Acute kidney injury (AKI) was recognized early in the pandemic as a severe complication of COVID-19, occurring in 32–57% of hospitalized patients and associated with significant mortality9,10,11,12. Evidence has established that patients who develop AKI during acute COVID-19 face an increased risk of subsequent CKD13. However, an important knowledge gap remains regarding the long-term renal consequences in COVID-19 survivors who did not experience AKI during their initial infection. A study of 1,008 COVID-19 patients followed over six months found no evidence of CKD development after acute illness, regardless of COVID-19 severity14. These findings suggest that kidney function might remain stable over time in patients who avoid acute renal dysfunction during their initial infection. This knowledge gap is particularly concerning given the global scale of SARS-CoV-2 infection and the substantial healthcare burden associated with CKD15.
Previous studies examining post-COVID renal dysfunction have been limited by short follow-up periods, small sample sizes, or focus on subgroups of patients with pre-existing AKI5,6,7,8. Additionally, many studies have not adequately controlled for pre-existing risk factors or baseline kidney function, making it difficult to establish the independent contribution of COVID-19 to CKD development. There is a pressing need for large-scale, well-controlled studies to quantify the risk of new-onset CKD following COVID-19 and to identify factors that might modify this risk.
This cohort study aimed to evaluate the association between COVID-19 infection and subsequent development of advanced CKD (i.e., stage 3–5) in a large, population-based sample, while accounting for relevant confounding factors. By analyzing longitudinal data from TrinetX, we sought to provide robust estimates of advanced CKD risk following COVID-19 infection and identify patient characteristics that might influence this risk.
Method
Setting and data source
This study utilized data from the TriNetX Healthcare Commercial Organizations (HCOs) database, a global federated network that includes electronic health records from 133 healthcare organizations predominantly located in the United States. While the authors are affiliated with institutions in Taiwan, the analysis was conducted remotely through secure access to the TriNetX platform, which provides de-identified patient data in compliance with international research standards. The de-identification process was validated through expert determination in accordance with the HIPAA Privacy Rule requirements. The TriNetX platform provides real-time access to de-identified electronic health records, including demographics, diagnoses (ICD-10 codes), procedures (CPT/ICD-10-PCS codes), medications, laboratory results, and clinical observations. Data collection and analysis were conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was not required for this retrospective study, as it involved secondary analysis of pre-existing data without any interventions or direct participant interaction. The study protocol was approved by the Institutional Review Board of Chi Mei Medical Center, which granted a waiver of informed consent in compliance with its observational research regulations (approval number No. 11302-E01).
Inclusion and exclusion criteria
We conducted a retrospective cohort study using data from the TriNetX database. From the initial population, we identified eligible participants as adults aged ≥ 18 years who had visited HCOs at least twice during 2022–2023. Within this population, we identified two groups of patients: those diagnosed with COVID-19 [ICD-10: U07.1] for the first time (COVID-19 group) and those diagnosed with influenza [ICD-10: J09-J11] for the first time (influenza group). The COVID-19 group included patients with confirmed SARS-CoV-2 infection through mRNA diagnostic testing. To prevent confounding from dual infections, patients in the COVID-19 group were excluded if they had an influenza diagnosis within one year before or after their COVID-19 diagnosis. Conversely, patients in the influenza group were excluded if they had a COVID-19 diagnosis within one year before or after their influenza diagnosis.
To minimize bias, patients with any history of advanced renal dysfunction in the year prior to their COVID-19 or influenza diagnosis were excluded. This included any estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m², documented proteinuria, a history of AKI, a diagnosis of CKD stages 3–5, or a history of hemodialysis or peritoneal dialysis. In addition, patients were excluded if they had a history of kidney transplantation, cystic kidney disease, or congenital kidney malformations; underwent any surgical procedure within 3 months before or up to 1 year after infection; experienced AKI; required renal dialysis; developed rhabdomyolysis, sepsis, or required intensive care unit admission within 1 month of infection; or had pre-existing heart failure or autoimmune disease. The one-month exclusion period for AKI was chosen based on previous studies showing that COVID-19-associated AKI typically occurred a median of 7–15 days from symptom onset16,17, making our exclusion period sufficient to capture acute kidney complications directly related to the infection.
Propensity score matching
To minimize selection bias and confounding factors, we performed 1:1 propensity score matching between the COVID-19 and influenza groups using logistic regression. The matching variables comprised demographic factors (age, sex, race) and laboratory values obtained at the closest time point before diagnosis, including body mass index (LOINC: 9083), hemoglobin (LOINC: 9014), serum albumin (LOINC: 9045), and HbA1c (LOINC: 9037). Comorbidities were matched and identified using ICD-10 codes from the three years prior to the diagnosis of COVID-19 or influenza and included essential hypertension [ICD-10: I10], neoplasms [ICD-10: C00-D49], obesity [ICD-10: E66], depression [ICD-10: F32], nicotine dependence [ICD-10: F17], diabetes [ICD-10: E08-E13], ischemic heart diseases [ICD-10: I20-I25], liver disease [ICD-10: K76], COPD [ICD-10: J44], cerebrovascular diseases [ICD-10: I60-I69], alcohol disorders [ICD-10: F10], atrial fibrillation [ICD-10: I48], NSAID use [ICD-10: Z79.1], and malnutrition [ICD-10: E40-E46]. The balance of covariates before and after matching was assessed using standardized mean differences, with values < 0.1 considered indicative of good balance.
Study outcomes
The primary outcome was the incidence of advanced CKD (i.e., stages 3–5) [ICD-10: N18.3-N18.5] occurring between 1 and 12 months after COVID-19 or influenza infection. Secondary outcomes included risks of AKI (ICD-10: N17) or single eGFR measurement < 60 mL/min/1.73 m² during the same follow-up period. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula18. For each patient, follow-up began one month after their initial COVID-19 or influenza diagnosis (index date) to exclude acute renal dysfunction, and continued until either advanced CKD development, death, loss to follow-up, or the end of the study period. We selected the 1–12 month follow-up period to allow sufficient time for the development of CKD while minimizing loss to follow-up. A minimum 1-month lag was implemented to exclude acute renal dysfunction directly related to the infection period. Additional analyses were performed for outcomes occurring between 1 and 6 months to assess earlier renal dysfunction.
Sensitivity analysis
We conducted four sensitivity analyses to evaluate the robustness of our findings. First, we performed propensity score matching that incorporated baseline eGFR values with previous variables to evaluate the consistency of our outcomes. For each patient, we identified the most recent eGFR value prior to the index infection date. Second, to evaluate whether the increased risk of CKD stage 3–5 was independent of AKI development, we conducted a sensitivity analysis excluding all patients who developed AKI during the one-year follow-up period. Third, we performed a sensitivity analysis without excluding patients with AKI, ICU admission, rhabdomyolysis, or sepsis within one month of infection. This analysis helps to evaluate whether our main findings persist when including the full spectrum of disease severity. Fourth, within the COVID-19 cohort, we compared the outcomes between hospitalized and non-hospitalized patients. We identified patients who were hospitalized within 14 days of their initial COVID-19 diagnosis. Using propensity score matching, we created a matched cohort of non-hospitalized COVID-19 patients. This analysis helps identify whether hospitalization after infection serves as a marker for an increased risk of subsequent kidney dysfunction, which could help clinicians prioritize monitoring resources. Using the same propensity score matching approach as our primary analysis, we assessed these outcomes at the 12-month follow-up.
Statistical analysis
Continuous variables are presented as mean ± standard deviation, and categorical variables are expressed as numbers and percentages. Between-group differences were assessed using standardized mean differences, with values less than 0.1 indicating good balance between matched cohorts. The cumulative incidence of renal dysfunction was estimated using the Kaplan-Meier method, and differences between the COVID-19 and influenza groups were compared using the log-rank test. A multivariable Cox proportional hazards model, which included all patients from both the COVID-19 and influenza cohorts, was constructed to identify independent risk factors for new-onset CKD stage 3–5 at the 12-month follow-up. The model included the following covariates: demographic factors (i.e., age at index, sex) and comorbidities (e.g., essential hypertension, diabetes mellitus, obesity, and neoplasms). The results are expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). All analyses were performed using the TriNetX analytics platform, which provides real-time statistical analyses. All statistical analyses were performed using the built-in analytics platform within TriNetX, which provides real-time statistical analysis. Statistical significance was set than 0.05.
Results
Patient selection
Of the 156,386,048 patients in 133 TriNetX healthcare organizations, 34,764,412 patients aged ≥ 18 years visited healthcare facilities at least twice between 2022 and 2023 (Fig. 1). Among them, 1,451,073 were diagnosed with first-time COVID-19 and 666,902 with first-time influenza. After applying the exclusion criteria (Supplemental Table 1), 636,264 COVID-19 patients and 141,737 patients with influenza remained eligible. Propensity score matching 1:1 by age, sex, race, and comorbidities yielded final analytical cohorts of 141,587 patients in each group.
Patient characteristics before and after matching
Prior to propensity score matching, our study included 636,264 patients in the COVID-19 group and 141,737 patients in the influenza group, respectively. Several notable differences in the baseline characteristics were observed between the groups (Table 1). The COVID-19 group was slightly older (mean age 46.9 ± 17.6 years vs. 42.6 ± 16.3 years) and had a marginally higher body mass index (29.8 ± 7.5 vs. 29.0 ± 7.2). While gender distribution was similar between groups (56.4% vs. 56.6% female), racial composition showed more variation, with the COVID-19 group having a higher proportion of White patients (57.7% vs. 49.6%) and Black or African American patients (14.2% vs. 9.9%), but a lower proportion of patients of unknown race (16.0% vs. 27.1%).
After 1:1 propensity score matching, we achieved well-balanced cohorts of 141,587 patients in each group, with standardized differences less than 0.1 for all baseline characteristics (Table 1). The matched cohorts had comparable demographic characteristics, including age (42.5 ± 16.5 vs. 42.6 ± 16.3 years), body mass index (28.7 ± 7.2 vs. 29.0 ± 7.2), gender distribution (56.7% vs. 56.6% female), and racial composition. Comorbidity profiles were also well-balanced between the matched groups, with similar prevalences of essential hypertension (13.2% vs. 13.4%), neoplasms (8.6% vs. 8.9%), and other chronic conditions. Laboratory values, including hemoglobin (13.6 ± 1.9 vs. 13.7 ± 1.8 g/dL), albumin (4.2 ± 0.6 vs. 4.2 ± 0.4 g/dL), and hemoglobin A1c (5.9 ± 1.4% vs. 6.0 ± 1.8%), showed no significant differences between the matched groups.
Outcome at 6-month and 12-month follow-up
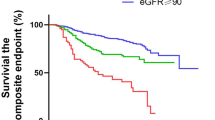
During the first 6 months of follow-up, patients in the COVID-19 group demonstrated significantly higher rates of renal dysfunction than those in the influenza group (Table 2). The incidence of AKI was threefold higher in COVID-19 patients (0.25% vs. 0.08%, HR 3.16, 95% CI 2.57–3.88, P < 0.0001), whereas advanced CKD occurred more than twice as frequently (0.14% vs. 0.07%, HR 2.25, 95% CI 1.76–2.87, P < 0.0001). Similarly, reduced eGFR (< 60 mL/min/1.73 m²) was substantially more common in the COVID-19 group (0.68% vs. 0.22%, HR 3.32, 95% CI 2.92–3.78, P < 0.0001). The disparity in renal dysfunction persisted through 12 months of follow-up (Table 2). Kaplan-Meier curves show the cumulative incidence of renal dysfunction in COVID-19 versus influenza patients (Fig. 2). The COVID-19 group maintained higher rates of AKI (0.45% vs. 0.15%, HR 3.04, 95% CI 2.61–3.55) (Fig. 2a), advanced CKD (0.25% vs. 0.13%, HR 2.02, 95% CI 1.69–2.42) (Fig. 2b), and reduced eGFR (1.23% vs. 0.43%, HR 3.01, 95% CI 2.74–3.30) (Fig. 2c). All differences at 12 months remained statistically significant (P < 0.0001), indicating a consistently elevated risk of renal dysfunction in COVID-19 patients compared with those with influenza.
Kaplan-Meier curves showing the cumulative incidence of renal dysfunctions in COVID-19 versus influenza patients. (a) Acute kidney injury (AKI); (b) Chronic kidney disease (CKD) stages 3–5; and (c) Reduced estimated glomerular filtration rate (eGFR < 60 mL/min/1.73 m²). Blue and red lines represent COVID-19 and influenza groups respectively, with shaded areas indicating 95% confidence intervals. Higher rates of all three outcomes were observed in the COVID-19 group throughout the 12-month follow-up period (all P < 0.0001).
Subgroup analysis of gender on clinical outcomes
In a subgroup analysis stratified by sex, both females (n = 80,632 per cohort) and males (n = 56,745 per cohort) showed significantly higher incidence rates of AKI, advanced CKD, and reduced eGFR in the COVID-19 group than in the influenza group during the 12-month follow-up period (all p < 0.0001) (Table 3). However, the HR for AKI and reduced eGFR were notably higher in males than in females. For AKI, the HR was 3.78 (95% CI 3.04–4.71) in males compared to 2.27 (95% CI 1.79–2.86) in females. Similarly, for reduced eGFR, the HR was 3.13 (95% CI 2.71–3.62) in males versus 2.84 (95% CI 2.52–3.19) in females. These findings suggest that while COVID-19 infection poses a significantly higher risk of developing renal dysfunction than influenza infection in both sexes, males may be more susceptible to AKI and reduced eGFR than females after COVID-19 infection.
Subgroup analysis of age on kidney dysfunction
Analysis of age-stratified outcomes at 12 months revealed distinct patterns of renal dysfunction between younger (18–45 years) and older (> 45 years) patients (Table 4). In the younger cohort of 67,859 patients per group, COVID-19 patients demonstrated a significantly higher risk of AKI (0.16% vs. 0.07%, HR 2.29, 95% CI 1.62–3.24, P < 0.0001) and reduced eGFR (0.23% vs. 0.05%, HR 4.47, 95% CI 3.09–6.49, P < 0.0001) than influenza patients. However, the difference in the incidence of advanced CKD was not statistically significant in this age group (0.03% vs. 0.02%, HR 1.53, 95% CI 0.76–3.07, P = 0.2311).
The older cohort (56, 782 patients per group) showed more pronounced differences in all kidney outcomes. COVID-19 patients over 45 years of age exhibited substantially higher rates of AKI (0.87% vs. 0.28%, HR 3.46, 95% CI 2.89–4.14, P < 0.0001), advanced CKD (0.56% vs. 0.29%, HR 2.09, 95% CI 1.74–2.53, P < 0.0001), and reduced eGFR (2.79% vs. 1.00%, HR 3.08, 95% CI 2.80–3.40, P < 0.0001) than their influenza counterparts. These findings suggest that older age may be an important risk modifier of renal dysfunction following COVID-19.
Sensitivity analysis
In sensitivity analyses (Supplemental Table 2), we first incorporated baseline eGFR as a matching variable, achieving well-balanced cohorts (COVID-19:96.9 ± 21.6 mL/min/1.73 m²; Influenza: 97.2 ± 19.9 mL/min/1.73 m², standardized difference: 0.0341). Despite this balance, COVID-19 patients continued to exhibit significantly higher 12-month risks for AKI (HR 2.80, 95% CI 2.44–3.21), advanced CKD (HR 2.38, 95% CI 2.04–2.77), and eGFR < 60 mL/min/1.73 m² (HR 2.78, 95% CI 2.56–3.02) (all P < 0.0001).
After excluding patients who developed AKI within one year post-infection, COVID-19 patients remained at elevated risk for advanced CKD (HR 1.88, 95% CI 1.59–2.22, P < 0.0001) and eGFR < 60 mL/min/1.73 m² (HR 2.62, 95% CI 2.40–2.85, P < 0.0001), suggesting that COVID-19 may contribute to chronic kidney dysfunction independent of AKI (Supplemental Table 2).
When including patients with initial severe disease (defined as AKI, ICU admission, rhabdomyolysis, or sepsis within one month of infection), COVID-19 patients consistently demonstrated higher risks for AKI (HR 2.27, 95% CI 2.03–2.54), advanced CKD (HR 1.68, 95% CI 1.48–1.91), and eGFR < 60 mL/min/1.73 m² (HR 2.74, 95% CI 2.53–2.96) (all P < 0.0001) (Supplemental Table 2).
Finally, in a matched comparison of COVID-19 patients with and without early hospitalization, those who were hospitalized had higher risks for AKI (HR 2.43, 95% CI 2.14–2.76, P < 0.0001) and eGFR < 60 mL/min/1.73 m² (HR 1.56, 95% CI 1.43–1.69, P < 0.0001). However, there was no significant difference in the risk of advanced CKD (HR 1.05, 95% CI 0.89–1.24, P = 0.591), suggesting that while early hospitalization may help identify patients at higher risk for acute complications and reduced eGFR, it does not necessarily translate into an increased risk of advanced CKD (Supplemental Table 2).
Cox proportional hazards analysis
Cox proportional hazards analysis revealed several significant risk factors for new-onset advanced CKD (Fig. 3). Demographic factors, including age at index (HR 1.07, 95% CI 1.07–1.08) and male sex (HR 1.18, 95% CI 1.10–1.26) showed mild but significant associations. Diabetes mellitus (HR 2.42, 95% CI 2.24–2.62) and essential hypertension (HR 2.14, 95% CI 1.97–2.33) emerged as the strongest clinical predictors. Several comorbidities also showed significant associations, including malnutrition (HR 1.77, 95% CI 1.43–2.18), obesity (HR 1.28, 95% CI 1.17–1.40), neoplasms (HR 1.18, 95% CI 1.09–1.27), depressive episodes (HR 1.16, 95% CI 1.05–1.29), and ischemic heart diseases (HR 1.10, 95% CI 1.00-1.21). Notably, nicotine dependence, alcohol-related disorders, cerebrovascular diseases, liver diseases, atrial fibrillation, and COVID-19 vaccination status were not significantly associated with kidney dysfunction (all P > 0.05). The analysis also confirmed an elevated risk in the COVID-19 cohort compared to the influenza cohort (HR 2.29, 95% CI 1.97–2.67, P < 0.0001).
Risk factors for new-onset chronic kidney disease (CKD) stage 3–5 at the 12-month follow-up. Forest plot showing adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) from multivariate Cox regression analysis. The vertical line at HR = 1 represents no effect; HRs greater than 1 indicate an increased risk. The variables are ordered by the magnitude of the effect size.
Discussion
In this large-scale matching cohort study, we investigated the association between COVID-19 and new-onset CKD. We specifically excluded patients who experienced AKI within one month of infection to focus on the direct viral impact on kidney function. Our findings revealed significantly higher risks of renal dysfunction (i.e., AKI, advanced CKD, and reduced eGFR) in COVID-19 patients than in those with influenza during the 6-month and 12-month follow-up periods. Gender-stratified analyses showed consistently elevated risks across all renal dysfunctions for both males and females. Age-stratified analyses revealed that while both age subgroups (i.e., 18–45 and > 45 years) showed increased risks of AKI and reduced eGFR, only patients over 45 years demonstrated a significantly higher risk of advanced CKD. Cox proportional hazards analysis identified several significant risk factors for new-onset CKD, with diabetes mellitus and essential hypertension emerging as the strongest predictors.
Our findings revealed significantly elevated risks of new-onset advanced CKD in COVID-19 patients without initial AKI compared to those with influenza at both the 6-month and 12-month follow-up periods. However, these results are in contrast with those of some previous studies. For instance, a study examining 1,008 COVID-19 patients over a 6-month period found no evidence of CKD development after acute illness, regardless of COVID-19 severity14. Similarly, Strohbehn et al. reported that among patients followed for ≥ 90 days, the rates of new-onset CKD were not significantly different between the COVID-19 and influenza cohorts19. The discrepancy between our findings and those of previous studies14,19 might be explained by the differences in follow-up time and sample size. Our observation of persistently higher risk across both time points (6-month HR 2.25; 12-month HR 2.02 for CKD stages 3–5) suggests that COVID-19’s impact on kidney function may manifest early and continue through at least one year. The consistent HRs observed between 6 and 12 months suggest that the risk stabilizes during this period rather than continuing to deteriorate. This temporal pattern has important implications for clinical monitoring, suggesting that kidney function surveillance may be most crucial in the first 6 months post-infection, while remaining important throughout the first year.
Our findings of increased CKD risk in COVID-19 patients without initial AKI suggest mechanisms distinct from acute damage pathways. This phenomenon can be explained by several pathophysiological processes. SARS-CoV-2 can directly infect kidney tissues through ACE2 receptors expressed in renal tubular cells and podocytes, initiating subtle but progressive tissue damage through local inflammation and fibrosis20. COVID-19-induced endothelial dysfunction and microthrombus formation can lead to chronic microvascular damage, whereas systemic inflammation characterized by elevated pro-inflammatory cytokines may promote ongoing oxidative stress and tissue remodeling21. Additionally, COVID-19 can induce autoimmune responses targeting kidney tissue and dysregulate the renin-angiotensin system, affecting long-term kidney homeostasis21. These mechanisms can operate independently of or synergistically with traditional acute kidney injury pathways, explaining the observed increased risk of CKD even in patients without initial AKI.
Our findings demonstrated significantly higher risks of AKI in COVID-19 patients than in influenza patients at both the 6-month (HR 3.16) and 12-month (HR 3.04) follow-up periods, maintaining consistent elevation over time. The magnitude of the increased AKI risk we observed is comparable to but somewhat higher than that reported by Xie et al. in their study, which found that COVID-19 patients had a 1.52 times higher AKI risk compared to influenza patients22. Our findings also complement the work of Bowe et al., who found that 30-day COVID-19 survivors had a 1.94 times higher risk of AKI than non-infected controls23. This sustained elevation in the risk of AKI in the current study suggests ongoing kidney vulnerability well beyond the acute phase of infection.
Our gender-stratified analyses revealed that males exhibited a substantially higher risk for AKI than females (HR 3.78 vs. 2.27), suggesting potential gender-specific vulnerabilities in acute kidney dysfunction after viral infection. Regarding CKD, a previous study reported that men generally experience approximately a fifth higher excess risk of incident CKD than women24, and Minutolo et al. also demonstrated higher risks for end-stage kidney disease in men25. However, the development of CKD in the current study showed similar patterns between men and women, indicating that long-term kidney outcomes may be less influenced by sex-specific factors in the context of COVID-19 infection.
Our Cox proportional hazards analysis revealed several significant risk factors for new-onset CKD following COVID-19, with diabetes mellitus and hypertension emerging as the strongest predictors. These findings align with the established literature on traditional CKD risk factors26,27,28 and emphasize the importance of maintaining effective control of these conditions in patients with a history of COVID-19. The significant association between malnutrition and CKD risk (HR 1.77) aligns with previous research showing that hypoalbuminemia, a key marker of malnutrition, is independently associated with incident CKD29. Erlinger et al. demonstrated that hypoalbuminemia was associated with CKD risk even after adjusting for multiple confounders, including diabetes and cardiovascular disease29. Similarly, the identified risk associated with obesity (HR 1.28) suggests that metabolic dysfunction may play a crucial role in post-COVID renal dysfunction. These findings have important implications for clinical practice, suggesting the need for particularly vigilant kidney function monitoring in COVID-19 patients with pre-existing diabetes or hypertension. The identification of these risk factors could help clinicians stratify patients for targeted preventive interventions and more intensive follow-up care.
To specifically evaluate the effect of COVID-19 on renal dysfunction, our study design included several methodological considerations. One key step was the exclusion of patients who had experienced AKI within one month of infection. This approach is essential, as AKI, irrespective of its underlying cause, is associated with a higher risk of CKD development30. By excluding these patients, we aimed to investigate whether COVID-19 could lead to chronic renal dysfunction through mechanisms independent of acute kidney damage. Similarly, we excluded patients who underwent surgical procedures within 3 months before or up to 1 year after infection because surgery can independently affect kidney function through multiple mechanisms31,32. This approach helped minimize confounding factors that could obscure the direct relationship between viral infection and kidney outcomes. In addition, the study period (2022–2023) captures contemporary COVID-19 variants and current treatment protocols, making findings relevant to present clinical practice.
The choice of influenza as a control group offers several advantages. First, influenza represents another viral respiratory infection with systemic implications, allowing us to differentiate COVID-19-specific effects from general post-viral complications33,34. Second, both conditions typically affect similar populations and receive comparable medical attention, thereby reducing the selection bias. Third, using influenza as a control helps account for healthcare-seeking behavior patterns among patients with respiratory infections.
Several limitations of this study should be considered when interpreting our findings. First, although our study utilized a large healthcare database, reliance on ICD-10 codes for outcome identification may have led to misclassification bias. The accuracy of COVID-19 and CKD diagnoses depends on proper coding practices, which can vary across healthcare organizations. Second, although we employed rigorous propensity score matching to control for known confounders, residual confounding from unmeasured variables may persist. For instance, detailed information on COVID-19 severity, viral variants, and socioeconomic factors was not available in our dataset. The TriNetX database reflects data as recorded in electronic health records by participating healthcare organizations, which may have missing data for certain laboratory values or demographic information. Because patients with incomplete records were not automatically excluded from the dataset, this missing data could affect the robustness of our findings, particularly in analyses requiring complete laboratory values or demographic information. Additionally, an important limitation was our inability to account for the frequency of eGFR measurements. Testing frequency can indicate both healthcare utilization patterns and underlying kidney disease risk, with more frequent baseline testing potentially leading to increased post-infection monitoring and detection of kidney dysfunction. Without these data from TriNetX, we cannot fully account for potential surveillance bias or adequately control for baseline kidney disease risk, as reflected in testing patterns. Third, our study’s follow-up period was limited to 12 months, which may not have been sufficient to capture the full spectrum of long-term renal dysfunction. The natural history of post-COVID kidney dysfunction might extend beyond this timeframe. Fourth, our study population was drawn from healthcare organizations within the TriNetX network, which may not be fully representative of the general population. Patients who seek care at these institutions may differ systematically from those who receive care elsewhere. Finally, while we excluded patients with acute renal dysfunction within one month of infection, we cannot completely rule out the possibility of subclinical kidney injury that may have influenced our findings.
Conclusion
This large-scale matched cohort study demonstrated that COVID-19 increases the risk of long-term renal dysfunction compared to influenza. Early hospitalization also emerged as a key predictor of subsequent kidney complications, with hospitalized COVID-19 patients showing significantly higher risks of AKI and eGFR < 60 mL/min/1.73 m² than non-hospitalized COVID-19 patients during the 12-month follow-up. Although the risk of CKD stages 3–5 was not significantly elevated, possibly due to limited statistical power, these findings suggest that post-COVID kidney monitoring should prioritize hospitalized patients, particularly those with additional risk factors such as diabetes and hypertension, enabling more efficient allocation of healthcare resources. Future research should focus on developing targeted interventions for these high-risk populations and understanding the underlying mechanisms driving different types of kidney dysfunction.
Data availability
The data that support the findings of this study are available from TriNetX Research Network, but restrictions apply to the availability of these data, which were used under a collaboration agreement for the current study and so are not publicly available. Data are however available from the author (Kuo-Chuan Hung) upon reasonable request and with permission of TriNetX. Access to the de-identified data requires either TriNetX network membership or establishment of a collaborative agreement with TriNetX.
References
Katz, G. M. et al. Understanding how Post–COVID-19 condition affects adults and health care systems. JAMA Health Forum. 4, e231933–e (2023).
Kopańska, M. et al. Effects of SARS-CoV-2 inflammation on selected organ systems of the human body. Int. J. Mol. Sci. ;23. (2022).
Philip, B. et al. COVID-19 and its long-term impact on the cardiovascular system. Expert Rev. Cardiovasc. Ther. 21, 211–218 (2023).
Rabaan, A. A. et al. SARS-CoV-2 infection and multi-organ system damage: A review. Biomol. Biomed. 23, 37–52 (2023).
Gu, X. et al. Association of acute kidney injury with 1-year outcome of kidney function in hospital survivors with COVID-19: A cohort study. EBioMedicine 76, 103817 (2022).
Zhang, Y. et al. Long-term renal outcomes of patients with COVID-19: A meta-analysis of observational studies. J. Nephrol. 36, 2441–2456 (2023).
Teng, L. et al. The pattern of cytokines expression and dynamic changes of renal function at 6 months in patients with Omicron COVID-19. J. Med. Virol. 95, e28477 (2023).
Atiquzzaman, M. et al. Long-term effect of COVID-19 infection on kidney function among COVID-19 patients followed in post-COVID-19 recovery clinics in British Columbia, Canada. Nephrol. Dial Transpl. 38, 2816–2825 (2023).
Pan, X. W. et al. Acute kidney injury during the COVID-19 outbreak. Nephrol. Dial Transpl. 35, 1635–1641 (2020).
Ng, J. H. et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am. J. Kidney Dis. ;77 (2021). :204 – 15.e1.
Gupta, S. et al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J. Am. Soc. Nephrol. 32, 161–176 (2021).
Fisher, M. et al. AKI in hospitalized patients with and without COVID-19: A comparison study. J. Am. Soc. Nephrol. 31, 2145–2157 (2020).
Nugent, J. et al. Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw. Open. 4, e211095 (2021).
Kemec, Z. & Akgul, F. Are patients with Covid-19 at risk of long-term chronic kidney disease? Niger J. Clin. Pract. 26, 341–346 (2023).
Jha, V. et al. Global economic burden associated with chronic kidney disease: A pragmatic review of medical costs for the inside CKD research programme. Adv. Therapy. 40, 4405–4420 (2023).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395, 1054–1062 (2020).
Cheng, Y. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 97, 829–838 (2020).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Strohbehn, I. A. et al. Acute kidney injury incidence, recovery, and Long-term kidney outcomes among hospitalized patients with COVID-19 and influenza. Kidney Int. Rep. 6, 2565–2574 (2021).
Diao, B. et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat. Commun. 12, 2506 (2021).
Legrand, M. et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol. 17, 751–764 (2021).
Xie, Y., Bowe, B., Maddukuri, G. & Al-Aly, Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: Cohort study. Bmj 371, m4677 (2020).
Bowe, B., Xie, Y., Xu, E. & Al-Aly, Z. Kidney outcomes in long COVID. J. Am. Soc. Nephrol. 32, 2851–2862 (2021).
Weldegiorgis, M. & Woodward, M. The impact of hypertension on chronic kidney disease and end-stage renal disease is greater in men than women: a systematic review and meta-analysis. BMC Nephrol. 21, 506 (2020).
Minutolo, R. et al. Sex differences in the progression of CKD among older patients: Pooled analysis of 4 cohort studies. Am. J. Kidney Dis. 75, 30–38 (2020).
Shen, Y. et al. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: A systematic review and meta-analysis. Endocrine 55, 66–76 (2017).
Hamrahian, S. M. & Falkner, B. Hypertension in chronic kidney disease. Hypertension: from basic research to clinical practice. :307 – 25. (2017).
Hill, N. R. et al. Global prevalence of chronic kidney Disease - A systematic review and Meta-Analysis. PLoS One. 11, e0158765 (2016).
Erlinger, T. P. et al. Leukocytosis, hypoalbuminemia, and the risk for chronic kidney disease in US adults. Am. J. Kidney Dis. 42, 256–263 (2003).
Ohlmeier, C. et al. Risk of chronic kidney disease in patients with acute kidney injury following a major surgery: A US claims database analysis. Clin. Kidney J. 16, 2461–2471 (2023).
Sear, J. Kidney dysfunction in the postoperative period. Br. J. Anaesth. 95, 20–32 (2005).
Turan, A. et al. Mild acute kidney injury after noncardiac surgery is associated with Long-term renal dysfunction: A retrospective cohort study. Anesthesiology 132, 1053–1061 (2020).
Shusterman, E. et al. Risk factors, clinical characteristics and prognostic value of acute kidney injury in COVID-19 compared with influenza virus and respiratory syncytial virus. J. Nephrol. 36, 1349–1359 (2023).
Quarg, C., Jörres, R. A., Engelhardt, S., Alter, P. & Budweiser, S. Characteristics and outcomes of patients hospitalized for infection with influenza, SARS-CoV-2 or respiratory syncytial virus in the season 2022/2023 in a large German primary care centre. Eur. J. Med. Res. 28, 568 (2023).
Funding
None.
Author information
Authors and Affiliations
Contributions
Author contribution: I-Wen Chen and Li-Chen Chang: Conceptualization. Kuo-Chuan Hung and Chun-Ning Ho: methodology and software. Kuo-Chuan Hung and Jheng-Yan Wu: validation. Ya-Wen Tsai: formal analysis. Chun-Ning Ho: investigation. Kuo-Chuan Hung and Ying-Jen Chang: resources. Ying-Jen Chang: data curation. Ying-Jen Chang and Kuo-Chuan Hung: writing—original draft preparation. Kuo-Chuan Hung and I-Wen Chen: writing—review and editing. Kuo-Chuan Hung and I-Wen Chen: visualization and supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Human ethics and consent to participate
Informed consent was not required for this retrospective study, as it involved secondary analysis of pre-existing data without any interventions or direct participant interaction. The study protocol was approved by the Institutional Review Board of Chi Mei Medical Center, which granted a waiver of informed consent in compliance with its observational research regulations (IRB Serial No. 11302-E01).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, IW., Chang, LC., Ho, CN. et al. Association between COVID-19 and the development of chronic kidney disease in patients without initial acute kidney injury. Sci Rep 15, 10924 (2025). https://doi.org/10.1038/s41598-025-96032-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96032-4
Keywords
This article is cited by
-
Long COVID and the kidney
Nature Reviews Nephrology (2025)
-
Long-term renal consequences of COVID-19. Emerging evidence and unanswered questions
International Urology and Nephrology (2025)