Abstract
The study aimed to fabricate and evaluate a bone tissue engineering scaffold made from a composite of polylactic-co-glycolic acid (PLGA), nano-hydroxyapatite (nHA), and graphene oxide (GO) using low-temperature 3D printing and freeze-drying techniques. The scaffolds were produced with varying compositions: PLGA alone and in combination with nHA and GO. The macro and microstructure, pore size, porosity, mechanical properties, and in vitro biocompatibility were assessed. Bone marrow mesenchymal stem cells (BMSCs) were co-cultured with the scaffolds to evaluate cell adhesion, proliferation, and cytotoxicity. The PLGA/nHA/GO composite scaffolds exhibited optimal pore size and microtopography, enhanced mechanical properties, excellent water absorption, and appropriate degradability. The co-culture with BMSCs demonstrated improved cell adhesion and proliferation, indicating good biocompatibility. The PLGA/nHA/GO composite scaffolds show potential as a bone tissue engineering material due to their favorable properties and biocompatibility, suggesting their suitability for bone defect repair applications.
Similar content being viewed by others
Introduction
Bone tissue engineering (BTE), proposed by Crane et al. in the 1990s1, aims to create functional bone grafts with osteoid histological features and biomechanical properties through interdisciplinary integration of materials science, engineering, and biological principles1. Recognized as the most promising approach for bone defect repair2, BTE remains a focal point in contemporary biomedical research. However, conventional fabrication methods face limitations in precise control over scaffold morphology, internal architecture, and microporous features3, leading to compromised pore interconnectivity and inadequate regulation of porosity parameters. Building on biomaterial properties and composite advantages, this study employs low-temperature 3D printing coupled with freeze-drying to fabricate BTE scaffolds. The design utilizes PLGA as structural matrix and nHA to replicate bone’s inorganic composition, while exploring graphene oxide (GO) supplementation. Using pure PLGA scaffolds as controls, we systematically investigated the synergistic effects arising from: (1) organic-inorganic PLGA/nHA integration, and (2) strategic GO incorporation at nanoscale concentrations. Comprehensive characterization of scaffold physico-chemical properties was performed across all experimental groups. Furthermore, in vitro cytocompatibility and osteogenic potential were assessed through rBMSC-scaffold coculture models, using rabbit bone marrow mesenchymal stem cells as biological evaluators.
The specific aims of this research are threefold: (1) to optimize the micro- and macro-structural properties of the scaffold; (2) to assess its mechanical properties, degradation characteristics, and biocompatibility; and (3) to preliminarily investigate its capacity to promote the proliferation and differentiation of bone marrow mesenchymal stem cells (BMSCs) in vitro.
Materials and methods
Experimental materials
-
(1)
PLGA (LA/GA = 75/25; Cat. No. R08060301) - Hangzhou Regenovo Biotechnology (Hangzhou, China).
-
(2)
nHA (200 nm particle size; Cat. No. RH299785) - Shanghai Yien Chemical Technology.
-
(3)
GO (Cat. No. RH322190) - Shanghai Yien Chemical Technology.
-
(4)
1,4-Dioxane (Cat. No. C11112807) - Shanghai Macklin Biochemical.
-
(5)
RSC-RBrBMSCs (Cat. No. ZQ0682) - Shanghai Zhong Qiao Xin Zhou Biotechnology.
-
(6)
rBMSC Medium (Cat. No. ZQ-1318) - Shanghai Zhong Qiao Xin Zhou Biotechnology.
Fabrication and morphological characterization of scaffolds
Materials for scaffolds in each group
PLGA group: PLGA pellets (2 g) were dissolved in 15 mL 1,4-dioxane under magnetic stirring (30 min, room temperature).
PLGA/nHA group: (1) Base solution: Prepared under identical conditions to PLGA group. (2) Addition: 0.5 g nHA powder. (3) Vortex dispersion (1 min). (4) Magnetic stirring until homogenization Mass ratio PLGA: nHA = 4:1 (w/w).
PLGA/nHA/GO group: (1) Base solution: Prepared under identical conditions to PLGA group. (2) Co-addition: nHA 0.5 g and GO 0.01 g. (3) Vortex dispersion (1 min). (4) Magnetic stirring until homogenization Mass ratios PLGA: nHA = 4:1; nHA: GO = 100:2 (w/w).
Printing of scaffolds
The 3D printer ( Bio-Architect wS, Hangzhou Jenofei Biological Technology Co., LtD, China) pre-cooled for half an hour, put the above materials into the printing cartridge, install the piston and the corresponding size of the needle, and segment the model according to the corresponding software, and alternately fill and print each three layers in one section. Based on the properties of composite materials, the optimal printing parameters were obtained after several previous printing explorations (Table 1).
The printing parameters are closely related to various factors, such as the properties of the printing material and the ambient temperature. Moreover, the platform temperature should facilitate the formability of the extruded material. Therefore, the parameters will depend on the actual formability during printing: First, gradient cooling of the platform was needed during the printing process. The platform temperature was set to − 12 °C when printing the first 4 layers, and manually reduced as the height further increased later in the printing process. Second, the air pressure should be increased appropriately when there was not much material left in the cartridge. Afar printing, the scaffold was placed in a -80 °C refrigerator for 2 h, then freeze-dried in a freeze dryer (FDU-2110, Tokyo Riken Instrument Co., LtD, Japan) for 24 h, and stored at -20 °C until use.
Morphological characterization of scaffolds
The general morphology, including the macrostructure and surface pores, of scaffolds in each group was observed under natural light with the naked eye, photographed and recorded.
Performance evaluation of scaffolds
Characterization of macroscopic pore size and porosity of scaffolds
Scaffold samples were randomly selected from each group. The surface of the scaffolds was photographed with a microscope. Four macroscopic pores in different areas were randomly selected on the front and side of each sample scaffold, respectively, for pore size measurement.
Three scaffold samples were randomly selected from each group to measure the porosity by optical coherence tomography (OCT) (GANYMEDE, Thorlabs GmbH).
Microscopic analyses of the scaffolds
Scaffold samples were randomly selected from each group. Samples were sputter coated with a slenderlayer of gold for 30 s. Subsequently, the microtopography of the scaffolds was observed and photographed under a field emission electron microscope (SU8020, Hitachi (China) Co., Ltd., China). Three parallel experiments were performed for each group.
Determination of mechanical properties of the scaffolds
Scaffold samples were randomly selected from each group and fixed on the mechanical testing machine (E43.104, Metus Industrial Systems (China) Co., LtD). According to ASTM D695, the compression modulus is tested at a compression rate of 1 mm/min with a 60% strain rate. The compression (mm) and load (kgf) values were recorded. The compressive modulus of the scaffold is the slope within the linear interval of the stress-strain curve at 0 to 10% strain. Three parallel experiments were performed for each group.
Determination of water absorption of scaffolds within 12 h
Scaffold samples were randomly selected from each group. The mass of the dry scaffolds was measured and recorded as m1. The scaffolds were immersed in deionized water at room temperature, and taken out after 3 h, 6 h, 9 h, and 12 h to gently wipe the unabsorbed water on the surface with filter paper. The mass of the wetted scaffolds was measured and recorded as m2. The water absorption of the scaffolds at each time point was calculated as follows:
Three parallel experiments were performed for each group.
Determination of degradation rate of scaffolds
The experimental procedure is the same as described in 2.3.5 The degradation rate of the scaffolds at each time point was calculated as follows:
The degradation medium used was “phosphate-buffered saline (PBS, pH 7.4)”.
pH changes during scaffold degradation
The pH values of the scaffold degradation solutions in each group were measured and recorded at the time points described above. The pH measurements were conducted using a “pH meter (Mettler Toledo FE28)” calibrated with standard buffer solutions (pH 4.0, 7.0, and 10.0).
Biocompatibility evaluation of scaffolds
Sterilization of scaffolds
The scaffold samples of each group were immersed in 75% ethanol and irradiated with ultraviolet light for 3 h. They were then washed with PBS 2–3 times to remove residual ethanol. Subsequently, the scaffolds were immersed in a penicillin-streptomycin solution and left overnight at 4 °C. Finally, they were rinsed twice with PBS before use.
Cell seeding on scaffolds
Rabbit bone marrow mesenchymal stem cells (rBMSCs) used in this study were purchased from Shanghai Zhongqiao Xinzhou Biotechnology Co., LTD. (Product number: ZQ0682).
One day before cell seeding, three sterilized scaffolds of each group were immersed in a 24-well plate containing rMBSC complete medium, and transferred to a new well plate after 24 h. The third passage rBMSCs were digested, centrifuged, and resuspended. Cell density was adjusted to 5 × 106cells/mL. Using the static surface seeding method, the cell suspension was dropped on the scaffolds of each group at a ratio of 0.3 µL/mm3. The well plate was cultured statically for 3 h in a cell incubator with 5% carbon dioxide at 37 °C. When the cells were well adhered, 1 mL of complete medium was added to each well to cover the scaffold. The well plate was then placed back in the incubator. Half of the medium was replaced every 2–3 days.
Determination of cell adhesion rate of scaffolds
At 1, 8, 16, and 24 h after seeding, the non-adhered cells on the scaffold surface were gently washed off with PBS. The scaffold-cell complex was then transferred to a new well plate to digest the cells adhered to the scaffold with 0.25% trypsin-EDTA. The surface and internal pores of the scaffold were pipettedup and down to ensure that all the adhered cells were detached. The number of cells in suspension was counted. The cell adhesion rate of scaffolds at each time point was calculated as follows: adhesion rate (%) = the number of digested cells/the total number of cells seeded × 100%. Three parallel experiments were performed for each group.
Determination of relative growth rate (RGR) of cells by CCK-8 assay and cytotoxicity classification
First, 96-well plates were inoculated with 100 µL of cell suspension per well containing the third passage rBMSCs at 5 × 104 cells/mL, and placed in an incubator. When the cell density at the bottom of the plate reached about 90% as seen under an inverted microscope, the medium was removed and replaced with the scaffold extract of each group at 100 µL/well. Three parallel wells were used for each group. The cultures in the scaffold extracts of each group were the experimental groups, and that in rBMSC complete medium was used as the control group. To reduce evaporation, PBS was added to the periphery of the wells to maintain moisture, and the plate was placed in the incubator near the water tray. After culturing for 24 h, the culture medium of each group was removed and replaced with freshly prepared mixed solution of culture medium and CCK-8 at a ratio of 10:1 at 100 µL/well. Triplicate wells containing only the mixed solution were used as the blank group. After the plate was incubated in the incubator for another 2 h, the optical density (OD) was measured at 450 nm using a microplate reader. The RGR was calculated as follows:
The cytotoxicity of scaffolds of each group was classified according to the national in vitro cytotoxicity classification (ISO 10993-5//GB/T16886. 5) (Table 2).
Determination of cell proliferation on scaffolds
Cells were seeded according to the method described in 2.4.4.The culture medium of each group was removed after 1, 3, and 5 days. The scaffold-cell complex was gently washed with PBS and transferred to a new 24-well plate containing 1 mL of freshly prepared mixed solution of medium and CCK-8 at a ratio of 10:1 per well. After the plate was incubated in the incubator for another 2 h, 100 µL of supernatant was pipetted from each well into a 96-well plate. Six parallel wells were used for each group. OD was measured at 450 nm using a microplate reader.
Statistical analysis
Data analysis was performed using SPSS 26.0. The experimental results are measurement data expressed as Data expressed as x ± s. The data of each group were firstly tested for normality and homogeneity of variance. If both were confirmed, one-way analysis of variance (ANOVA) was employed for comparison among groups, and the least significant difference (LSD)-t test for comparison between groups. Dunnett’s T3 test was used for comparison among groups when homogeneity of variance was not achieved; and Kruskal-Wallis H test was used when normality was not achieved. The test level was α = 0.05, and P < 0.05 was considered statistically significant. Some experimental results were plotted using GraphPad Prism 8.
Results
Fabrication and morphological characterization of scaffolds
All scaffold groups demonstrated consistent extrudability with: (1) Continuous filament deposition; (2) Zero delamination events; (3) 100% structural integrity retention: (4) Nozzle clogging frequency < 0.1%. They were finally formed into a 3D cuboid structure. The PLGA and PLGA/nHA scaffolds were milky white as a whole as seen with the naked eye under natural light. It became slightly whiter when nHA was added. The PLGA/nHA/GO scaffolds were grayish, which was related to the color of GO powder. In addition, the PLGA scaffolds had severe contraction and large overall structural deformation. The PLGA/nHA and PLGA/nHA/GO scaffolds had a regular shape, which was basically consistent with the design specifications. Because borderless printing and a raster angle of 0°/90° were used, there were interconnected square pores on the surface and interior of the scaffolds of all the three groups (Fig. 1).
Performance evaluation of scaffolds
Characterization of macroscopic pore size and porosity of scaffolds
Because the scaffold material was printed at low temperature by extrusion, and the material was continuously solidified, there was inevitably be a time delay. Moreover, the material itself was affected by gravity. Although there was essentially no effect on the approximately square shape of the pores of the scaffolds in the top view, measurements are needed to determine whether the side pores were compressed or not. Therefore, the pore size in the top view and the longitudinal size of the side pores in the front view of the scaffolds of each group were randomly measured (see Fig. 2a for some example images of each group). The macroscopic pore size of the scaffolds of the three groups are shown in Fig. 2b. The PLGA/nHA/GO group had the smallest pore size in the top view, followed by the PLGA/nHA group, and the PLGA group had the largest one, with significant differences among the groups (P < 0.05). There was no significant differences in the pore size among the groups in the front view (P > 0.05). The porosity of the scaffolds in the three groups is shown in Fig. 2c. There is no significant difference among the three groups (P > 0.05).
Microtopography of scaffolds under scanning electron microscopy
As revealed by scanning electron microscopy, the scaffolds of the three groups had a continuous network structure with clear lines and no breakage. The were interconnected square or rectangular holes inside the scaffolds. When observed at a higher magnification, microscopic pore structures were found on the surface of the scaffolds of all the three groups. Comparatively speaking, the PLGA scaffolds had an overall smoother surface, with micropores of a smaller size and at a shallower depth. The PLGA/nHA and PLGA/nHA/GO composite scaffolds had a rough surface with a large number of densely distributed micropores of different sizes ranging from 0 to 20 nm. Moreover, the composite scaffolds containing GO had an even rougher coral-like surface (Fig. 3).
Mechanical properties of scaffolds
The stress-strain curves of the scaffolds of the three groups are shown in Fig. 4. The compression moduli arepresented in Table 3. The PLGA scaffolds had the smallest compressive modulus (P < 0.05). The PLGA/nHA/GO scaffolds had a slightly smaller compressive modulus than the PLGA/nHA scaffolds but with no significant difference (P > 0.05). It was basically consistent with the mechanical properties of cancellous bone.
Water absorption of scaffolds within 12 h
The water absorption of the scaffolds of the three groups continued to increase in the first 6 h, and remained in an equilibrium state with a slow increase in the later 6-h period. At 3 h, there was no significant difference in the water absorption among the three groups (P > 0.05). However, the PLGA scaffolds had slightly higher water absorption (P > 0.05), which may be related to its larger macroscopic pore size. The water absorption of the scaffolds of the three groups increased the fastest at 3–6 h, with a faster increase in that of the PLGA/nHA and PLGA/nHA/GO composite scaffolds. The highest water absorption was measured in the PLGA/nHA/GO scaffolds within 12 h (P < 0.05) (Fig. 5).
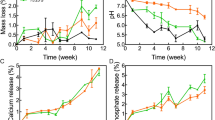
Mass loss rate of scaffolds during degradation
The mass loss of scaffolds increased with the degradation time in all the three groups. In the first 2 weeks of degradation, that is, at the initial stage of implantation, all the three groups of scaffolds had a small mass loss with no significant differences among groups (P > 0.05). At all time points from 4 weeks of degradation to the end of the degradation test, the mass loss of the pure PLGA scaffolds was much higher than those of the two groups of composite scaffolds (P < 0.05); and the mass loss of the PLGA/nHA/GO scaffolds was slightly but not significantly higher than that of the PLGA/nHA scaffolds (P > 0.05) (Fig. 6a).
Degradation rate of scaffolds
The degradation rates of scaffolds increased with the degradation time in all the three groups. In the first 2 weeks of degradation, that is, at the initial stage of implantation, all the three groups of scaffolds exhibited low degradation rates with no significant differences among groups (P > 0.05). At all time points from 6 weeks of degradation to the end of the degradation test, the degradation rate of the pure PLGA scaffolds was much higher than those of the two groups of composite scaffolds (P < 0.05); and the degradation rate of the PLGA/nHA/GO scaffolds was slightly higher than that of the PLGA/nHA scaffolds (P < 0.05) (Fig. 6b).
pH changes during scaffold degradation
The initial pH of the scaffold degradation solution was 7.4 and basically decreased with the degradation time in all the three groups. In the first 2 weeks of degradation, that is, at the initial stage of implantation, all the three groups exhibited no significant changes in pH (P > 0.05). From 4 weeks of degradation to the end of the degradation test, the pH of the pure PLGA group gradually decreased to weak acidity overtime, and was much lower than that of the two composite groups at all time points in this period (P < 0.05). The pH of the PLGA/nHA and PLGA/nHA/GO groups remained stable between 7.1 and 7.4 within the test period, which was similar to the pH of body fluid (Fig. 6c).
Biocompatibility of scaffolds
Cell adhesion rate of scaffolds
The cell adhesion rates of scaffolds increased with the seeding time in all the three groups. When the cells were seeded on the scaffolds for 1 h, the adhesion rate was low in all the three groups, without no significant difference among the groups (P > 0.05). At 8 h, the PLGA/nHA and PLGA/nHA/GO groups exhibited better early adhesion ability than the pure PLGA group (P < 0.05). After 8 h, the adhesion rates increased slowly in the three groups. The highest adhesion rate was observed in the PLGA/nHA/GO group (P < 0.05), and the lowest in the PLGA group (P < 0.05) (Fig. 7a).
Cytotoxicity of scaffolds
There were no significant differences in the RGR of cells in the scaffold extracts among the three groups at 24 h (P > 0.05). According to the cytotoxicity classification (Table 4), the cytotoxicity of the scaffold materials was grade 0 in all the three groups. It suggested that the scaffold materials in all groups had negligible cytotoxicity and high biosafety.
Cell proliferation on scaffolds
As the culture time increased, the cells continued to proliferate on the scaffolds in all the three groups. After 1 day of seeding, OD depended on the early adhesion of cells on the scaffolds. At all the subsequent time points, OD was much higher in the PLGA/nHA/GO group than in the other two groups (P < 0.05), and the lowest OD was measured in the PLGA group (P < 0.05) (Fig. 7b).
Discussion
Fabrication of scaffolds
The molecular weight, particle size, and ratio of materials may affect the properties of the scaffold. The regeneration and repair of bone tissue defects generally takes 3–6 months under physiological conditions. The degradation rate of the BTE scaffold should match the bone regeneration rate to achieve stress transfer. As noted above, the degradation rate of PLGA can be regulated by adjusting the LA/GA ratio and molecular weight. Middleton et al. found that the proportion of LA was proportional to the degradation time. The degradation time was approximately 1–2 months at a LA/GA ratio of 50/50, 4–5 months at 75/25, and 5–6 months at 85/153. Therefore, PLGA (75/25) was used as the matrix material in this study.
In addition, in terms of the ratio of materials, studies have shown that the addition of 20–30% nHA to PLGA improved the mechanical properties of the composite scaffolds. However, when the proportion of nHA increased to 40%, the materials could not be uniformly dispersed, which affected the microscopic morphology of the scaffold. Partial aggregation of materials also weakened the bonding between the organic and inorganic phases, and caused a significant decrease in the mechanical strength of the composite scaffolds4,5.
Based on the above data analysis, LA/GA = 75/25 and PLGA: nHA = 4:1 (w: w) were used in this study to provide suitable mechanical properties and degradation rate for BTE scaffolds. In addition, a small amount of GO was used as a reinforcing phase and to ensure biosafety.
According to our preliminary study, nHA: GO = 100:2 (w: w) provides the best mechanical properties and biocompatibility without compromising the structural integrity of the scaffold.
Performance of scaffolds
Macroscopic pore size and porosity of scaffolds
An ideal BTE scaffold should mimic the structure of natural bone. Interconnected pores and appropriate pore size and porosity result in a large SSA, which facilitates the adhesion and migration of cells and the transfer of nutrients and metabolic waste, and guides osteogenesis and angiogenesis. Macroscopic pore size and porosity are also closely related to the mechanical properties of the scaffold. Too large pore size and high porosity will reduce its mechanical strength. However, there is no consensus on the appropriate pore size of scaffolds. The pore size of cancellous bone is 10–400 μm. Too small pores will impede ingrowth of bone and blood vessels, but too large pores can impair the mechanical properties of the scaffold. It has been reported that scaffolds with a pore size greater than 200 μm have osteoconductive properties, whereas a pore size of 200–400 μm promotes bone regeneration and is most conducive to osteogenesis6. Guda et al. found that a pore size greater than 300 μm was suitable for the ingrowth of bone and blood vessels7. Mehdizadeh et al. demonstrated that a pore size of 275–400 μm promoted the ingrowth of blood vessels while maintaining the mechanical properties of the scaffold through computer simulation8. The pure PLGA scaffolds fabricated in this study had the largest pore size with large standard deviation and poor uniformity due to the characteristics of the material itself, and severe contraction of the overall scaffold and filament diameter. The pore size of PLGA/nHA and PLGA/nHA/GO composite scaffolds decreased with the addition of nHA and GO. In particular, the pore size of the PLGA/nHA/GO scaffolds in the top and front views is the most similar to previously reported values and had high uniformity. Cancellous bone is a highly porous environment with 50–90% porosity. In addition, Jeong et al. suggested that scaffolds with 70–75% porosity had the most favorable mechanical properties and biodegradability9. In this study, all the three groups of scaffolds achieved a porosity of more than 70%, which basically satisfies the porosity requirements of BTE scaffolds.
Microtopography of scaffolds
During bone regeneration using an implanted BTE scaffold, the most important is to retain bone cells on the surface of the scaffold to allow them to migrate through the interconnected pores, and finally proliferate and differentiate into bone. Therefore, the surface microtopography of the material is also very important. In this study, the surfaces of the three groups of scaffolds all had microscopic pore structures, which are similar to that of cancellous bone. Macroscopic and microscopic pores have a synergistic effect. The presence of nanoscale micropores yields a higher SSA, which facilitates cell adhesion and increases the adsorption area of biomacromolecules and drugs10,11. Scanning electron microscopy revealed that the PLGA scaffolds had an overall smooth surface with small, shallow, and foam-like micropores, exhibiting a dense surface structure and poor microtopography. In contrast, the PLGA/nHA and PLGA/nHA/GO composite scaffolds had a rough surface with densely distributed micropores of different sizes ranging from 0 to 20 nm. Moreover, the composite scaffolds containing GO had an even rougher coral-like surface, indicating a more favorable microtopography for promoting cell adhesion and proliferation.
Mechanical properties of scaffolds
BTE scaffolds should have acceptable mechanical properties to offer temporary mechanical support for the defect area, and provide space for bone ingrowth while maintaining local stability. The mechanical properties of scaffolds are also closely related to pore size and porosity. Too large pore size or high porosity will reduce its mechanical strength12. There is no standard mechanical properties for scaffolds due to the different mechanical properties of bones in different parts of the body. The compressive modulus is the ratio of normal stress to normal strain of an elastically deformed substance. The greater the compressive modulus, the higher the deformation resistance of the scaffold13. In this study, the lowest compression modulus was measured in the pure PLGA scaffolds. This is not only related to the properties of the material itself, but also to the large pores, thin pore walls, and high porosity. After adding nHA, the compressive modulus of the composite scaffolds was improved, which is consistent with previous findings4,5. The compression modulus of the PLGA/nHA/GO scaffolds decreased slightly compared with that of the PLGA/nHA scaffolds. This may be related to the destruction of the pore wall by the large number of micropores on the surface. Nevertheless, it essentially conforms to the mechanical properties of cancellous bone, and has a suitable initial mechanical strength. To sum up, both groups of composite scaffolds could effectively provide sufficient partial structural support for the bone defect.
Water absorption of scaffolds
The hydrophilicity of BTE scaffolds is directly proportional to early cell adhesion14. In addition, scaffolds containing micropores have a larger SSA, and interconnected pores facilitate cell adhesion15. However, too high water absorption will increase the degradation rate. As PLGA is a hydrophobic material, the water absorption of the pure PLGA scaffolds was relatively low within 24 h. After nHA with high hydrophilicity was added, the hydrophobicity was improved, which may be related to the interaction between the P-OH group on the surface of nHA and water16. The addition of GO, which has a large number of oxygen-containing functional groups, further improved the hydrophilicity of the scaffold. The PLGA/nHA/GO composite scaffolds had the highest water absorption within 24 h. The increased SSA of the composite also has a positive effect on the increase in water absorption.
Degradability of scaffolds
BTE scaffolds should also have suitable biodegradability. They should maintain structural stability during early implantation, and then be gradually degraded by body fluid to reduce stress shielding while providing space for bone regeneration17. Degradable scaffold materials avoid a second surgery for material removal. However, the degradation of the scaffold should match the bone regeneration rate. If the degradation is too fast, the bone has not fully regenerated to replace the scaffold, which will result in the early loss of mechanical support for the defect area. However, too slow degradation will hamper the ingrowth and integration of new bone and blood vessels, leading to prolonged recovery time18. In addition, the biosafety of degradation products should also be taken into account. In this study, the body fluid environment after the scaffold was placed was simulated in vitro to investigate the changes in the mass and pH of the scaffold in each group during degradation.
Mass changes in scaffolds during degradation
The mass loss and degradation rate of scaffolds increased with the degradation time in all the three groups. During early implantation, the two groups of composite scaffolds had small mass loss and low degradation rate, thereby providing structural stability. Overtime, the mass loss and degradation rate of almost all the scaffolds in the pure PLGA group were much higher than those of the two composite groups, which may lead to early collapse of the scaffolds and fail to meet the requirements of BTE applications. The experimental results showed that the addition of nHA slowed down the degradation of the scaffold to match the bone regeneration rate. After adding GO, the mass loss and degradation rate of the composite scaffolds increased, which may be related to a large SSA and a large contact area with the degradation solution. In addition, the water absorption of the material also affects the degradation of the scaffold.
pH changes during scaffold degradation
Some by-products will be separated from the main body of the scaffold during degradation. These by-products may have adverse effects on the human body by changing the pH of the microenvironment. Therefore, BTE scaffolds should maintain appropriate pH during degradation. The experimental results showed that the pH of the pure PLGA scaffolds decreased rapidly with time to reach weak acidity levels within the observation time. Although the degradation products of PLGA can be excreted by metabolism, the acidic substances produced during degradation may stimulate local aseptic inflammation. Moreover, the acidic substances will catalyze the hydrolysis of the polymer, accelerate the degradation of PLGA, and thus may cause the early collapse of the scaffold19. The pH of the two groups of scaffolds containing nHA was mild throughout the degradation, which is similar to the pH of the human microenvironment and more favorable for cell proliferation and viability. It indicates that the weak basicity of nHA buffered the acidic by-products of PLGA degradation. The oxygen-containing functional groups of GO can interact with water to produce acidic substances20. However, the addition of a small amount of GO did not significantly affect the pH change during degradation. The slight decrease in pH maybe related to fast degradation. Nevertheless, given the constant acidic degradation of PLGA as a matrix material, further experiment is needed to examine the pH changes at later time points.
Biocompatibility of scaffolds
Biocompatibility is a prerequisite for the implantation of BTE scaffolds. It is the basis for distinguishing them from other materials. Non-biocompatible materials can cause immune rejection and other safety concerns21. At present, in vitro cell experiments are commonly used to evaluate the in vitro biocompatibility of BTE scaffolds22. BMSCs are highly proliferative and have multi-lineage differentiation potential. They are widely used as the precursor cells of osteoblasts and are ideal seed cells in BTE23. In addition, consideration should be given to providing an experimental basis foundation for subsequent research on BTE scaffolds. Experiments in large animals have a high cost and require a long time for bone repair. By contrast, experiments in rodents are more cost-effective. However, due to their primitive bone structure, bone repair in rodents is different from that in humans. Rabbits have a similar bone structure to humans, and are the most commonly used animal for the modeling of bone defects. Therefore, rBMSCs were used for co-culture to evaluate the in vitro cytocompatibility of scaffolds so as to provide an experimental basis for subsequent research on scaffold osteogenesis.
Cell adhesion rate of scaffolds
An ideal bone material should facilitate cell adhesion. Cell adhesion to the scaffold is aprerequisite for subsequent cell proliferation and differentiation, and final bone regeneration. Cell adhesion rate indirectly reflects the biocompatibility of scaffolds24. In addition to the possible interaction between the scaffold material and the cells, the cell adhesion rate is closely related to the scaffold structure. An interconnected porous structure and rough surface facilitate cell adhesion and prevent cell detachment25. In addition, the hydrophilicity of the scaffold promotes early cell adhesion. In this study, the addition of nHA led to an improvement of early adhesion, and the addition of GO led to a further improvement. It demonstrates that the excellent microstructure and hydrophilicity of the scaffold promote cell adhesion, and facilitate subsequent proliferation and differentiation.
Cytotoxicity of scaffolds
The biosafety of PLGA and nHA in BTE has been widely recognized. However, scaffold material combination may involve the incorporation or release of cytotoxic substances. Moreover, the biosafety of GO is controversial. Therefore, it is necessary to assess the cytotoxicity of the scaffold material. Biosafety of the fabricated scaffold is a prerequisite for its application to biomedicine26. The cytotoxicity test showed that there was no significant difference in the RGR of cells in scaffold extracts among the three groups. All the three groups showed grade 0 of cytotoxicity. It suggests that the scaffold materials in all groups had negligible cytotoxicity to rBMSCs and high biosafety.
Cell proliferation on scaffolds
An ideal biological scaffold material should promote the proliferation of seeded cells. Carly cell proliferation on scaffolds is firstly related to adhesion. An interconnected porous structure also provides space for cell migration and nutrient transport. Low proliferation was observed in the PLGA group during the observation time. The addition of nHA increased the surface roughness of the scaffold, which in turn increased the contact area between the nanoscale surface and the cells, thereby promoting cell adhesion and proliferation; the calcium and phosphorus released by nHA also promoted cell proliferation27. Cell proliferation on the scaffolds was further enhanced with the addition of GO. In addition to the above reasons, it may also be related to the antioxidant and anti-apoptotic activities of GO28.
Limitations and directions for further research
When evaluating the mechanical properties of BTE scaffolds, most studies ignore the difference between in vitro and in vivo mechanical strength. Future research can be pursued in this direction. When evaluating degradability, this study only investigated the mass and pH changes of the scaffolds in simulated buffer solution in vitro. However, the degradation solution did not contain certain enzymes in body fluids that may affect the degradation of the scaffolds. In vitro degradation experiments cannot completely replace in vivo degradation kinetics. Therefore, in vivo experiments are needed to further verify the degradability of the scaffolds29.
Conclusions
In this study, with appropriate proportions of PLGA, nHA, and GO as raw materials, novel PLGA, PLGA/nHA, and PLGA/nHA/GO 3D porous scaffolds with stable morphology and both micro- and nano-pores were fabricated using low-temperature 3D printing combined with freeze-drying by adjusting the printing parameters.
The performance of the three groups of scaffolds was evaluated in experiments. The PLGA/nHA/GO composite scaffold was shown to have more favorable pore size and microtopography, suitable initial mechanical strength, excellent water absorption, appropriate degradability, and good in vitro cytocompatibility, which better satisfy the requirements of BTE scaffolds. It has the potential to be developed as a BTE scaffold material.
Data availability
Although the data can be provided upon reasonable request, it is more prudent not to publicly release all raw data considering privacy and data security. If there is a reasonable need, please contact the corresponding author. Xiaohe Li (798242742@qq.com).
References
Crane, G. M., Ishaug, S. L. & Mikos, A. G. (Nature Publishing Group US New York, (1995).
Dubey, S. K. et al. Uncovering the diversification of tissue engineering on the emergent areas of stem cells, nanotechnology and biomaterials. Curr. Stem Cell Res. Therapy. 15, 187–201 (2020).
Middleton, J. C. & Tipton, A. J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 21, 2335–2346 (2000).
Wu, X. et al. Preparation of mesoporous nano-hydroxyapatite using a surfactant template method for protein delivery. J. Bionic Eng. 9, 224–233 (2012).
Xie, X. H. et al. Structural and degradation characteristics of an innovative porous PLGA/TCP scaffold incorporated with bioactive molecular Icaritin. Biomed. Mater. 5, 054109 (2010).
Vinatier, C., Mrugala, D., Jorgensen, C., Guicheux, J. & Noël, D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 27, 307–314 (2009).
Guda, T. et al. Hydroxyapatite scaffold pore architecture effects in large bone defects in vivo. J. Biomater. Appl. 28, 1016–1027 (2014).
Mehdizadeh, H., Sumo, S., Bayrak, E. S., Brey, E. M. & Cinar, A. Three-dimensional modeling of angiogenesis in porous biomaterial scaffolds. Biomaterials 34, 2875–2887 (2013).
Jeong, J. C., Lee, J. & Cho, K. Effects of crystalline microstructure on drug release behavior of Poly (ε-caprolactone) microspheres. J. Controlled Release. 92, 249–258 (2003).
Dai, C. et al. Three-dimensional high-porosity Chitosan/honeycomb porous carbon/hydroxyapatite scaffold with enhanced osteoinductivity for bone regeneration. ACS Biomaterials Sci. Eng. 6, 575–586 (2019).
Chen, G. & Kawazoe, N. Porous scaffolds for regeneration of cartilage, bone and osteochondral tissue. Osteochondral Tissue Engineering: Nanatechnol. Scaffolding-Related Developments Translation, 171–191 (2018).
González, S. G., Vlad, M. D., López, J. L. & Aguado, E. F. Novel bio-inspired 3D porous scaffold intended for bone-tissue engineering: design and in Silico characterisation of histomorphometric, mechanical and mass-transport properties. Mater. Design. 225, 111467 (2023).
Koh, I., López, A., Helgason, B. & Ferguson, S. J. The compressive modulus and strength of saturated calcium sulphate dihydrate cements: implications for testing standards. J. Mech. Behav. Biomed. Mater. 34, 187–198 (2014).
Chernozem, R. et al. Piezoelectric 3-D fibrous Poly (3-hydroxybutyrate)-based scaffolds ultrasound-mineralized with calcium carbonate for bone tissue engineering: inorganic phase formation, osteoblast cell adhesion, and proliferation. ACS Appl. Mater. Interfaces. 11, 19522–19533 (2019).
Zhang, S. et al. 3D-Printed polyurethane Tissue‐Engineering scaffold with hierarchical microcellular foam structure and antibacterial properties. Adv. Eng. Mater. 24, 2101134 (2022).
Huang, Y., Ren, J., Chen, C., Ren, T. & Zhou, X. Preparation and properties of Poly (lactide-co-glycolide)(PLGA)/nano-hydroxyapatite (NHA) scaffolds by thermally induced phase separation and rabbit MSCs culture on scaffolds. J. Biomater. Appl. 22, 409–432 (2008).
Sharma, S. et al. Critical review of biodegradable and bioactive polymer composites for bone tissue engineering and drug delivery applications. Polymers 13, 2623 (2021).
Srinath, P., Abdul Azeem, P. & Venugopal Reddy, K. Review on calcium silicate-based bioceramics in bone tissue engineering. Int. J. Appl. Ceram. Technol. 17, 2450–2464 (2020).
Ma, Z. et al. Performance of 3D printed PCL/PLGA/HA biological bone tissue engineering scaffold. Polym. Compos. 42, 3593–3602 (2021).
Dimiev, A. M., Alemany, L. B. & Tour, J. M. Graphene oxide. Origin of acidity, its instability in water, and a new dynamic structural model. ACS Nano. 7, 576–588 (2013).
Swetha, S., Lavanya, K., Sruthi, R. & Selvamurugan, N. An insight into cell-laden 3D-printed constructs for bone tissue engineering. J. Mater. Chem. B. 8, 9836–9862 (2020).
N’gatta, K. M. et al. 3D printing of cellulose nanocrystals based composites to build robust biomimetic scaffolds for bone tissue engineering. Sci. Rep. 12, 21244 (2022).
Stamnitz, S. & Klimczak, A. Mesenchymal stem cells, bioactive factors, and scaffolds in bone repair: from research perspectives to clinical practice. Cells 10, 1925 (2021).
Khan, M. U. A. et al. Bioactive scaffold (sodium alginate)-g-(nHAp@ SiO2@ GO) for bone tissue engineering. Int. J. Biol. Macromol. 222, 462–472 (2022).
Han, J. et al. Surface roughness and biocompatibility of Polycaprolactone bone scaffolds: an energy-density-guided parameter optimization for selective laser sintering. Front. Bioeng. Biotechnol. 10, 888267 (2022).
Li, Y., Tan, Z., Zhang, J., Mu, J. & Wu, H. Physical and chemical properties, biosafety evaluation, and effects of nano natural deer bone meal on bone marrow mesenchymal stem cells. Front. Bioeng. Biotechnol. 10, 891765 (2022).
Shi, J. et al. Frontiers of hydroxyapatite composites in bionic bone tissue engineering. Materials 15, 8475 (2022).
Abdelhalim, A. O. et al. Graphene oxide enriched with oxygen-containing groups: on the way to an increase of antioxidant activity and biocompatibility. Colloids Surf., B. 210, 112232 (2022).
Oh, S. H., Kang, S. G. & Lee, J. H. Degradation behavior of hydrophilized PLGA scaffolds prepared by melt-molding particulate-leaching method: comparison with control hydrophobic one. J. Mater. Science: Mater. Med. 17, 131–137 (2006).
Funding
This research was funded by “Grassland Talent” project for youth innovation and entrepreneurship talent in Inner Mongolia Autonomous Region (2020), Project Leader: Xiaohe Li; Science research project of Inner Mongolia Autonomous Region Mongolian Medicine and Pharmaceutical Collaborative Innovation Center in 2021, Project Leader: Xiaohe Li; Inner Mongolia Medical University 2021 annual school-level research key project (YKD2021ZD001); Project Leader: Xiaohe Li; Inner Mongolia Higher Education Innovation Team Development Program (NMGIRT2227), Project Leader: Xiaohe Li; Innovation Team Development Plan for Higher Education Institutions in Inner Mongolia Autonomous Region(NMGIRT2419), Project Leader: Haiyan Wang, Inner Mongolia Autonomous Region key research & development & achievement transformation plan (2023 Science and Technology to support the Yellow River Basin ecological protection and high-quality development) project(2023YFHH0003), Project Leader: Xiaohe Li; Yong Scientific and Technological Talent Program of Institutes of Higher Education of Inner Mongolia Education Department (NJYT24031), Project Leader: Enhejirigala; Doctoral initiation program of Inner Mongolia Medical University (YKD2023BSQD012), Project Leader: Enhejirigala; Research Project of InnerMongolia Medical University (YKD2024MS006), Project Leader: Enhejirigala ; The central government guides local funds for scientific and technological development (2024SZY0127), Project Leader:Enhejirigala.
Author information
Authors and Affiliations
Contributions
L.T. wrote the manuscript.G.S. was responsible for data aggregation and statistical analyses. H.W., E.J. and X.L. provided directions for writing and submitting the manuscript. Z.Q.,K.Z.,L.B.,Y.L. and L.S. were responsible for data key point labeling.Q.L.,Y.Z.,Y.M.,J.L.,X.W. and Y.F. were responsible for the collection, aggregation, and data analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Biomedical Ethics Committee of Inner Mongolia Medical University (approval number: YKD202405069).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tong, L., Shi, G., Liu, Q. et al. Fabrication and evaluation of 3D printed PLGA/nHA/GO scaffold for bone tissue engineering. Sci Rep 15, 12446 (2025). https://doi.org/10.1038/s41598-025-96099-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96099-z