Abstract
Despite numerous studies on second-line therapies in metastatic pancreatic cancer, there is no randomized study evaluating the efficacy of gemcitabine plus nab-paclitaxel as a second-line treatment. This study aims to examine the efficacy of gemcitabine plus nab-paclitaxel in second-line therapy. In this retrospective study, a total of 218 patients from 23 centers were included. The primary endpoint was progression-free survival (PFS), secondary endpoints included overall survival (OS), treatment efficacy based on ECOG performance status (PS), and tumor marker (CEA, CA 19 − 9) levels. In the second-line treatment with gemcitabine plus nab-paclitaxel, the median PFS was 5.1 months (95% CI, 5.6 to 7.1), and the median OS was 8.6 months (95% CI, 7.3 to 10.0). Median PFS was 6.6 months in patients with normal CEA levels compared to 4.4 months in patients with high CEA levels (P = 0.01). Median PFS was 6 months in patients with ECOG PS 0–1 compared to 3.8 months in patients with PS 2 (P < 0.01). This study demonstrates the contribution of gemcitabine plus nab-paclitaxel in both OS and PFS in second-line treatment of metastatic pancreatic cancer. It was found to be a good option especially for young patients with good ECOG PS.
Similar content being viewed by others
Introduction
Pancreatic cancer represents the 12th most common type of cancer worldwide, with 511,000 new cases annually. Its mortality rate is nearly equal to its incidence rate, which positions it as the sixth leading cause of cancer-related death1. Unfortunately, the prognosis is quite poor, with a five-year survival rate of 13% for all stages, which drops below 5% at the metastatic stage1.
Surgical resection represents the sole curative option for pancreatic cancer. However, 85% of patients are not eligible for resection at the time of diagnosis2. In cases of pancreatic cancer for which local treatment is not a suitable option, multiple-agent cytotoxic chemotherapy drugs are typically employed. Initially, the median survival with single-agent gemcitabine or fluoropyrimidine drugs was approximately six months. However, with multi-agent combinations, the median OS can approach one year3,4. The selection of these agents depends on various factors, primarily performance status. International guidelines recommend FOLFIRINOX (a combination of folinic acid, 5-fluorouracil [5-FU], irinotecan, and oxaliplatin), gemcitabine plus nab-paclitaxel, or Liposomal irinotecan + 5-FU + leucovorin + oxaliplatin (NALIRIFOX) regimens for first-line treatment in patients with good performance status who can tolerate multi-agent chemotherapy5.
A number of studies have been conducted to compare these therapies in first-line settings. In the JCOG1611, GENERATE study, the median overall survival was 17.1 months in the Nab-paclitaxel plus gemcitabine arm, 14.0 months in the modified FOLFIRINOX arm and 13.6 months in the S-IROX (S-1, irinotecan and oxaliplatin) arm, with the longest value observed in the Nab-paclitaxel plus gemcitabine arm6. In the NAPOLI-3 study, the overall survival rates were 11.1 months with NALIRIFOX (liposomal irinotecan, oxaliplatin, leucovorin, fluorouracil) and 9.2 months with nab-paclitaxel-gemcitabine7.
The combination therapies of FOLFIRINOX and Nab-paclitaxel plus gemcitabine have been demonstrated in clinical trials to yield superior survival outcomes compared to single-agent gemcitabine3,4. Despite their superiority over gemcitabine, the majority of patients with pancreatic cancer experience disease progression due to the aggressive nature of the disease. Post-progression treatment options are more limited, with single-agent gemcitabine or 5-FU-based drugs or combination therapies for patients with a favorable performance status being utilized. In rare cases, targeted agents may be employed for specific genetic alterations (RET, TRK fusion, MSI, BRCA, etc.)5.
Although gemcitabine plus nab-paclitaxel has been demonstrated to be more efficacious than gemcitabine monotherapy in first-line treatment, there is currently no randomized study evaluating its efficacy as a second-line treatment. Prospective single-arm studies comprising a limited number of patients have reported overall response rates (ORR) of 10–20% and OS ranging from 7.6 to 8.9 months with gemcitabine plus nab-paclitaxel in second-line treatment following FOLFIRINOX failure8,9. With single-agent gemcitabine in this stage, ORR is around 10%, and median OS is below 6 months10. Although patient selection bias cannot be ignored, indirect comparisons suggest that gemcitabine plus nab-paclitaxel is numerically better.
The primary objective of this study is to evaluate the efficacy of gemcitabine plus nab-paclitaxel as a second-line treatment for metastatic pancreatic cancer in patients who have progressed on FOLFIRINOX. Secondarily, the tolerability, frequency of side effects, and which patient groups the treatment is more effective for will be investigated.
Results
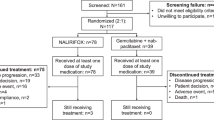
A total of 218 patients were included in this study between December 2014 and April 2023. Of these, 134 (61.5%) were male and 84 (38.5%) were female, resulting in a male-to-female ratio of 1.6. The flow chart illustrating the study population, including the criteria for patient inclusion and exclusion, is presented in Fig. 1. The median follow-up period was 16.9 months, with a range of 5.1 to 62.6 months. The median age at diagnosis was 60 years (range, 33.8 to 76.6 years). At the time of diagnosis, 171 patients (78.4%) were in the metastatic stage. By the cut-off date, 184 patients (84.4%) had died, and 34 (15.6%) were still alive. The general demographic characteristics of the patients during first-line treatment are provided in Table 1.
In the metastatic stage, the median PFS with first-line treatment was 6.8 months (95% confidence interval [CI], 6.1 to 7.4), and the median OS was 17.9 months (95% CI, 16.2 to 19.6).
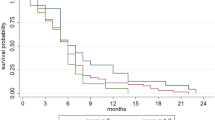
In the second-line treatment, patients received a median of 6 cycles (range, 1–36 cycles) of gemcitabine plus nab-paclitaxel. The median follow-up time after second-line treatment was 6 (1–36) months. The general characteristics and treatment responses of patients during second-line therapy are shown in Table 2. The median PFS for patients receiving gemcitabine plus nab-paclitaxel was 5.1 months (95% CI, 5.6 to 7.1). Response rates and survival rates with gemcitabine plus nab paclitaxel treatment are shown in Table 3. In second-line treatment, 34 patients (15.6%) were still alive, with a median OS of 8.6 months (95% CI, 7.3 to 10.0). When comparing PFS based on response status at 3 months, patients with partial response had a median PFS of 9.7 months (95% CI, 8.3 to 10.1), and those with stable disease had a median PFS of 7.3 months (95% CI, 6.5 to 8.1), which was statistically significant (P:0.01) (Fig. 2).
The relationship between tumor marker levels (CEA and CA 19 − 9) and survival was analyzed by comparing high and normal levels before the first cycle of gemcitabine plus nab-paclitaxel. Patients with normal CEA levels (less than 5 ng/ml) exhibited a median PFS of 6.6 months (95% CI 5.3 to 7.9), whereas those with elevated CEA levels (equal to or greater than 5 ng/ml) demonstrated a median PFS of 4.4 months (95% CI 3.9 to 4.8). This difference was statistically significant (P = 0.01) (Fig. 3). With regard to overall survival (OS), patients with normal CEA levels exhibited a median OS of 12.1 months (95% CI 9 to 15.2), in comparison to 7.9 months (95% CI 6.8 to 9) for those with elevated CEA levels. This difference was statistically significant (P < 0.001) (Fig. 4). No significant differences were observed in PFS and OS between patients with normal and elevated CA 19 − 9 levels.
At the commencement of gemcitabine plus nab-paclitaxel treatment, 68.3% of patients (n = 149) exhibited an ECOG PS of 0 or 1, while 31.7% (n = 69) demonstrated an ECOG PS of 2. In the survival analysis, patients with a superior ECOG PS (0 or 1) The median PFS was 6 months (95% CI, 5.1 to 7) for patients with a performance status (PS) of 0 or 1, compared to 3.8 months (95% CI 3 to 4.6) for those with PS 2, which was statistically significant (P < 0.01) (Fig. 5). With regard to OS, patients with an ECOG PS of 0–1 exhibited a median OS of 10.2 months (95% CI, 8.1 to 12.4), in comparison to 6.8 months (95% CI 7.3 to 8.2) for those with an ECOG PS of 2. This difference was statistically significant (P < 0.01) (Fig. 6).
In the univariate analysis, the survival rates were found to decline in a linear fashion as CEA levels increased (hazard ratio [HR] = 1.02 95% CI: 1.01–1.03]; P < 0.001). In the categorical classification according to gender, ECOG PS, and metastatic status at diagnosis, patients with good ECOG PS and no metastatic disease at diagnosis exhibited superior survival outcomes. The details of the univariate and multivariate analyses are provided in Table 4. A stratified univariate analysis according to high and low CEA and CA 19 − 9 levels revealed that patients with low CEA levels exhibited superior survival outcomes compared to those with high CEA levels (HR = 0.65 [95% CI: 0.48–0.89]; P = 0.007). No statistically significant difference was observed in CA 19 − 9 levels (P = 0.2). The results of the categorical univariate analysis according to high and low CEA and CA 19 − 9 levels are presented in Table 5.
In patients receiving gemcitabine plus nab-paclitaxel treatment, the incidence of neutropenia was observed in 28.9% (n = 63), anemia in 19.7% (n = 43), and thrombocytopenia in 20.1% (n = 44). A dose reduction due to toxicity was performed in 63 patients (28.9%), and treatment was discontinued in 20 patients (9.2%) due to toxicity. No deaths were attributable to gemcitabine plus nab-paclitaxel treatment. The incidence of treatment-related adverse events is presented in Table 6. During the course of treatment, granulocyte colony-stimulating factor (G-CSF) support was provided to 38.1% of patients (n = 83).
Discussion
The results of this retrospective multicenter observational study, which analyzed data from 218 patients who received gemcitabine plus nab-paclitaxel as second-line treatment following FOLFIRINOX progression, indicated an ORR of 23.9%, a PFS of 5.1 months, and an OS of 8.6 months. Notwithstanding the paucity of treatment options for this disease and the absence of optimal sequential data, our findings lend support to the use of gemcitabine plus nab-paclitaxel in second-line treatment for patients who have progressed on FOLFIRINOX and a favorable performance status.
Despite the investigation of second-line treatments, including liposomal irinotecan and FOLFOX, which have demonstrated the potential to enhance survival outcomes, there remains a paucity of definitive recommendations regarding the optimal second-line treatment for metastatic pancreatic cancer11,12. The combination of gemcitabine plus nab-paclitaxel as a second-line treatment has yet to be approved or included in clinical guidelines. The lack of consensus on second-line treatment recommendations and the unmet needs of many patients have prompted the need for further investigation. The objective of this study was to evaluate the efficacy and tolerability of gemcitabine plus nab-paclitaxel in second-line treatment for patients who have progressed on first-line therapy.
In the PRODIGE 4 study, which evaluated the efficacy of FOLFIRINOX in first-line treatment, the median PFS was 6.4 months, and OS was 11.1 months4. In our study, patients who received FOLFIRINOX in first-line treatment had a median PFS of 6.8 months and a median OS of 17.9 months. According to a meta-analysis by Nichetti et al., NALIRIFOX, FOLFIRINOX, Gemcitabine plus Nab-Paclitaxel studies were examined in first-line treatment13. According to this meta-analysis, overall survival was 10.4–11.7 months. In our study, we found that the PFS value was compatible with the literature, while the OS value was longer than that observed in previous studies. The longer OS data may be attributed to several factors, including the better ECOG PS of our patients, their younger age compared to previous studies, the use of an effective combination such as gemcitabine plus nab-paclitaxel in the second line after FOLFIRINOX treatment in the first line, and the administration of a third line treatment to almost half of the patients.
The primary objective of this study was to evaluate the efficacy of gemcitabine plus nab-paclitaxel in second-line treatment. The results demonstrated a median PFS of 5.1 months. In the largest randomized study of gemcitabine plus nab-paclitaxel in first-line treatment of metastatic pancreatic cancer, the MPACT study, the PFS was 5.5 months, and OS was 8.5 months3. Despite being a second-line treatment in our study, the PFS was 5.1 months and OS was 8.6 months, comparable to the MPACT study. In studies in which the gemcitabine plus nab-paclitaxel combination was used in second-line treatment, PFS was found to be between 3.5 and 5.7 months, and OS between 7.1 and 18 months. These findings indicate that the use of this combination in second-line treatment resulted in shorter survival times than first-line use8,13,14,15. Our study aligns with the literature regarding both PFS and OS.
The PRODIGE65 study, which examined the efficacy of gemcitabine plus paclitaxel in second-line treatment for pancreatic cancer, randomly assigned patients who had progressed after FOLFIRINOX to receive gemcitabine plus paclitaxel in one arm and gemcitabine in the other arm. Although there was no statistically significant difference in OS between the gemcitabine plus paclitaxel arm (6.4 months and 5 months, respectively), there was a statistically significant difference in PFS of 3.1 months and 2 months, respectively16. In our study, the PFS in patients receiving gemcitabine plus nab-paclitaxel was 5.1 months, which was superior to the PFS data observed in patients receiving gemcitabine plus paclitaxel in the PRODIGE 65 study.
Although there is no randomized study on gemcitabine plus nab-paclitaxel in second-line treatment, several small studies have shown this combination to be more effective than gemcitabine monotherapy, providing a partial survival benefit10,14,17,18,19. In a literature review, we found qualitative similarities between our study and the one conducted by Zaibet et al., which compared the efficacy of gemcitabine plus nab-paclitaxel with gemcitabine monotherapy in patients who progressed on FOLFIRINOX. According to this study, combination therapy was superior to monotherapy in terms of both overall and progression-free survival14. Our study reported a higher ORR of 23.9%, compared to 12% in the Zaibet et al. study.
In a phase 2 study, Huffman et al. evaluated the efficacy of NPC-1 by adding NPC-1 antibody to gemcitabine plus nab paclitaxel in second-line treatment of patients who progressed on FOLFIRINOX. In this study, both overall survival and progression-free survival were better in the NPC-1 arm, but there was no statistical difference20. A study with a methodological approach analogous to our own was conducted, in which the efficacy of Gemcitabine plus nab paclitaxel treatment in the second line in patients who progressed after first-line FOLFIRINOX treatment was measured. The study included 108 patients, and the results showed a progression-free survival (PFS) of 4.6 months and an overall survival (OS) of 9.8 months21. Our study demonstrated comparable outcomes in terms of both OS and PFS.
Numerous studies have shown that patients with good ECOG PS have better overall and progression-free survival than those with poor PS1,19,22. In our study, Cox regression analysis indicated that patients with ECOG PS 0–1 had significantly longer PFS and OS than those with PS 2, consistent with the literature.
Patients with high levels of CEA and CA 19 − 9 at the start of gemcitabine plus nab-paclitaxel treatment had worse PFS and OS. The MPACT study also found that patients with high, non-decreasing CA 19 − 9 levels had worse survival3,14. Other studies have similarly linked high CA 19 − 9 levels with poor prognosis14,23. Our findings also indicated that elevated tumor marker levels were associated with diminished survival, which corroborates the results of previous studies. In this regard, we postulate that high tumor marker levels are associated with poor survival and possess prognostic significance.
When our study was analyzed in terms of side effects and safety, neutropenia was the most common side effect observed in 28.9% patients with grade 3 and above. When previous studies were analyzed, neutropenia rates were found to be between 13 and 38% and our study was similar to the literature3,23,24. The frequencies of grade 3–4 anemia and thrombocytopenia were also similar to those reported in the literature3,14.
Neuropathy was a major concern since all patients had received FOLFIRINOX in first-line treatment. Neuropathy rates of 4% for taxane regimens and 0-7.5% for oxaliplatin are known12,19,25,26,27. In our study, 13.8% of patients experienced grade 2–3 neuropathy, likely due to the sequential administration of oxaliplatin and taxane.
Although our study is retrospective in nature, its multicenter design and use of real-world data render it a valuable contribution to the existing literature on this topic. A review of the existing literature revealed a dearth of studies examining the combination of gemcitabine and nab-paclitaxel in the second-line treatment of metastatic pancreatic cancer. Consequently, we posit that our study, which demonstrated both PFS and OS benefits in 218 patients from 23 centers, will contribute to the existing literature on this topic.
It is possible to mention some limitations of this study. The primary limitations of our study include the lack of randomization due to its retrospective nature and the absence of a control group receiving an alternative treatment. Additionally, patients who were deceased or had a poor performance status (PS) following first-line treatment were not included, and the metastasis sites and number of metastases were not specified.
Conclusion
In this study, we evaluated the efficacy of the gemcitabine plus nab-paclitaxel combination in second-line treatment for metastatic pancreatic cancer following FOLFIRINOX progression. Our findings revealed a PFS of 5.1 months and an OS of 8.6 months. The majority of observed adverse effects were manageable. Our findings indicate that the gemcitabine plus nab-paclitaxel combination is both efficacious and tolerable, rendering it a promising option, particularly for younger patients with a favorable ECOG PS. We propose that this study should be supported by prospective investigations.
Methods
This study is a retrospective analysis of data from 23 cancer diagnosis and treatment centers in various provinces in Turkey. In this multicenter study, patients with a histologically confirmed diagnosis of pancreatic adenocarcinoma, diagnosed between December 2014 and April 2023, were screened. Subsequently, a total of 218 patients who received gemcitabine in combination with nab-paclitaxel as second-line therapy following the progression of FOLFIRINOX during the metastatic phase were included in the study. Patients aged 18 or over with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2 were included in the study. Patients with a secondary malignancy, ECOG PS 3–4, or those who received nab-paclitaxel in treatments other than second-line were excluded.
Chemotherapy regimens
For the Gem-Nab group, treatment consisted of a 60-min infusion of Nab-paclitaxel at a dose of 125 mg/m² followed by a 30-min infusion of Gem at a dose of 1000 mg/m² on days 1, 8 and 15 every 4 weeks, as previously reported3. Treatment was delivered until either disease progression or unacceptable treatment toxicity was reached.
Metastasis confirmation was done via computed tomography (CT) or positron emission tomography (PET-CT). Response evaluation was performed every 8–12 weeks based on the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria28. PFS was calculated from the start of chemotherapy to radiological progression, clinical progression, or death from any cause. OS was calculated from the start of chemotherapy to death.
Hemogram and biochemical blood tests were examined before each treatment cycle. Chemotherapy-related toxicities were evaluated in line with the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) V4.03 and managed by dose reduction of one or both drugs at the discretion of the physician.
In the study, tumor marker levels were measured before starting gemcitabine plus nab-paclitaxel, and survival relationships were analyzed in patients with high and normal levels. The upper limit for carcinoembryonic antigen (CEA) was 5 ng/ml, and for carbohydrate antigen 19 − 9 (CA 19 − 9) was 37 U/ml. The relationship between OS and PFS was examined in patients with good versus poor ECOG PS (ECOG PS 0–1 vs. PS 2).
Statistical analysis
All analyses were conducted using IBM SPSS Statistics 25.0 The normality of the numerical data was assessed using the Kolmogorov-Smirnov test. Median values with minimum and maximum values were given for non-normally distributed data. Kaplan–Meier calculations with the log-rank test were used for the analysis of OS and PFS. Univariate and multivariate analyses were performed using a Cox proportional-hazard model. A comprehensive review of the extant literature was conducted to identify factors that may influence survival in patients with pancreatic cancer. Subsequently, univariate analysis was performed on the identified factors. Variables that demonstrated significance in the univariate analysis were selected for further investigation. Multivariate analysis was then performed, incorporating the selected variables to elucidate their relationship and potential interactions. Hazard ratios (HR) and 95% confidence intervals (CI) were reported. Overall, p-values ≤ 0.05 were considered statistically significant.
Conference presentation
This study has been accepted as a poster at European Society for Medical Oncology 2024 (ESMO 2024) congress and will be presented with the number FPN 1523.
Data availability
The datasets generated during and / or analysed during the current study are available from the corresponding author on reasonable request.
Change history
06 November 2025
The original online version of this Article was revised: In the original version of this Article Nilüfer Avcı, İlkay Gültürk, Semiha Urvay, Mustafa Özgür Arıcı, Mehmet Emin Kalender and Mustafa Yıldırım were incorrectly affiliated with Affiliation 1. Their correct Affiliations are: Nilüfer Avcı - Department of Medical Oncology, Medicana Private Hospital, Bursa, Turkey; İlkay Gültürk - İstanbul Regional Training Research Hospital Oncology Department, İstanbul, Turkey; Semiha Urvay - Department of Medical Oncology, Kayseri Acibadem Private Hospital, Kayseri, Turkey; Mustafa Özgür Arıcı - Antalya Regional Training Research Hospital Oncology Department, Antalya, Turkey; Mehmet Emin Kalender - Department of Medical Oncology, Maddem private Hospital, Isparta, Turkey; Mustafa Yıldırım - Department of Medical Oncology, Faculty of Medicine, Sanko University, Gaziantep, Turkey. The original Article has been corrected.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 74, 229–263 (2024).
Arnold, M. et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 20, 1493–1505 (2019).
Von Hoff, D. D. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 369, 1691–1703 (2013).
Conroy, T. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 (2011).
Buckley, C. W. & O’Reilly, E. M. Next-generation therapies for pancreatic cancer. Expert Rev. Gastroenterol. Hepatol. 18, 55–72 (2024).
Ohba, A. et al. 1616O Nab-paclitaxel plus gemcitabine versus modified FOLFIRINOX or S-IROX in metastatic or recurrent pancreatic cancer (JCOG1611, GENERATE): A multicentred, randomized, open-label, three-arm, phase II/III trial. 34, S894 (2023).
Wainberg, Z. A. et al. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): a randomised, open-label, phase 3 trial. 402, 1272–1281 (2023).
Portal, A. et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br. J. Cancer. 113, 989–995 (2015).
Mita, N. et al. Second-line gemcitabine plus nab-paclitaxel for patients with unresectable advanced pancreatic cancer after first-line FOLFIRINOX failure. J. Clin. Med. 8, 761 (2019).
Sarabi, M. et al. Use of gemcitabine as a second–line treatment following chemotherapy with Folfirinox for metastatic pancreatic adenocarcinoma. Oncol. Lett. 13, 4917–4924 (2017).
Wang-Gillam, A. et al. NAPOLI-1 phase 3 study of liposomal Irinotecan in metastatic pancreatic cancer: final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer. 108, 78–87 (2019).
Oettle, H. et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J. Clin. Oncol. 32, 2423–2429 (2014).
Nichetti, F. et al. NALIRIFOX, FOLFIRINOX, and gemcitabine with nab-paclitaxel as first-line chemotherapy for metastatic pancreatic cancer: a systematic review and meta-analysis. JAMA Netw. Open. 7, e2350756–e2350756 (2024).
Zaibet, S. et al. Gemcitabine + nab-paclitaxel or gemcitabine alone after FOLFIRINOX failure in patients with metastatic pancreatic adenocarcinoma: a real-world AGEO study. Br. J. Cancer. 126, 1394–1400 (2022).
Zhang, Y., Hochster, H., Stein, S. & Lacy, J. Gemcitabine plus nab-paclitaxel for advanced pancreatic cancer after first-line FOLFIRINOX: single institution retrospective review of efficacy and toxicity. Experimental Hematol. Oncol. 4, 1–5 (2015).
De La Fouchardière, C. et al. Gemcitabine and Paclitaxel versus gemcitabine alone after 5-Fluorouracil, oxaliplatin, and Irinotecan in metastatic pancreatic adenocarcinoma: A randomized phase III PRODIGE 65-UCGI 36-GEMPAX UNICANCER study. 42, 1055–1066 (2024).
Gilabert, M. et al. Evaluation of gemcitabine efficacy after the FOLFIRINOX regimen in patients with advanced pancreatic adenocarcinoma. Medicine 96, e6544 (2017).
Girardi, D. M. et al. Second-Line treatment for advanced pancreatic adenocarcinoma: is there a role for gemcitabine?? J. Gastrointest. cancer. 50, 860–866 (2019).
Soares, H. P. et al. A phase II study of capecitabine plus docetaxel in gemcitabine-pretreated metastatic pancreatic cancer patients: captere. Cancer Chemother. Pharmacol. 73, 839–845 (2014).
Huffman, B. M. et al. Effect of a MUC5AC antibody (NPC-1 C) administered with second-line gemcitabine and nab-paclitaxel on the survival of patients with advanced pancreatic ductal adenocarcinoma: a randomized clinical trial. JAMA Netw. Open. 6, e2249720–e2249720 (2023).
Chae, H. et al. Efficacy and safety of second-line nab-paclitaxel plus gemcitabine after progression on FOLFIRINOX for unresectable or metastatic pancreatic ductal adenocarcinoma: multicenter retrospective analysis. Therapeutic Adv. Med. Oncol. 12, 1758835920923424 (2020).
Petrillo, A. et al. Nab-paclitaxel plus gemcitabine as first line therapy in metastatic pancreatic cancer patients relapsed after gemcitabine adjuvant treatment. Med. Oncol. 36, 83 (2019).
De Vita, F. et al. NAB-paclitaxel and gemcitabine in metastatic pancreatic ductal adenocarcinoma (PDAC): from clinical trials to clinical practice. BMC cancer. 16, 1–8 (2016).
Vogl, U. M. et al. Nab-paclitaxel and gemcitabine or FOLFIRINOX as first-line treatment in patients with unresectable adenocarcinoma of the pancreas: does sequence matter? BMC cancer. 19, 1–8 (2019).
Yoo, C. et al. A randomised phase II study of modified FOLFIRI. 3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br. J. Cancer. 101, 1658–1663 (2009).
Zaanan, A. et al. FOLFOX as second-line chemotherapy in patients with pretreated metastatic pancreatic cancer from the FIRGEM study. BMC cancer. 14, 1–8 (2014).
Pelzer, U. et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur. J. Cancer. 47, 1676–1681 (2011).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer. 45, 228–247 (2009).
Acknowledgements
The authors thank all of the patients and their families.
Author information
Authors and Affiliations
Contributions
YS, OK, MNA, MU, ZU, AUK, ME, YE and HY contributed to the study conception and design. Data acquisition was performed by YS, AAS, MG, MY, MEK, MOA, SU, NA, IG, MS, MNB, MBA, MO, AA, MA, TA, BME, HA, HA, MS, BUK, EO, SA, SY, HY, SG, NM, IA, AB, SI, ST, . Quality control of data, data analysis and interpretation were performed by YS, YE, ZU, OK, and MNA. Statistical analysis was performed by YS, OK, MNA and MU. The frst draf of the manuscript was written by YS, OK, YE, MU, MNA and HY. The manuscript was critically reviewed by all authors. All authors have reviewed the article. They agree that the article is appropriate. It agrees to be published as such.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and informed consent
The study protocol was approved by The Van Regional Training and Research Hospital (IRB No. 2023 / 19/ 04 -13.09.2023). Due to the retrospective nature of the study, the need for informed consent was waived with the decision of the Ethics Committee of Van Regional Training and Research Hospital. The study was performed in accordance with the ethical guidelines of the World Medical Association’s Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sezgin, Y., Karhan, O., Aldemir, M.N. et al. Efficacy of gemcitabine plus nab-paclitaxel in second-line treatment of metastatic pancreatic cancer. Sci Rep 15, 11675 (2025). https://doi.org/10.1038/s41598-025-96157-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96157-6