Abstract
Acute Kidney Injury (AKI) is a common condition with significant impact on morbidity, mortality, and healthcare costs. This study explores the epidemiology of AKI, highlighting key factors and outcomes. In a retrospective study we evaluated patients admitted to hospital from 2016 to 2019, excluding those with pre-existing chronic kidney disease (CKD) stages 4–5. Data were extracted from hospital databases, with AKI defined by changes in serum creatinine (sCr) according to KDIGO criteria. Additionally, AKI was classified as “de novo” or as AKI on CKD in the subgroup of patients with available pre-hospital eGFR. Outcomes included mortality, hospital stay duration (LOS), AKI recovery, and persistent AKI. Of 87,087 patients, 17,946 (20.6%) developed AKI. AKI patients were older, with more comorbidities, and had significantly higher mortality (17.7% vs. 4.3%, p < 0.001). AKI was associated with in-hospital mortality (HR 1.23, 95% CI 1.16–1.30), longer LOS, and ICU admission. Mortality increased with AKI severity. Considering the 34,285 patients (39% of the total cohort) with pre-hospital eGFR, AKI occurred in 17.3% patients without previous CKD and in 31.1% of patients with previous CKD. These patients presented higher incidence of ICU admission and mortality. Additionally, 17.6% of AKI patients had persistent kidney dysfunction at discharge, often requiring extended hospitalization and ICU care. The substantial impact of AKI on both short- and potentially long-term health emphasizes the importance of early detection, personalized management, and structured follow-up to enhance outcomes and reduce CKD progression risk.
Similar content being viewed by others
Introduction
Acute Kidney Injury (AKI), one of the most frequent and probably underestimated syndromes in hospitalized patients, is commonly defined as an abrupt decline of renal function evaluated by the increase of serum creatinine levels and by the concomitant decrease of urinary output categorized according to the KDIGO 2012 criteria1. Previous studies clearly showed that the most severe forms of AKI (stages 2–3 KDIGO) are associated with increased risk of mortality, length of hospital stay and healthcare costs mainly due to frequent re-hospitalizations and development of comorbidities2. Moreover, AKI represents an independent risk factor for the progression toward chronic kidney disease (CKD)3,4. AKI has recently emerged as a major public health concern with high human and financial costs. In the US, AKI leads to an increase of the hospitalization costs that ranges $5.4 to $24 billion: the higher costs are related to the need of renal replacement therapies (RRT) leading to a longer hospital stay5.
Moreover, we have recently found a significant discrepancy in AKI epidemiology depending on the definition used. Specifically, reliance solely on administrative data, as opposed to diagnosis based on serum creatinine changes, substantially underestimates AKI incidence in hospitalized patients6.
Given these challenges, there is an urgent need for new studies to accurately assess the actual incidence of AKI. This would provide hospitals and policymakers with the necessary data to develop integrated healthcare strategies aimed at reducing AKI-related costs.
This is particularly relevant for elderly patients who are more prone to develop AKI in consideration of the loss of renal functional reserve due to aging and the presence of different chronic comorbidities such as diabetes, hypertension and heart failure7. In a European hospitalized cohort, the average age of AKI patients was 76 years8. Moreover, it has been shown that age-related yearly incidence of AKI increased from 17 per million in adults under age 50 to 949 per million in those aged 80–899.
Most of the published studies on AKI referred to critically ill patients admitted to Intensive Care Units (ICU) in which the main driver of renal dysfunction is sepsis10. However, recent reports showed that AKI is a frequent complication also in low- and medium-intensity care settings, representing the first cause of Nephrology consultancy worldwide11,12,13.
To date, we still have some unmet needs in the management of AKI in hospitalized patients: first, an earlier diagnosis of AKI may limit the worsening of the syndrome with the need of RRT, decreasing the length of hospital stay, thus improving outcomes. Secondly, the identification of AKI patients more prone to the progression toward CKD at hospital discharge remains challenging both for clinical and organizational causes14. AKI patients should enter a program of specialized follow-up aimed to assess renal function and the development of distant organ complications15,16.
This study aimed to assess in-hospital incidence of AKI using a standardized epidemiologic model, paving the way for improved AKI recognition, specialized post-discharge care, and biomarker discovery to better understand the progression from AKI to CKD.
Materials and methods
Study design and patient enrolment
We performed a retrospective observational study in patients aged ≥ 18 years hospitalized at the Policlinico Universitario “San Martino”, Genova (Italy) and at the Azienda Ospedaliera Universitaria “Maggiore della Carità”, Novara (Italy), from January 1, 2016, to December 31, 2019. Patients were included at the time of first hospital admission. All subjects with chronic kidney disease (CKD) stage 4 and 5 identified by the ICD- 9-CM (International Classification of Disease, 9 th Revision, Clinical Modification) diagnosis codes reported on the Hospital Discharge Form (HDF) and/or in regular outpatient clinic pre-dialysis follow-up, were not included in the research algorithm. Both institutional review boards approved the study protocol (Genova: N. Registro CER Liguria: 515/2020; Novara: Protocollo 530/CE, Studio n. CE 220/19 NOV-AKI Study) and waived the need for informed consent. The study was performed in accordance with the Declaration of Helsinki.
Data collection and definitions
All data were obtained from the hospital electronic database. We exported the following demographic, clinical and laboratory data: age, sex, comorbidities, serum creatinine (sCr), admission ward, length of hospital stay (LOS), death, and outcome. The most frequent comorbidities were identified by using ICD- 9-CM codes17. The values of sCr were collected at different time points: hospital admission, hospital discharge, peak and nadir levels during hospitalization. Only patients with at least two sCr determinations were admitted to the study.
For the primary objectives of the study, the lowest sCr value recorded during hospitalization was assumed as the baseline for all included patients18.
AKI incidence was then determined based on changes in sCr, calculated as the ratio of peak sCr to the lowest sCr during hospitalization (peak sCr/lowest sCr).
We defined and graded AKI according to the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline, based only on sCr changes. In accordance with KDIGO, stage 1, 2 or 3 AKI was defined as sCr increase of 1.5 to 1.9 times, 2 to 2.9 times, and ≥ 3 times (or need of dialysis) in respect to baseline sCr level respectively1. Urine output was not considered due to the retrospective nature of the study and to the limited collected data outside the Intensive Care Unit (ICU).
Notably, based on the available data, we were unable to determine which patients required dialysis during hospitalization. However, it is likely that these patients, considering their low baseline creatinine levels—potentially influenced by dialysis—were classified within the AKI stage 3 group.
When available in the laboratory database, we also collected sCr and estimated Glomerular Filtration Rate (eGFR by using CKD-EPI Formula) performed from day 7 to 180 before hospitalization to identify pre-existing CKD defined as eGFR < 60 ml/min/1.73 m2. Then, in the subgroup of patients with available pre-hospital eGFR, we differentiated and compared patients with “de novo” AKI defined as an AKI episode in absence of pre-existing CKD, and AKI on CKD defined as AKI that developed in patients with pre-existing CKD.
AKI persistence or recovery were defined by calculating the ratio between sCr at discharge and the lowest sCr during hospitalization (discharge sCr/lowest sCr): since the inclusion in the KDIGO criteria requires a discharge sCr/lowest sCr ≥ 1.5, we considered patients with persistent AKI accordingly. Conversely, patients with a ratio < 1.5 were classified as recovery AKI.
Outcomes
The following outcomes were considered: a) incident in-hospital AKI; b) overall mortality; c) length of hospital stay (LOS); d) type of hospital discharge (protected vs. at home); e) persistence or recovery AKI at hospital discharge.
Statistical analysis
Normally distributed variables are presented as mean ± 1SD and compared using an independent or paired t-test as appropriate. Logarithmically transformed values of skewed variables were used for the statistical analysis. Comparisons between groups were made by analysis of variance. Comparisons of proportions were made using the χ2-test or Fisher’s exact test when appropriate. The incidence rate of AKI was calculated. Univariate and multivariate logistic regression analyses were used to describe the relationship between all available clinical variables of biological relevance and the presence of AKI. Odds ratios and 95% confidence intervals were calculated by exponentiation of logistic regression coefficients. Time-to-event analyses were performed using: (i) the Kaplan–Meier method for survival curves estimation and log-rank test to compare them; (ii) univariate and multivariate Cox regression models: risk was reported as hazard ratios (HR) along with their 95% confidence intervals (CI). Covariates included all available clinical variables with biological plausibility. The time variable was defined as the interval time between the baseline date and the date of endpoint occurrence or the last available follow-up. Power analysis showed that the number of individuals in the database (n = 87,087) represented a sample largely sufficient to avoid b error also after stratification by AKI. Statistical calculations were performed by STATA package, version 14.2 (StataCorp, 4905 Lakeway Drive, College Station, Texas 77,845 USA). The null hypothesis was rejected for values of p < 0.05.
Results
Patients’ characteristics
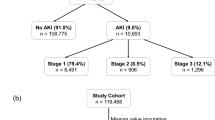
We collected data from 87,087 hospitalized patients with a mean age of 69.2 ± 17.7 years, 43,467 (49.9%) males. As reported in the HDF, 8455 (9.7%) patients were diabetic, 7767 (8.9%) had heart failure (HF) and 5924 (6.8%) had pre-existing CKD. Sepsis occurred in 3361 (3.9%) cases. At hospital admission, mean sCr was 1.12 ± 0.98 mg/dl, corresponding to an eGFR of 90.1 ± 16 ml/min. Most of the patients were admitted to low or medium intensity care settings, whereas 3147 (3.6%) to ICU (Table 1).
Incidence and risk factors for AKI
AKI developed in 17,946 (20.6%) patients. In respect to the No-AKI population, patients with AKI were significantly older and with a higher prevalence of females. Moreover, in respect to the No-AKI group, AKI patients presented a higher prevalence of all the recorded comorbidities, including CKD (9 vs 6.1% of the No-AKI group, p < 0.001), diabetes (11.2 vs 9.3%, p < 0.001), heart failure (14.7 vs 7.4%, p < 0.001) and sepsis (10.7 vs 2.1%, p < 0.001) (Table 1).
As expected, analyzing admission data, we found that patients experiencing AKI showed higher sCr levels (1.55 ± 1.53 vs 1 ± 1 mg/dl, p < 0.001) and lower eGFR (81.6 ± 16 vs 91 ± 15 ml/min, p < 0.001) when compared with the No-AKI group. AKI incidence was significantly higher in patients admitted to ICU (47%) and emergency wards (25%) compared with general internal medicine and surgery units (10.4% and 16%, respectively).
We then analyzed the clinical determinants of AKI by univariate and multivariate logistic regression analyses (Table 2). For the latter, two independent models were generated since the variables CKD reported in the HDF and admission sCr were found to be correlated during preliminary analysis. The results of logistic regressions demonstrated that in both univariate and multivariate analyses, demographic characteristics (age, female sex), length of hospital stay, ICU admission, main comorbidities (except for diabetes), and admission sCr were significantly associated with the risk of AKI development (Table 2).
Correlation of outcomes with AKI development
The in-hospital mortality rate was significantly higher in the AKI group compared to the No-AKI group (Table 3). Additionally, we observed that a higher number of AKI patients were admitted to ICU compared to low- and medium-intensity care settings. These patients also experienced a significantly longer LOS compared to No-AKI patients. At hospital discharge, AKI patients still exhibited elevated sCr levels and required a higher number of protected follow-up to ensure the continuity of care.
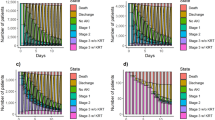
We also investigated mortality risk factors in the study cohort by using both univariate and multivariate Cox analyses. Univariate analysis revealed that age, male sex, ICU admission, sCr at admission, and the occurrence of AKI, were all significantly associated with mortality. In the multivariate analysis, the development of AKI remained a significant and independent predictor of mortality risk (HR 1.23; 95% CI 1.16–1.30) (Table 4). This finding was further confirmed by Kaplan–Meier survival analysis (Fig. 1).
Correlation of outcomes with AKI KDIGO stages
The following step was to evaluate the impact of AKI severity on patients’ outcomes. We observed that 10,679 patients (59.5%) developed stage 1 AKI, 4611 (25.7%) stage 2, and 2656 (14%) stage 3, respectively (Table 5). Notably, the mean age was lower in the stage 3 group. As expected, age stratification revealed an increased incidence of AKI in patients in the older quartiles in accordance with the probable reduction of renal functional reserve (Fig. 2).
AKI stages were also characterized by a different prevalence of comorbidities: when compared to stage 1 and stage 2, patients with stage 3 AKI showed a lower prevalence of diabetes and cardiac diseases and a concomitant higher prevalence of sepsis and pre-existing CKD. Moreover, a higher prevalence of stage 3 AKI was observed in patients admitted to ICU: conversely, patients hospitalized in low- and medium-intensity care settings showed a higher prevalence of stage 1 AKI (Table 5).
The in-hospital mortality rate was significantly higher in stage 3 AKI group (Table 6): the 90-day Kaplan–Meier survival analysis confirmed a progressive increase in mortality in the most severe forms of AKI (Fig. 3). Furthermore, stage 3 AKI patients were more commonly admitted to ICU and had a longer LOS. The stage 3 group presented higher sCr levels at discharge and required more frequently protected assistance for the continuity of care (Table 6).
Outcomes in de-novo AKI vs. AKI on CKD
Pre-hospitalization sCr levels were available in 34,285 patients, constituting 39% of the total cohort.
These patients were analyzed to compare the clinical characteristics and outcomes of de novo AKI and AKI superimposed on pre-existing CKD.
Specifically, among this subgroup, 24,588 patients (71.8%) had no previous CKD, while 9697 patients (28.2%) presented with CKD before hospital admission, with a mean eGFR of 40.2 ± 14.1 ml/min.
De-novo AKI occurred in 17.3% (n. 4263) of patients without prior CKD, and AKI on CKD in 31.1% (n. 2795) of patients with pre-existing CKD.
AKI on CKD patients were older, prevalently males and with a higher prevalence of diabetes and HF (Table 7).
AKI on CKD patients presented higher sCr levels at admission and developed more severe AKI forms during hospitalization. The in-hospital mortality rate was significantly higher in AKI on CKD patients (Table 8): the 90-day Kaplan–Meier survival analysis confirmed these data (Fig. 4). Moreover, AKI on CKD patients were more frequently admitted to ICU, although the LOS did not differ in comparison to de-novo AKI patients. Last, AKI on CKD patients showed higher sCr levels at discharge.
Determinants and outcomes of Renal recovery or Persistent AKI
Finally, in the cohort of 14,774 AKI patients who survived hospitalization, we separately evaluated the incidence and determinants of renal recovery in comparison to the persistence of AKI at discharge. Overall, 12,175 (82.4%) patients experienced renal recovery, whereas 2599 (17.6%) had persistent AKI, still meeting the diagnostic criteria for KDIGO AKI according to serum creatinine levels at hospital discharge (Table 9).
There was no significant difference in sex distribution between these two groups. However, patients with persistent AKI were slightly but significantly younger, had a higher prevalence of HF, and a lower prevalence of CKD compared to those who recovered.
At admission, patients who recovered from AKI had significantly higher sCr levels compared to those with persistent AKI. Additionally, AKI severity differed between the groups, with a higher incidence of stages 2–3 AKI in the persistent AKI group (persistent severe AKI).
Multivariate logistic regression analysis indicated that the probability of renal recovery was significantly and positively associated with older age and a diagnosis of CKD (as reported in administrative records) (Table 10). Conversely, renal recovery was negatively associated with LOS, a history of HF, and ICU admission. Furthermore, clinical outcome analysis revealed that patients with persistent AKI were more frequently admitted to the ICU, had longer hospitalizations, and more often required a protected discharge post-hospitalization care (Table 11).
Discussion
The aim of this study was to evaluate the incidence of AKI in patients admitted to two large University Hospitals in Italy between January 1, 2016, and December 31, 2019. We analyzed the clinical factors and outcomes associated with AKI development. The findings confirmed previous studies that showed AKI as an independent risk factor for increased in-hospital mortality, longer LOS, and progression to CKD. Using KDIGO criteria based solely on sCr values, we found an AKI incidence of 20.6% among hospitalized patients, which aligns with global AKI studies in our geographical region (South-West Europe)13. This supports the reliability of using sCr changes alone to define AKI. The most common form of AKI was stage 1, accounting for around 60% of cases.
We identified several risk factors for AKI, such as age, sex, and comorbidities. AKI patients were typically older and had a higher burden of comorbidities compared to non-AKI patients. In accordance with previous epidemiologic data including the 0by25 project of the International Society of Nephrology (ISN), AKI incidence was higher in patients admitted to the ICU, although about 20% of AKI cases also occurred in low- and medium-intensity care settings12. This highlights the need for further research on AKI in non-critically ill patients, an area currently underexplored in global AKI research.
The study also confirmed that AKI severity significantly impacts clinical outcomes19. AKI patients, especially those with severe forms, had worse conditions at discharge, with higher sCr levels and a greater need for protected follow-up. These patients may require long-term management, which has economic and social implications, and increases the risk of developing acute kidney disease (AKD) and progressing to CKD20. The relationship between AKI and CKD is complex and bidirectional, with CKD being a well-established risk factor for AKI21. We investigated this issue in our cohort, in which, in accordance with this selection strategy that excluded stages 4–5 CKD, only patients with mild forms of CKD were included.
Specifically, to ensure accuracy in estimating pre-hospitalization kidney function, we focused on comparing the characteristics and outcomes of de-novo AKI and AKI on CKD exclusively within the subgroup of patients with available pre-hospitalization eGFR.
In this cohort of 34,285 patients, our findings confirmed that individuals with pre-existing mild CKD exhibited a higher incidence of AKI.
These patients were older, had more comorbidities, and presented with elevated sCr at admission. They also developed more severe forms of AKI, which worsened clinical outcomes, highlighting the role of even mild CKD as an additional risk factor for AKI and its complications.
We also evaluated renal recovery in AKI survivors at hospital discharge. While most recovered their baseline kidney function, a significant portion (17.6%) still exhibited persistent AKI. Patients who recovered were generally older, had higher CKD prevalence, and had worse kidney function at admission but experienced less severe AKI during hospitalization. A plausible explanation is that many of these patients may have developed AKI in the community before hospitalization, which tends to have better outcomes as it is often caused by reversible factors22,23.
Interestingly, our findings partly contradict those of other studies, such as a recent review by Saraiva et al., which identified older age, diabetes, and CKD as risk factors for persistent AKI in non-renal transplant patients24. However, differences in the definitions of persistent AKI and patient populations likely explain these discrepancies. Indeed, while prior studies have largely focused on ICU patients25,26, our data pertains to a general hospital population, where ICU admissions represent a small minority.
Despite these differences, our results align with existing literature demonstrating that persistent AKI is associated to severe forms of the disease and worse outcomes, such as longer hospital stays, greater need for intensive care, and poorer discharge conditions27,28.
However, it should be emphasized that in this study, persistent AKI was defined based on creatinine levels at discharge, regardless of the length of hospitalization. Thus, we were unable to precisely determine the incidence of AKD, which current concepts on AKI trajectories recognize as a risk factor for subsequent progression to CKD20. Indeed, the formal definition of AKD (i.e., the persistence of kidney damage beyond 7 days from the onset of AKI) requires precise temporal data regarding the timing of AKI onset, which is lacking in our cohort29.
Another important factor to consider is the potential impact of AKI on renal function at discharge, highlighting the significance of renal functional reserve (RFR), defined as the kidney’s ability to increase GFR under physiological or pathological conditions30. Loss of RFR is an early step toward GFR decline and progression to CKD following an AKI episode31. This issue is likely to become increasingly important due to population growth, aging, and the rising incidence of comorbidities such as hypertension, diabetes, and obesity32.
Moreover, the worsening environmental conditions, such as global warming, pollution, and nephrotoxic drug use, will probably further worsen this scenario33.
The above-described epidemiological and clinical findings are confirmed at cellular and molecular level and nowadays AKI should be considered as a sort of accelerator of the aging processes within the kidney, leading to structural and functional changes, particularly in severe and persistent cases34,35. For this reason, these patients should receive specialized nephrological follow-up to optimize post-AKI care.
This study provided a comprehensive analysis of AKI intrahospital epidemiology, though we acknowledge certain limitations. The use of sCr-based AKI definitions may not fully capture all AKI cases, such as community-acquired AKI6.
Moreover, there are evidence suggesting that certain clinical conditions, such as sepsis and prolonged hospitalization—particularly in critical care settings—can affect serum creatinine production and its reliability as a marker of kidney function36,37.
However, in our study, we analyzed a large cohort of patients admitted to various hospital wards, where the occurrence of muscle loss is likely less pronounced compared to patients in the ICU. Nonetheless, the influence of muscle mass and nutritional status on serum creatinine is a well-known limitation of this marker. This limitation highlights the need for research and identification of novel renal biomarkers38.
Additionally, we lack information on AKI etiology, which could influence outcomes. The use of administrative codes to describe comorbidities may introduce biases, and we have no follow-up data, which are essential to understanding the full impact of in-hospital AKI on future health outcomes39.
Despite these limitations, our results are based on real-world data from two Italian university hospitals, making them valuable for further exploration. We addressed the unmet need in AKI management, particularly concerning the progression to CKD40. The recent UK ARID study demonstrated that CKD progression was significantly higher in hospitalized patients with severe AKI, supporting our findings15. Based on these considerations, the Italian Society of Nephrology’s Working Group on AKI and Extracorporeal Blood Purification Therapies recommends raising AKI awareness among clinicians, stakeholders, and policymakers. This consciousness derives from the analysis of epidemiological data coming from “real-life” evidence of AKI incidence not only focused on Intensive Care Units. Moreover, the application of the KDIGO criteria during the whole hospitalization will allow to identify patients with persistent and severe forms of AKI more prone to progress toward AKD and subsequently CKD41. Then, in these high-risk patients, establishing specialized post-AKI outpatient clinics, in collaboration with nephrologists and general practitioners, is crucial in warranting a proper follow-up. In this setting, given the advanced age of many AKI patients, incorporating telemedicine and digital health approaches could further enhance post-AKI care42. Moreover, creating biobanks to preserve plasma and urine samples for biomarker discovery would be a key step forward in improving AKI management and outcomes. Without these measures, the impact of AKI on mortality during hospitalization and CKD progression after discharge will likely worsen.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl (2011) 2, 4 (2012).
Shiao, C.-C. et al. Long-term remote organ consequences following acute kidney injury. Crit. Care 19, 438 (2015).
Coca, S. G., Singanamala, S. & Parikh, C. R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 81, 442–448 (2012).
Heung, M. & Chawla, L. S. Acute Kidney Injury: Gateway to Chronic Kidney Disease. Nephron Clin. Pract. 127, 30–34 (2014).
Silver, S. A. & Chertow, G. M. The Economic Consequences of Acute Kidney Injury. Nephron 137, 297–301 (2017).
Esposito, P. et al. Recognition patterns of acute kidney injury in hospitalized patients. Clin. Kidney J. https://doi.org/10.1093/ckj/sfae231 (2024).
Yokota, L. G. et al. Acute kidney injury in elderly patients: Narrative review on incidence, risk factors, and mortality. Int. J. Nephrol. Renovasc. Dis. 11, 217–224 (2018).
Ali, T. et al. Incidence and Outcomes in Acute Kidney Injury. J. Am. Soc. Nephrol. 18, 1292–1298 (2007).
Groeneveld, A. B. J., Tran, D. D., van der Meulen, J., Nauta, J. J. P. & Thijs, L. G. Acute Renal Failure in the Medical Intensive Care Unit: Predisposing, Complicating Factors and Outcome. Nephron 59, 602–610 (1991).
Pickkers, P. et al. Acute kidney injury in the critically ill: an updated review on pathophysiology and management. Intensive Care Med. 47, 835–850 (2021).
Mehta, R. L. et al. Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int. 66, 1613–1621 (2004).
Mehta, R. L. et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: A multinational cross-sectional study. The Lancet 387, 2017–2025 (2016).
Hoste, E. A. J. et al. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 14, 607–625 (2018).
Neyra, J. A. et al. Challenges in the Care of Patients with AKI Receiving Outpatient Dialysis: AKINow Recovery Workgroup Report. Kidney360 5, 274–284 (2024).
Horne, K. L. et al. A comprehensive description of kidney disease progression after acute kidney injury from a prospective, parallel-group cohort study. Kidney Int. 104, 1185–1193 (2023).
Silver, S. A. et al. Association of an Acute Kidney Injury Follow-up Clinic With Patient Outcomes and Care Processes: A Cohort Study. Am. J. Kidney Dis. 81, 554-563.e1 (2023).
International Classification of Diseases - 9th revision - Clinical Modification 2007. https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=2251.
Esposito, P. et al. Changes of Acute Kidney Injury Epidemiology during the COVID-19 Pandemic: A Retrospective Cohort Study. J. Clin. Med. 11, 3349 (2022).
Ikizler, T. A. et al. A prospective cohort study of acute kidney injury and kidney outcomes, cardiovascular events, and death. Kidney Int. 99, 456–465 (2021).
Kurzhagen, J. T., Dellepiane, S., Cantaluppi, V. & Rabb, H. AKI: An increasingly recognized risk factor for CKD development and progression. J. Nephrol. 33, 1171–1187 (2020).
Belayev, L. Y. & Palevsky, P. M. The link between acute kidney injury and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 23, 149–154 (2014).
Wonnacott, A., Meran, S., Amphlett, B., Talabani, B. & Phillips, A. Epidemiology and Outcomes in Community-Acquired Versus Hospital-Acquired AKI. Clin. J. Am. Soc. Nephrol. 9, 1007–1014 (2014).
Mesropian, P. D. et al. Community-acquired acute kidney injury: A challenge and opportunity for primary care in kidney health. Nephrology 21, 729–735 (2016).
Saraiva, I. E. et al. Risk Factors and Outcomes Associated With the Development of Persistent Acute Kidney Injury in Non-Renal Solid Organ Transplant Recipients: Systematic Review and Meta-Analysis. Clin. Transplant. https://doi.org/10.1111/ctr.15444 (2024).
Kellum, J. A. Persistent Acute Kidney Injury*. Crit. Care Med. 43, 1785–1786 (2015).
Samoni, S., De Rosa, S., Ronco, C. & Castellano, G. Update on persistent acute kidney injury in critical illnesses. Clin. Kidney J. 16, 1813–1823 (2023).
Venkatachalam, M. A., Weinberg, J. M., Kriz, W. & Bidani, A. K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 26, 1765–1776 (2015).
Perinel, S. et al. Transient and Persistent Acute Kidney Injury and the Risk of Hospital Mortality in Critically Ill Patients. Crit. Care Med. 43, e269–e275 (2015).
Molinari, L. et al. Distribution of Acute and Chronic Kidney Disease Across Clinical Phenotypes for Sepsis. Chest 166, 480–490 (2024).
Sharma, A., Mucino, M. J. & Ronco, C. Renal Functional Reserve and Renal Recovery after Acute Kidney Injury. Nephron. Clin. Pract. 127, 94–100 (2014).
Husain-Syed, F. et al. Persistent decrease of renal functional reserve in patients after cardiac surgery-associated acute kidney injury despite clinical recovery. Nephrol. Dial. Transplant. 34, 308–317 (2019).
Chang-Panesso, M. Acute kidney injury and aging. Pediatr. Nephrol. 36, 2997–3006 (2021).
Francis, A. et al. Chronic kidney disease and the global public health agenda: An international consensus. Nat. Rev. Nephrol. 20, 473–485 (2024).
Franzin, R. et al. Targeting Premature Renal Aging: From Molecular Mechanisms of Cellular Senescence to Senolytic Trials. Front. Pharmacol. https://doi.org/10.3389/fphar.2021.630419 (2021).
Agarwal, A. et al. Cellular and Molecular Mechanisms of AKI. J. Am. Soc. Nephrol. 27, 1288–1299 (2016).
Prowle, J. R. et al. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin. J. Am. Soc. Nephrol. 9, 1015–1023 (2014).
Doi, K. et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J. Am. Soc. Nephrol. 20, 1217–1221 (2009).
Strauß, C., Booke, H., Forni, L. & Zarbock, A. Biomarkers of acute kidney injury: From discovery to the future of clinical practice. J. Clin. Anesth. 95, 111458 (2024).
Esposito, P. et al. Renal Outcomes of Dialysis-Dependent Acute Kidney Injury in Noncritically Ill Patients: A Retrospective Study. Blood Purif https://doi.org/10.1159/000517707 (2022).
Yeh, H.-C., Ting, I.-W., Huang, H.-C., Chiang, H.-Y. & Kuo, C.-C. Acute Kidney Injury in the Outpatient Setting Associates with Risk of End-Stage Renal Disease and Death in Patients with CKD. Sci. Rep. 9, 17658 (2019).
Liu, K. D. et al. AKI!Now Initiative: Recommendations for Awareness, Recognition, and Management of AKI. Clin. J. Am. Soc. Nephrol. 15, 1838–1847 (2020).
Kashani, K. B. et al. Digital health and acute kidney injury: consensus report of the 27th Acute Disease Quality Initiative workgroup. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-023-00744-7 (2023).
Acknowledgements
Authors are members of the AKI and Extracorporeal Blood Purification Therapies of the Italian Society of Nephrology (SIN); VC is vice-chair of the ERAKI Working Group of the European Renal Association (ERA).
Author information
Authors and Affiliations
Contributions
Concept and design: PE, FC, GC, VC. Collection, acquisition, analysis, or interpretation of data: all the authors. Drafting of the manuscript: MF, FP, VZ, EN, ADQ. Critical revision of the manuscript: MM, EL, VC, GC. All the authors give their agreement for all aspects of the work and ensure the accuracy and integrity of any part of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esposito, P., Cappadona, F., Prenna, S. et al. Acute kidney injury in hospitalized patients with real-life analysis of incidence and clinical impact in Italian hospitals (the SIN-AKI study). Sci Rep 15, 14261 (2025). https://doi.org/10.1038/s41598-025-96236-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96236-8