Abstract
This study aimed to characterize ophthalmological manifestations and associated inflammatory markers in EVD survivors in post-treatment era. Case-control study of ophthalmological manifestations and plasma inflammatory biomarker profile in EVD survivors (n = 120) from the 2018–2020 outbreak in DRC, their gender- and age-matched close contacts (n = 120) and non-contact (healthy) controls (n = 120). Expressions of inflammatory markers were assessed using the Olink Explore 384 Assay and compared across study groups before and after stratification by treatment with monoclonal antibodies (mAB114, ZMapp, or Regeneron) or antiviral drug (Remdesivir). Protein profiling was carried out using the Olink statistical package. Mean age (years) was comparable among survivors (29.7 ± 10.6), close contacts (28.9 ± 11.1) and non-contact controls (29.3 ± 10.6) (p = 0.85). Mean time from disease onset to clinical assessment was 3.5 ± 0.5 (2.5–4.2) years in survivors. Optic neuropathy was more common in survivors (6.7%) than in close contacts (0.8%) and non-contacts (0.0%) (p = 0.003). Survivors with optic neuropathy had significantly worse visual acuity in both eyes than those without optic neuropathy (all p < 0.001). Clinical evidence of past anterior uveitis was observed in 2.5% of survivors, 2.9% of close contacts, and 1.8% of healthy controls (p = 0.86). Plasma circulating DGKZ, INFGR1, ERBB3, and MICA-MICB showed differential expression patterns between survivors and controls (all p < 0.05). However, no clear separation could be detected on principal component analysis of multiplexed proteomic data between survivor and control samples. Three proteins (ITM2A, CLEC4D, NCLN) were differentially expressed and related to optic neuropathy. The comparison between treatment groups revealed a trend toward lower protein inflammatory markers in survivors treated with Remdesivir than those treated with monoclonal antibodies. We conclude that in treated EVD survivors, optic neuropathy was the only neuro-ophthalmologic abnormality. Uveitis was far less frequent than reported in West African cohorts. ITM2A, CLEC4D, and NCLN were differentially expressed in EVD survivors with optic neuropathy long after the acute phase of the infection. The true meaning of these findings will need further investigations.
Similar content being viewed by others
Introduction
Ebola virus (EBOV), an RNA virus belonging to the filovirus family, causes outbreaks of hemorrhagic fever with very high mortality rates in humans when left untreated1. Since the first outbreak in 1976 in the northwestern Democratic Republic of the Congo (DRC), several outbreaks have occurred intermittently in sub-Saharan Africa (SSA), with the DRC recording the largest number and highest burden of disease2. The 2018–2020 outbreak in Eastern DRC was the country’s largest and the second in Ebola history after the outbreak that predominantly affected three West African countries (Sierra Leone, Liberia, and Guinea) and sent isolated cases into neighboring countries and the Western hemisphere, leading to a declaration of global public health emergency. These outbreaks have left a staggering number of survivors, thus offering the possibility of better characterizing the spectrum of clinical manifestations, including long-term sequelae of Ebola virus disease (EVD), of developing preventive and therapeutic countermeasures, of demonstrating the persistence of EBOV in some immune privileged sites, and of advancing our understanding of the pathogenesis of the EVD.
Characteristics of acute EVD include non-specific manifestations such as a febrile syndrome (fever, asthenia, myalgia, headache) and other specific manifestations such as those involving the central nervous system (CNS)3and the eye4. Neurological and ophthalmological symptoms and signs may occur early in the acute phase, during convalescence or long after discharge, some persisting as sequelae. Because it has generally been difficult to thoroughly assess patients during the acute phase of the infection due to the risk of infection transmission to medical staff and the cumbersomeness of examining patients with full protective equipment, neurological and ophthalmological manifestations have mostly been evaluated after discharge from Ebola treatment centers (ETC).
Neuro-ophthalmological abnormalities have been reported in a limited number of studies in EDV survivors. In the PREVAIL III study, rates of optic nerve changes were comparable in EVD survivors and controls5. Ocular smooth pursuit and conjugate gaze deficits were described in an American healthcare worker evacuated from Sierra Leone to the US with laboratory-confirmed EVD and meningoencephalitis6. Impaired saccades and eye pursuits were reported in a cohort of Liberian survivors7. A United States physician developed a sight-threatening hypertensive panuveitis and optic neuropathy more than two months after the onset of the acute phase of infection8. Meningoencephalitis associated with EBOV infection with paralysis of IIIrd, VIth, and VIIth cranial nerves were described in a Scottish nurse working in Sierra Leone nine months after initial discharge9. In an uncontrolled sample of 96 Liberian Ebola survivors, neuro-ophthalmologic manifestations included optic neuropathy and ocular motility disorders10. Moreover, in a recent post-hoc analysis of data from the Ebola Virus Persistence in Ocular Tissues and Fluids (EVICT) study11, we also reported optic nerve changes and ocular motility abnormalities12. Few other clinical studies have reported cranial nerve abnormalities in EVD survivors, but failed to specify which nerve was affected13,14. In experimental models of EVD in non-human primates, imaging signs suggestive of optic neuritis with papilledema were observed in one surviving animal15, whereas post-mortem histopathological signs compatible with optic neuritis were observed in another16.
Although both the West African and the Eastern DRC outbreaks were caused by the Zaire Ebola virus (ZEBOV) strain, patients in West Africa only received supportive care. In contrast, those in DRC benefited from effective therapies17, and more than 300,000 people in the community, including contacts, received the recombinant vesicular stomatitis virus (rVSV)-vectored vaccine against ZEBOV virus disease (rVSV-ZEBOV) with robust antibody response and persistence18. Since immunization and antiviral therapy implemented during the 2018–2020 outbreak in Eastern DRC may reduce the incidence of EVD manifestations, the present study was the first attempt, in EVD post-treatment area, to unveil the profile of ophthalmological manifestations in survivors from the outbreak. Given that EBOV infection induces a robust immune activation expressed by increased production of inflammatory mediators19, we also assessed the expression levels of plasma circulating proteins including markers of inflammation in relation to EVD survivor status and ophthalmological manifestations.
Methods
Ethical considerations, study design and study area
This study received approval from Institutional Review Boards of the Oregon Health and Sciences University and the DRC Ministry of Health (MoH). Written informed consent was obtained from each participant and the study conformed with the principles of the Helsinki Declaration. A case control design enrolled EVD survivors from the 2018–2020 outbreak in DRC, their gender and age-matched close contacts and no-contact controls in September 2022 in Beni, Nord Kivu Province, DRC (eligibility criteria vide infra). All participants were residents of the area spanning from Beni (population ≈ 450,000) to Mangina (epicenter of the outbreak, population ≈ 40,000). Both cities were seriously affected by the outbreak, and each had an ETC.
Sample size determination
To detect differences between pairs of groups on dichotomous outcomes, we used preliminary data from a previous study on the rate of uveitis (33.3%) in EBOV survivors and 15.4% in their close contacts14. To detect such differences as statistically significant with a power of 0.80 or greater with standard deviation difference in group means of 0.42 in two-sided tests at 0.05 significance level, 90 participants were required per group at a single timepoint (i.e. baseline). In anticipation of possible loss to follow-up over time for multiple reasons (i.e. withdrawal, relocation, and death), we estimated that an addition of 25% (same as in longitudinal studies in West Africa) to the baseline sample size was justified, bringing the sample size to 120 in each group.
Recruitment and eligibility
The DRC MoH EBOV outbreak surveillance database, which contained information on all cases (survivors) and respective contacts (close contacts) from the beginning to the end of the outbreak, was used to recruit 120 survivors and 120 close contacts. In addition, 120 presumably healthy controls (non-contacts) from the same community, with no history of contact with survivors or their contacts, were also recruited. A survivor was defined as an individual who: (1) survived EBOV infection, (2) had symptoms meeting the case definition of acute EVD, (3) tested positive for the presence of EBOV (RT-PCR) at the time of the outbreak, and (4) who was registered as survivor in the outbreak surveillance database as evidenced by the survivor certificate and the membership card issued by the Ebola Survivors Association. A close contact was defined as an individual who: (1) resided in the outbreak area since the onset of the outbreak and has been in close contact (living in the same household or caring for a survivor) with a confirmed survivor, (2) never developed symptoms meeting the case definition of EVD, (3) tested negative for the presence of EBOV. A healthy control was someone who: (1) had also resided in the outbreak area since the onset of the outbreak, (2) had not been in contact with a confirmed survivor or a confirmed contact, (3) had never developed or experienced Ebola-like symptoms or any risk factor for CNS and ocular morbidity (including but not limited to a history of malnutrition, cerebral malaria, psychosis, head trauma, seizures, loss of consciousness, high blood pressure, diabetes, trachoma, syphilis, and HIV). Survivors were treated with either monoclonal antibodies (mAB114, ZMapp, or Regeneron) or antiviral drug (Remdesivir) during hospitalization. Survivors’ close contacts and healthy controls receive the rVSV-ZEBOV vaccine.
Ophthalmologic evaluation
Prior to the actual ophthalmologic examination, participants underwent an interview to verify their status (survivor, contact or healthy control). Information was also collected on ocular symptoms and diagnosis (if known) at the time of EVD onset as well as during and after ETC stay. Past and current treatments were also recorded. Both eyes of study participants underwent a detailed ophthalmological examination by licensed ophthalmologists (JCM, AKK, and NNK) who were blinded to study participants’ status as survivors, close contacts or healthy controls, their vaccination status and survivors’ treatment group. The examination included the following: (1) measurement of Snellen best corrected visual acuity, (2) careful assessment of eyelid statics and dynamics, (3) color vision testing using the 14-plate Ishihara booklet (Kanehara & Co., Ltd., Tokyo, Japan), (4) contrast sensitivity assessment (Vistech Consultants, Inc., Dayton, OH, USA), (5) measurement of intraocular pressure (IOP) with Tonopen (Reichert, Depew, New York, NY, USA), (6) assessment of pupil sizes and pupillary light reflexes primarily for the possible presence of an afferent pupillary defect, (7) examination of ocular motility (conjugate movements in all directions of gaze) to assess the integrity of extraocular muscles (EOM) and specific cranial nerves, smooth pursuits movements, saccades, and vergences by direct observation of eyes’ movements without recording), (8) measurement of central corneal thickness by ultrasound pachymetry (Pachmate 2, DGH Technology, Inc., Exton, PA, USA), and (9) assessment of basal tear production using Schirmer’s test. Slit lamp biomicroscopy was used to examine the conjunctiva, cornea, anterior chamber, iris, lens, and anterior vitreous after pupil dilation. The fundus (posterior vitreous, retina/choroid, retinal vasculature, and optic nerve) was examined first by fundus biomicroscopy with a + 90D Volk lens (Volk, Mentor, OH, USA) and then by indirect ophthalmoscopy (Keeler, Malvern, PA, USA) augmented with a + 28D Volk lens. Study outcomes included abnormalities in eyelid dynamics (i.e. ptosis), optic nerve changes (i.e. edema, atrophy, hyperemia, cupping), uveitis, cataracts, and disorders of ocular motility and EOM function.
Plasma collection, storage, and biomarkers screening
For all participants, peripheral venous blood samples were collected into vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA). Plasma was obtained after blood centrifugation (3000 rpm for 15 min), then stored at −80 °C until testing. The Olink Proximity Extension Assay (PEA) Inflammation Panel (Olink Explore 384 Inflammation Panel I, Olink proteomics, Uppsala, Sweden), which contains number of circulating target proteins, was used to analyze the samples from a randomly selected subset of the study population made of 56 survivors and 69 controls (32 close contacts and 37 healthy controls). This assessment was performed only at the time of this study. Stratification of the subset of survivors was based on treatment received during stay in the ETC and resulted in 16 subjects treated with mAb114, 21 with Regeneron, 7 with ZMapp, and 12 with Remdesivir. The O-link assay on 125 samples allowed profiling of 363 markers of inflammation per sample (Supplementary Table 1). While antibodies against EBOV were evaluated at disease onset in survivors and their close contacts, they were not evaluated in the context of this study.
Statistical analysis
Descriptive statistics are provided as means for quantitative variables and frequency and/or proportion for categorical variables. Means were compared with either Student t-test or ANOVA. The proportions of patients with a given abnormality between groups were compared using Pearson Chi-square or Fisher-Freeman-Halton exact test using SPSS version 28.0. Protein expression levels were compared between groups with Welch sample Student t-test or ANOVA using the statistical application of the Olink platform. For inflammatory protein levels, both adjusted and unadjusted p-values were computed, but only the adjusted p-value was ultimately considered to decide whether a protein was significantly differentially expressed or not. Principal component analysis (PCA) was also performed using the Olink statistical package and the first 2 principal components (PC1 and PC2) plotted. Contributions to the components were calculated as the variable loading divided by the total column variance explained by that component. The level of statistical significance was set at < 0.05.
Results
General characteristics of the study participants
An overview of the demographics and ocular complaints of the study participants is shown in Table 1. Mean time from EVD onset to clinical assessment in survivors was 3.5 ± 0.5 (2.5–4.2) years. Mean age did not differ significantly between survivors, close contacts, and health controls (ANOVA, p = 0.85). Female and male participants were equally distributed in the three groups (χ2 = 0.00, p = 1.00). Children (5–17 years) accounted for 15.8% of survivors and were equally distributed (χ2 = 0.82, p = 0.94). The proportion of subjects complaining of blurred vision was significantly different between groups (χ2 = 7.02, p = 0.029). More precisely, this proportion was higher among survivors than among close contacts (p = 0.008), but comparable to that of healthy controls (p = 0.27). Similar comparisons regarding ocular pain, ocular redness, and myodesopsia showed no significant differences among these three groups (all p > 0.05).
Ophthalmological findings
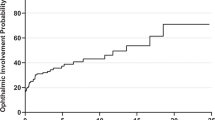
Among survivors, only 8/120 (6.7%) had some type of neuro-ophthalmological abnormality versus 4/116 (3.3%) among close contacts, and 3/117 (2.5%) among healthy controls (χ2 = 2.92, p = 0.23). However, optic neuropathy was significantly more frequent in survivors (n = 8, 6.7%) than close contacts (1/120; 0.8%) and healthy controls (0/120; 0.0%) (Fisher-Freeman-Halton = 10.3, p = 0.003). It was bilateral in 7 of the 8 survivors. Visual acuity of survivors was significantly lower in eyes with than in those without optic neuropathy (0.64 ± 0.30 vs. 0.98 ± 0.07 for OD and 0.66 ± 0.29 vs. 0.97 ± 0.11 for OS, both p < 0.001). The characteristics of survivors with optic neuropathy are shown in Table 2. None of the survivors or close contacts demonstrated ptosis, compared to only one healthy control with the abnormality (0.3%) (Fisher-Freeman-Halton = 1.9, p = 0.32). Abnormal ocular motility was observed only in close contacts (3/117 or 2.5%) and healthy controls (3/111 or 2.6%) (Fisher-Freeman-Halton = 3.14, p = 0.21).
The numbers of color plates correctly identified by OD were 12.7 ± 1.5 (survivors), 12.7 ± 1.8 (close contacts), and 12.7 ± 1.9 (healthy controls), p = 0.94. The figures for OS were 12.9 ± 1.6, 12.9 ± 1.9 and 13.1 ± 1.7, respectively, p = 0.51. No differences were observed in the proportions of people with abnormal color vision between survivors (2.5%), close contacts (5.8%) and healthy controls (5.8%) (χ2 = 1.98, p = 0.37).
Contrast sensitivity also showed no differences between the three groups at all spatial frequencies in both eyes (all p > 0.05). However, significantly more survivors (62.5%) had abnormal contrast sensitivity than close contacts (47.5%) (χ2 = 5.46, p = 0.020), but not healthy controls (61.5%) (χ2 = 0.018, p = 0.89).
None of the participants had signs of active uveitis. Clinical markers of past anterior uveitis were observed in 3/120 (2.5%) survivors, 3/104 (2.9%) close contacts, and 2/114 (1.8%) healthy controls (Fishier-Freeman-Halton = 0.65, p = 0.86). Among participants with peripheral chorioretinal scars, the number was proportionally higher among survivors (5/120; 4.2%) than among close contacts (1/120; 0.8%) and non-contact controls (1/114; 0.9%), but the difference was not statistically significant (Fisher-Freeman-Halton = 3.59, p = 0.23). Overall, 8 (6.7%) survivors had evidence of past uveitis, regardless of uveitis type. Among them, 5 had signs of anterior and posterior uveitis whereas 3 presented only markers of anterior uveitis. Cortical lens opacities, observed in 2 survivors and 2 close contacts (Fisher-Freeman-Halton = 1.65, p = 0.78), were unilateral in one and bilateral in the other case in each group. The survivor with unilateral cataract also had evidence of past ipsilateral anterior uveitis whereas the other with bilateral cataract had unilateral optic atrophy without evidence of past intraocular inflammation.
Inflammatory biomarker analysis
Of the 384 inflammatory proteins measured, 363 (94.5%) proteins passed stringent data cleaning and their levels were compared between 56 survivors, 32 close contacts, and 37 healthy controls (Fig. 1, Supplementary CSV file). Because of the reduced sample size, all subjects without a history of EVD (close contacts and healthy controls) were grouped together into a single control group. When the comparison was made between these three groups, only diacylglycerol kinase zeta (DGKZ) (p = 0.022), interferon-γ receptor subunit 1 (IFNGR1) (p = 0.024); receptor tyrosine-protein kinase erb-3 (ERBB3) (p = 0.025) and major histocompatibility complex class I chain-related protein-A and B (MICB-MICA) (p = 0.044) were differentially expressed, of which DGKZ was upregulated and the other three were downregulated (Fig. 2). However, adjusted p-values showed no statistically significant differences between the two groups (all p > 0.05). The comparison of expressions of these four proteins between EVD survivors and controls is shown in Fig. 3. On PCA (Fig. 4), the main source of variance (PC1) explained 19.1% of the variance but did not allow a separation between the survivor and control samples. PC2, which explained 9.7% of the variance, also showed no separation between the two groups.
Volcano plot depicting differential expression of inflammatory proteins in EVD survivors vs. controls (combined close contacts and healthy controls). Scattered points represent individual proteins. The x-axis displays log2 fold changes (L2fc) in protein expression, with positive and negative values indicating upregulated and downregulated expressions, respectively. P-values are shown on -Log10 scale on the y-axis. Proteins determined to be significantly and differentially regulated are indicated in the uppermost segment (above the dashed line). Proteins differentially expressed at p < 0.050, but not reaching the adjusted threshold, are in the central segment, close to 0. Proteins not differentially expressed are scattered away from the central segment and below the dashed line.
Between-treatment groups analysis identified the following 10 proteins as significantly differentially expressed: chemokine CXLC9, interleukin 10 receptor subunit beta (IL10RB), lymphocyte antigen 6 family member D (LY6D), chemokine CXLC8, neurofascin (NFASC), interleukin 5 (IL5), serine protease 8 (PRSS8), Fc receptor like 3 (FCRL3), Fc receptor like 6 (FCLR6), and interleukin 2 (IL2). However, no differences in expression were detected after adjustment. No differences between samples from the four treatment groups could be discerned, but there was trend toward lower inflammatory marker levels in survivors treated with Remdesivir vs. those treated with mAb114, Regeneron, or ZMapp (Fig. 5). The expression of inflammation-related proteins was assessed in patients with optic neuropathy, anterior uveitis, and chorioretinitis. However, the limited size of the cohort examined for inflammatory markers and the low frequency of these neuro ophthalmic conditions precluded in-depth statistical analysis of this data. The full dataset of inflammatory marker analysis is nevertheless available to be explored in Supplementary CSV file.
Inflammation-Related protein expression in optic atrophy and uveitis
The expression of inflammation-related proteins was assessed in patients with optic neuropathy, anterior uveitis, and chorioretinitis. A total of 18 proteins were differentially expressed in participants with optic neuropathy, but only integral membrane protein 2 A (ITM2A), C-type lectin domain family 4 member D (CLEC4D), and nicalin (NCLN) remained so after adjustment. Fifty-six proteins with significantly different expressions between EVD survivors and controls were related to chorioretinitis. Of these, 36 remained differentially expressed after adjustment. The numbers of differentially expressed proteins before and after adjustment for anterior uveitis were 29 and 14, respectively (Table 3). Only three proteins, EPH receptor A1 (EPAH1), hematopoietic cell-specific Lyn substrate 1 (HCLS1), and regulator of G protein signaling 8 (RGS8), were consistently and differentially expressed in chorioretinitis and anterior uveitis before adjustment; this number dropped to one (EPHA1) after adjustment. None of the inflammatory markers was simultaneously differentially expressed in optic neuropathy and anterior or posterior uveitis after adjustment.
Discussion
In this case-control study, we described for the first time the ophthalmological manifestations and changes in inflammatory markers in EVD survivors of the 2018–2020 Ebola outbreak in Eastern DRC, their close contacts and presumed community healthy controls. Although there have been some reports of neuro-ophthalmological abnormalities in EVD survivors, the uniqueness of the present study lies in the following: (1) all survivors in Eastern DRC received Ebola treatment (monoclonal antibodies or antiviral drug) during the acute phase of the illness, unlike EVD survivors of the 2014–2016 outbreak in West Africa from which all available reports have originated, (2) it studies for the first time the expression of inflammation-related proteins in relation to optic neuropathy versus uveitis in the context of treated EVD.
In this study, we found that 6.7% of EVD survivors had optic neuropathy, a significantly higher proportion compared to close contacts and/or presumably healthy community controls on average 3.5 years after the onset of an EVD outbreak. While various ophthalmological abnormalities have previously been reported in EVD survivors in West Africa, only three studies have documented optic neuropathy. In Liberia, baseline findings of the PREVAIL III study showed no difference in proportion between survivors and close contacts with optic disc swelling5. It should be noted that this assessment took place on average one year after the onset of the outbreak. In another Liberian cohort of 96 survivors examined by Shantha et al., optic neuropathy was diagnosed in only 3.1% of the subjects10. We recently re-analyzed EVICT study data of 115 EVD survivors from Sierra Leone assessed between March 2015 and March 2016, and found that 7.0% had an optic nerve abnormality12. The observation that survivors in this cohort from DRC who showed optic neuropathy had significantly lower visual acuity than those without optic neuropathy is consistent with our recent findings in the EVICT cohort from Sierra Leone12. Unlike the two studies from Sierra Leone, no cases of ocular motility abnormalities of any type were observed in our cohort of EVD survivors of the 2018–2020 Ebola outbreak in Eastern DRC.
The pathogenesis of optic neuropathy in patients with EVD is not clear but remains under investigation. Although the virus has never been isolated from the optic nerve in humans, its replication has been detected in aqueous humor in a single patient with severe panuveitis and optic neuropathy8as well as the CSF of patients with encephalitis20,21. In non-human primates, EBOV genetic material was isolated from the vitreous of one animal that developed panuveitis with papilledema15. In another study of archived tissues of rhesus macaques with experimental EVD, EBOV RNA was present in the ciliary body, choroidal vessels, and optic nerve leptomeninges of animals that died 5–11 days after exposure. In animals that died 16–24 days after exposure, EBOV genomic material was detected in the leptomeninges of 13 of 14 animals, including in the vitreous body of one. In surviving animals, genetic viral material was isolated almost exclusively in the vitreous body22. Thus, inflammation leading to optic neuritis in the context of posterior uveitis, panuveitis, or encephalitis is one plausible explanation. Reduced blood perfusion to the optic nerve due to hypovolemia secondary to gastrointestinal disturbance is another viable mechanism of optic neuropathy in the acute phase of infection. The presence of viral material in the leptomeninges surrounding the optic nerve also suggests the possibility of optic neuropathy in case of EBOV-induced leptomeningitis22. The virus in the rVSVΔG-ZEBOV-GP vaccine has been shown to infect the neuroretina and the CNS in mice, and to cause neuroretinitis and neurodegeneration, respectively23. Whether the single case of optic neuropathy among close contacts was vaccine-induced remains an open question. However, for future studies we encourage a prospective monitoring and reporting system for all subjects receiving vaccines against EBOV to better assess the spectrum and incidence of adverse events.
Functional deficits such as abnormal color discrimination and reduced contrast sensitivity thresholds can be indicative of neuro-ophthalmologic abnormality, particularly optic neuropathy. The high number of previous studies on ophthalmologic findings in EVD survivors contrasts strikingly with the paucity of data on color vision and contrast sensitivity in these subjects. Since these functional tests were not included in most assessment protocols, there was no mention of contrast sensitivity performance in EVD survivors in previous studies and only one reported a 28.9% prevalence of color vision impairment in Liberian survivors compared to 19% in close contacts5. Surprisingly, our results showed lower contrast thresholds in similar proportions of EVD survivors and healthy controls, although higher than those observed in close contacts. Therefore, the relationship between EVD survivorship and impaired color vision or abnormal contrast sensitivity could not be established in this study.
The prevalence of uveitis (anterior and posterior combined) was 6.7%, which is significantly lower than found in West African cohorts5,10,14,24,25,26,27. Steptoe et al. described the topographical pattern of chorioretinal scars in Sierra Leonian EVD survivors as numerous, greyish, and non-pigmented lesions following the anatomic course of retinal ganglion cell axons28,29. Neither the small number of the lesions (often a single scar, except one patient who had 2 small scars in one eye) nor their pigmented appearance in our cohort of EDV survivors matched the characteristics described in Sierra Leone. In addition, the proportion of patients with those lesions was higher in Sierra Leone than RDC. The protective effect of antiviral treatment in this Congolese cohort should not be neglected to explain the difference between the two zones. Possible evidence was provided by Reyard et al.’s work on non-human primates. They showed that early control of viral load through antiviral therapy produces lower levels of pro-inflammatory cytokines in treated than in untreated animals30. While we did not observe a statistically significant difference in protein expression between survivors and controls, there was a tendency toward reduced inflammatory markers in survivors who received Regeneron compared to those who received monoclonal antibodies.
EBOV-induced cytokine storm is a well-known immunological phenomenon that plays a key role in survival, severe morbidity and mortality in humans and non-human primates31,32. While the host inflammatory response has been studied in humans in relation to disease severity and host survival31,33,34, little is known about the profile of inflammatory markers in EVD survivors in relation to ocular disease and sequelae long after recovery. Even though EVD is characterized by a massive production of both pro- and anti-inflammatory cytokines, we did not know which of them are specific to ocular complications such as uveitis and optic neuropathy. For this reason, we chose to run the entire panel of inflammatory proteins to determine which factors may or may not be related to outcome. We found that only three inflammatory markers (ITM2A, CLEC4D, and NCLN) were differentially expressed in patients with optic neuropathy. The function of ITM2A is not yet well defined, but previous studies have identified it in the brain endothelial cells of humans, rats, and pigs35,36,37, with high expression, particularly in humans and rats37,38. Although ITM2A is also known to be involved in immunoglobulin production through activation of T-cells39, the relationship between its overexpression in EVD and neurological or ophthalmological abnormalities is unclear and deserves further investigations. In humans, CLEC4D has been shown to increase significantly following rVSVΔG-ZEBOV-GP immunization. It is believed to upregulate the host innate immune system after immunization, probably by binding to the EBOV glycoprotein and inducing activation of innate immune response cells (monocytes, macrophages, and dendrite cells)40. In non-human primates, it was shown to be upregulated and to promote inflammation in animals that later died from EBOV infection41. CLEC4D overexpression observed in EVD survivors in our cohort may therefore be indicative of previous severe inflammation during the acute phase of infection. It is therefore reasonable to think that inflammation could have played a role in the occurrence of optic neuropathy observed in our cohort. This observation requires caution as an immunization randomized control trial using three vaccine regimens against Zaire Ebola virus disease (Ad26.ZEBOV followed by MVA-BN-Filo; rVSVΔG-ZEBOV-GP followed by placebo; and rVSVΔG-ZEBOV-GP followed by rVSVΔG-ZEBOV-GP)42 took place in Eastern Congo at the time of the outbreak suggesting that immunization status should be considered among possible triggers of changes in inflammation markers.
It was interesting to note that the 14 inflammatory proteins differentially expressed in anterior uveitis were different from the 36 proteins differentially expressed in chorioretinitis. Moreover, these numbers were higher than the 3 proteins differentially expressed in optic neuropathy. The higher numbers may reflect the complexity of the EVD-related uveitis and the need for more research to better understand the pathogenesis of this condition.
Given that the outbreak resulted in approximately 1200 survivors, studying a tenth of them could be a limitation of this study. Another limitation is that the long-time interval from disease onset to assessment (3.5 years) is likely sufficient for expression of most disrupted proteins to return to their pre-EVD levels. Plasma protein evaluation is of great interest early during the acute phase and convalescence as protein levels may be associated with survivorship and be used for prognosis. As such, serial assessment of these proteins over time would have been ideal to monitor progress starting at onset of the disease. In the present study, it was performed only once long after onset of the disease, which is an additional limitation of this study. However, the findings still showed that survivors had some disturbed plasma proteins compared to controls. The possibility that optic neuropathy and uveitis may be due to other conditions (i.e. nutritional deficiencies, HIV, toxoplasmosis) should not be discounted as serologic tests to exclude them were not performed. For example, Steptoe et al. reexamined 57 EVD survivors in Sierra Leone, among whom 17% had EVD-related chorioretinal lesions at an initial examination. While there were no new EVD chorioretinal lesions approximately 1 year later, 3.5% of the study participants showed new toxoplasmosis-like retinal lesions and 73% tested positive for T. gondii antibodies43. We utilized the rate of uveitis from a previous study in West Africa to detect a significant difference in optic neuropathy rates between groups in the present study. While this approach may be inaccurate, it was more practical than calculating a sample size for each clinical entity (anterior uveitis, posterior uveitis, optic neuropathy) as we did not anticipate observing such small numbers of EVD survivors with ocular sequalae. We also acknowledge that although there were significant differences between values of inflammation-related protein markers in survivors and controls with optic neuropathy and anterior uveitis, the sample sizes were too small. While it is unclear whether the outcomes of the present study were significantly impacted by these limitations, caution is required when interpreting the findings. In light of the characteristics of the retina lesions described by Steptoe et al.28,29, it remains unknown if retinal imaging in our cohort could have helped discriminate between EVD- and non-EVR-related lesions. However, the inclusion of two gender and age-matched different control groups (close contacts and presumably community healthy controls) and the screening of a wide range of inflammatory proteins exceeds these limitations.
In conclusion, in this cohort of EVD survivors treated with monoclonal antibodies or antiviral drug remdesivir, 6.7% of subjects had bilateral optic neuropathy and a similar proportion of them had clinical markers of past uveitis. Whether the low prevalence of uveitis in our cohort may be explained by prior antiviral treatment during the acute phase of infection remains to be established. Expression of ITM2A, CLEC4D, and NCLN appear to be linked to optic neuropathy 3.5 years after the onset of the outbreak. Expression of 14 and 36 other proteins were linked to anterior and posterior uveitis, respectively. Whether the same pattern of protein expression could have been seen at disease onset remains unknown and deserves further investigation. The observation that approximately 6.7% of EVD survivors had markers of optic neuropathy and lower visual acuity approximately 3.5 years after the outbreak highlights the importance of early assessment during the acute phase, control of virus replication and inflammation, and periodic follow-up of survivors starting immediately after discharge to allow timely initiation of treatment.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. The full dataset of inflammatory marker analysis is provided in a Supplementary CSV file.
References
Centers for Disease, C. Prevention. Update: outbreak of Ebola viral hemorrhagic fever–Zaire, 1995. MMWR Morb Mortal. Wkly. Rep. 44, 468–469 (1995).
Centers for Disease Control and Prevention. Ebola outbreak history. (2024). Available at https://www.cdc.gov/ebola/outbreaks/index.html. Accessed August 1.
Billioux, B. J., Smith, B. & Nath, A. Neurological complications of Ebola virus infection. Neurotherapeutics 13, 461–470. https://doi.org/10.1007/s13311-016-0457-z (2016).
Shantha, J. G., Crozier, I. & Yeh, S. An update on ocular complications of Ebola virus disease. Curr. Opin. Ophthalmol. 28, 600–606. https://doi.org/10.1097/ICU.0000000000000426 (2017).
Eghrari, A. O. et al. Characterization of ebola virus-associated eye disease. JAMA Netw Open. https://doi.org/10.1001/jamanetworkopen.2020.32216 (2021).
Chertow, D. S. et al. Severe meningoencephalitis in a case of Ebola virus disease: A case report. Ann. Intern. Med. 165, 301–304. https://doi.org/10.7326/M15-3066 (2016).
Bowen, L. et al. Survivors of Ebola Virus Disease Have Persistent Neurologic Deficits. Neurology https://doi.org/10.1212/WNL.86.16_supplement.S53.003 (2016).
Varkey, J. B. et al. Persistence of Ebola virus in ocular fluid during convalescence. N Engl. J. Med. 372, 2423–2427. https://doi.org/10.1056/NEJMoa1500306 (2015).
Jacobs, M. et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet 388, 498–503. https://doi.org/10.1016/S0140-6736(16)30386-5 (2016).
Shantha, J. G. et al. Ophthalmic manifestations and causes of vision impairment in Ebola virus disease survivors in Monrovia, Liberia. Ophthalmology 124, 170–177. https://doi.org/10.1016/j.ophtha.2016.10.011 (2017).
Shantha, J. et al. Ebola virus persistence in ocular tissues and fluids (EVICT) study: reverse Transcription-Polymerase chain reaction and cataract surgery outcomes of Ebola survivors in Sierra Leone. EBioMedicine 30, 217–224. https://doi.org/10.1016/j.ebiom.2018.03.020 (2018).
Nguyen, N. V. et al. Neuro-ophthalmology implications in a cohort of Ebola virus disease survivors from the West African Ebola outbreak, Sierra Leone. Invest. Ophthalmol. Vis. Sci. 64 (2023).
Kelly, J. D. et al. Neurological, cognitive, and psychological findings among survivors of Ebola virus disease from the 1995 Ebola outbreak in Kikwit, Democratic Republic of congo: A Cross-sectional study. Clin. Infect. Dis. 68, 1388–1393. https://doi.org/10.1093/cid/ciy677 (2019).
Group, P. I. S. et al. A longitudinal study of Ebola sequelae in Liberia. N Engl. J. Med. 380, 924–934. https://doi.org/10.1056/NEJMoa1805435 (2019).
Worwa, G. et al. Persistent intraocular Ebola virus RNA is associated with severe uveitis in a convalescent rhesus monkey. Commun. Biol. 5, 1204. https://doi.org/10.1038/s42003-022-04158-2 (2022).
Alves, D. A. et al. Necrotizing scleritis, conjunctivitis, and other pathologic findings in the left eye and brain of an Ebola Virus-Infected rhesus macaque (Macaca mulatta) with apparent recovery and a delayed time of death. J. Infect. Dis. 213, 57–60. https://doi.org/10.1093/infdis/jiv357 (2016).
Mulangu, S. et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl. J. Med. 381, 2293–2303. https://doi.org/10.1056/NEJMoa1910993 (2019).
Hoff, N. A. et al. Immunogenicity of rVSVDeltaG-ZEBOV-GP Ebola vaccination in exposed and potentially exposed persons in the Democratic Republic of the congo. Proc. Natl. Acad. Sci. U S A. https://doi.org/10.1073/pnas.2118895119 (2022).
McElroy, A. K. et al. Human Ebola virus infection results in substantial immune activation. Proc. Natl. Acad. Sci. U S A. 112, 4719–4724. https://doi.org/10.1073/pnas.1502619112 (2015).
de Greslan, T. et al. Ebola Virus-Related encephalitis. Clin. Infect. Dis. 63, 1076–1078. https://doi.org/10.1093/cid/ciw469 (2016).
Sagui, E. et al. Severe Ebola virus infection with encephalopathy: evidence for direct virus involvement. Clin. Infect. Dis. 61, 1627–1628. https://doi.org/10.1093/cid/civ606 (2015).
Zeng, X. et al. Identification and pathological characterization of persistent asymptomatic Ebola virus infection in rhesus monkeys. Nat. Microbiol. 2, 17113. https://doi.org/10.1038/nmicrobiol.2017.113 (2017).
McWilliams, I. L. et al. Pseudovirus rVSVDeltaG-ZEBOV-GP Infects Neurons in Retina and CNS, Causing Apoptosis and Neurodegeneration in Neonatal Mice. Cell Rep 26, 1718–1726 e1714, (2019). https://doi.org/10.1016/j.celrep.2019.01.069
Hereth-Hebert, E. et al. Ocular complications in survivors of the Ebola outbreak in Guinea. Am. J. Ophthalmol. 175, 114–121. https://doi.org/10.1016/j.ajo.2016.12.005 (2017).
Mattia, J. G. et al. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect. Dis. 16, 331–338. https://doi.org/10.1016/S1473-3099(15)00489-2 (2016).
Shantha, J. G. et al. Ophthalmic sequelae and psychosocial impact in pediatric Ebola survivors. EClinicalMedicine 49, 101483. https://doi.org/10.1016/j.eclinm.2022.101483 (2022).
Tiffany, A. et al. Ebola virus disease complications as experienced by survivors in Sierra Leone. Clin. Infect. Dis. 62, 1360–1366. https://doi.org/10.1093/cid/ciw158 (2016).
Steptoe, P. J. et al. Multimodal imaging and Spatial analysis of Ebola retinal lesions in 14 survivors of Ebola virus disease. JAMA Ophthalmol. 136, 689–693. https://doi.org/10.1001/jamaophthalmol.2018.1248 (2018).
Steptoe, P. J. et al. Novel retinal lesion in Ebola survivors, Sierra Leone, 2016. Emerg. Infect. Dis. 23, 1102–1109. https://doi.org/10.3201/eid2307.161608 (2017).
Reynard, S. et al. Early control of viral load by favipiravir promotes survival to Ebola virus challenge and prevents cytokine storm in non-human primates. PLoS Negl. Trop. Dis. 15, e0009300. https://doi.org/10.1371/journal.pntd.0009300 (2021).
Baize, S. et al. Inflammatory responses in Ebola virus-infected patients. Clin. Exp. Immunol. 128, 163–168. https://doi.org/10.1046/j.1365-2249.2002.01800.x (2002).
Bixler, S. L. & Goff, A. J. The role of cytokines and chemokines in filovirus infection. Viruses 7, 5489–5507. https://doi.org/10.3390/v7102892 (2015).
Colavita, F. et al. Inflammatory and humoral immune response during Ebola virus infection in survivor and fatal cases occurred in Sierra Leone during the 2014(-)2016 outbreak in West Africa. Viruses 11 https://doi.org/10.3390/v11040373 (2019).
Reynard, S. et al. Immune parameters and outcomes during Ebola virus disease. JCI Insight. https://doi.org/10.1172/jci.insight.125106 (2019).
Li, J. Y., Boado, R. J. & Pardridge, W. M. Rat blood-brain barrier genomics. II. J. Cereb. Blood Flow. Metab. 22, 1319–1326. https://doi.org/10.1097/01.WCB.0000040944.89393.0f (2002).
Pardridge, W. M. Blood-brain barrier genomics. Stroke 38, 686–690. https://doi.org/10.1161/01.STR.0000247887.61831.74 (2007).
Zhang, Y. et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89, 37–53. https://doi.org/10.1016/j.neuron.2015.11.013 (2016).
McKenzie, A. T. et al. Brain cell type specific gene expression and Co-expression network architectures. Sci. Rep. 8, 8868. https://doi.org/10.1038/s41598-018-27293-5 (2018).
Kirchner, J. & Bevan, M. J. ITM2A is induced during thymocyte selection and T cell activation and causes downregulation of CD8 when overexpressed in CD4(+)CD8(+) double positive thymocytes. J. Exp. Med. 190, 217–228. https://doi.org/10.1084/jem.190.2.217 (1999).
Martinez-Murillo, P. A. et al. Refined innate plasma signature after rVSVDeltaG-ZEBOV-GP immunization is shared among adult cohorts in Europe and North America. Front. Immunol. 14, 1279003. https://doi.org/10.3389/fimmu.2023.1279003 (2023).
Menicucci, A. R., Jankeel, A., Feldmann, H., Marzi, A. & Messaoudi, I. Antiviral innate responses induced by VSV-EBOV vaccination contribute to rapid protection. mBio https://doi.org/10.1128/mBio.00597-19 (2019).
Team, P. S. et al. Randomized trial of vaccines for Zaire Ebola virus disease. N Engl. J. Med. 387, 2411–2424. https://doi.org/10.1056/NEJMoa2200072 (2022).
Steptoe, P. J. et al. Evolving longitudinal retinal observations in a cohort of survivors of Ebola virus disease. JAMA Ophthalmol. 138, 395–403. https://doi.org/10.1001/jamaophthalmol.2020.0173 (2020).
Acknowledgements
We thank Francis Isse and Ange Mubiala (National Institute for Biomedical Research in DRC) for remotely troubleshooting issues related to data entry software malfunction and data upload to the server during field work, and with data processing. We also thank Dr. Jeremie Muhindo, Director of the Beni General Hospital for providing the space to conduct the study.
Author information
Authors and Affiliations
Contributions
J.C.M., J.C.S.M., D.T.K. conceived and designed this study. D.R.M., M.J.B., S.A., B.J.G., D.O., J.G.S., S.Y., D.N.M. critically reviewed the study design and provided guidance for improvement. J.C.M., A.K.K., J.M.K., M.V.M., R.O.K., N.N.K., D.T.K. collected the data. J.C.M., A.K.K., J.B.K. processed the data. J.C.M., D.R.M. analyzed the data. J.C.M., D.R.M, J.G.S, S.Y., D.T.K. interpreted the data. J.C.M drafted the manuscript, and all other authors critically reviewed it and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mwanza, JC., Kahindo, A.K., Mbusa-Kombi, J. et al. Ophthalmological manifestations and plasma markers of inflammation in Ebola survivors in post-treatment era. Sci Rep 15, 14966 (2025). https://doi.org/10.1038/s41598-025-96256-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96256-4