Abstract
Research suggests a potential link between varicella zoster virus (VZV) and Parkinson’s disease (PD), but the causal relationship between anti-VZV IgG levels and PD is not well understood. Using two-sample Mendelian Randomization (MR), we assessed the causal impact of anti-VZV IgG levels on PD risk and progression. Our study found a significant association between higher anti-VZV IgG levels and an increased risk of PD. For PD progression, higher anti-VZV IgG levels were linked to a greater risk of constipation, insomnia, and Restless Legs. These findings remained consistent after sensitivity analyses. In conclusion, our study suggests that elevated anti-VZV IgG levels may contribute to an increased risk and progression of PD, supporting a potential causal link that warrants further mechanistic investigation.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, with incidence expected to double in the next 30 years, significantly impacting society1. Effective treatments for the advanced stages of the disease are lacking. PD primarily involves the loss of dopaminergic neurons in the substantia nigra2. Although extensive research has been conducted, the precise cause of PD remains unclear. Recent studies suggest that infections might contribute to the development of the disease3. Neurotropic viruses, including varicella-zoster virus (VZV), are potential contributors but have been less studied compared to genetic and environmental factors3.
VZV causes chickenpox initially and can become latent in sensory ganglia, reactivating later as herpes zoster. Some studies have detected VZV DNA in the brains of Parkinson’s patients, suggesting a possible role in the disease’s development4. Viruses can affect PD through multiple mechanisms. For example, in rat experiments, viral infections such as HCV altered dopaminergic neurotransmission and induced dopaminergic neurotoxicity (60% of dopaminergic neurons died)5. Influenza A virus can enter the substantia nigra of the mouse brain through selective and reproducible invasion and ultimately cause cell death6. Viral infections trigger inflammatory and autoimmune processes in PD patients. A novel genetic association with PD has been identified in human leukocyte antigens and supports the involvement of the immune system in PD7. Inflammation caused by viral infections can trigger misfolding, aggregation, and multiplication of α-synuclein, which promotes the development of PD8. In addition, VZV may induce a molecular mimicry effect that leads to an indiscriminate attack by the immune system on the patient’s own tissues, leading to the worsening of Parkinson’s disease9,10,11,12.
Traditional observational studies often struggle with confounding factors and biases, complicating the determination of causal relationships in complex diseases like PD. These genetic variants, serving as instrumental variables, are randomly allocated during meiosis, thereby remaining independent of confounders and immune to reverse causation13. It provides a valuable alternative by using genetic variants as proxies for exposures, thereby mimicking the randomization of controlled experiments14,15,16,17,18,19,20.
This paper investigates the causal effects of VZV-induced antigen–antibody reactions on PD using MR. By utilizing large genetic datasets and sophisticated statistical techniques, we aim to address the limitations of conventional studies and provide more reliable insights into this potential causal relationship.
Genetic variants linked to VZV infection and the immune response can be identified through genome-wide association studies (GWAS). The alleles of this exposure-associated genetic variant are randomly allocated and not subject to reverse causation13. By using these variants as proxies for VZV-related immune responses, we can evaluate their impact on the risk and progression of PD with reduced bias15.
Understanding the causal relationship between VZV-induced immune responses and PD could significantly enhance our understanding of the disease’s development. It may reveal new therapeutic targets and preventive strategies. Here, we hypothesized that elevated anti-VZV IgG levels may contribute to an increased risk and progression of PD.
This study seeks to advance our understanding of PD etiology and explore new avenues for effective prevention and treatment. The application of MR in this context holds significant promise for clarifying the intricate relationship between infections and neurodegenerative diseases.
Methods
Study design
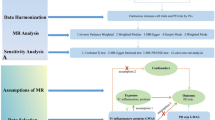
Figure 1 illustrates the diagram of the study design. (1) MR analysis identifying the causal effects of herpes zoster (caused by VZV infection) on risk of PD. (2) MR analysis identifying the causal effects of anti-VZV IgG levels on risk of PD. (3) MR analysis identifying the causal effects of anti-VZV IgG levels on progression of PD.
Acquisition of instrumental variables
Instrumental variables (IVs) were sourced from published genome-wide association studies (GWASs). Only pooled GWAS data from European populations were used to minimize bias related to population stratification. In Mendelian randomization studies, the predominant focus on European populations stems from the current predominance of European-centric genetic data repositories (e.g., GWAS catalogues), where genetic instruments (IVs) have been more thoroughly characterized and validated. Critical methodological considerations include fundamental differences in linkage disequilibrium patterns across populations and population-specific gene-environment interactions, which may violate core MR assumptions (particularly instrument independence) when extrapolating findings to non-European ancestries. While this practice raises concerns about generalizability, it reflects a pragmatic trade-off prioritizing internal validity given existing data limitations for diverse populations. Future progress requires systematic expansion of multi-ancestry genomic initiatives to reconcile scientific rigor with health equity, enabling robust trans-ethnic causal inference while preserving methodological integrity.
Acquisition of instrumental variables related to VZV infection
Genetic variants related to herpes zoster (caused by VZV infection) were obtained from the FinnGen Biobank. Herpes zoster diagnoses were based on International Classification of Diseases (ICD) codes from the Finnish Register of Inpatients, Outpatients, and Causes of Death. The dataset included 2080 cases and 211,856 controls from individuals of Finnish ancestry21.
Initially, we used a GWAS significance threshold of P < 5.0 × 10−8 to identify SNPs associated with herpes zoster (caused by VZV infection). However, this did not yield enough SNPs. Since elevated anti-varicella-zoster virus (VZV) IgG levels also indicate VZV infection and are a more objective measure, we used these levels to study the causal effects of VZV infection on PD.
Summary data on SNPs associated with anti-VZV IgG levels were obtained from the GWAS meta-analysis by Guillaume et al. based on the UK Biobank database. The UK Biobank recruited over 500,000 UK adults between 2006 and 2010, with a subset of 9724 participants (not overlapping with FinnGen Biobank database) providing serum samples for serologic measurements of 20 different microorganisms22. These samples were tested for total antibody levels against various antigens, including anti-VZV IgG. A total of 31 SNPs associated with anti-VZV IgG levels were identified through application of a GWAS significance threshold (P < 5.0 × 10⁻⁸).
We used Eq. (1) to calculate the F-statistic for each SNP associated with VZV infection, including only those with F-statistics greater than 10 to avoid weak IV effects.
where R2 represents the proportion of variance in the exposure attributed to the SNP, and N is the sample size for the GWAS of the exposure.
Linkage disequilibrium (LD) refers to the tendency of neighboring genetic variants to be inherited together23. Confounders in high LD with selected variants could introduce bias. To minimize this risk, we applied stringent statistical thresholds (R2 < 0.001, window size > 10,000 kb) to exclude variants with high LD24.
We used summarized data on PD to assess the effects of the selected SNPs on the outcome phenotype. Exposure and outcome databases were harmonized to ensure consistent alignment of effector alleles, and palindromic SNPs were excluded. Reverse MR analysis was conducted to rule out the possibility that PD could influence VZV infection (Table 1).
Acquisition of instrumental variables related to PD
The study examined the risk and progression of PD through two main aspects. GWAS summary data from Nalls et al. 2019 meta-analysis were used to investigate the causal relationship between anti-VZV IgG levels and PD risk25. This analysis included 17 European PD cohorts (no participant overlapping with UK Biobank or MRC IEU OpenGWAS datasets) with 37,688 patients (either self-reported or clinically diagnosed), 18,618 proxy cases (undetected but with a first-degree relative confirmed) from the UK Biobank, and over 1.4 million controls. Additional data on 33,647 patients with PD and 449,056 controls were sourced from the MRC IEU OpenGWAS database26.
PD progression is marked by changes in scores from scales like the Unified Parkinson’s Disease Rating Scale (UPDRS) and the Montreal Cognitive Assessment (MOCA), as well as by worsening symptoms such as constipation and depression. Iwaki et al. conducted a GWAS analysis on more than 20 clinical progression phenotypes involving 4093 patients with PD across 12 cohorts with 22,307 observations over a median of 3.81 years27. Supplementary Table 1 summarizes the GWAS pooled data we collected for the two exposures and 20 clinical phenotypes. The 20 clinical phenotypes included 11 binary outcomes: PD Risk at onset, Hoehn-Yahr stage 3 or higher (HY3), constipation, dementia, depression, Rapid Eye Movement Sleep Behavioral Disorder (RBD), insomnia, hyposmia, restless legs, and motor fluctuations or dyskinesia. Additionally, there were 9 continuous outcomes: PD Age at onset, UPDRS I-IV and total, MoCA, MMSE, and SEADL28.
Assessment of instrumental variables and sensitivity analysis
For MR to yield unbiased results, instrumental variables (IVs) must meet three key assumptions: (1) they must be significantly correlated with the exposure, (2) they must be independent of any confounders, and (3) they should influence the outcome only through their effect on the exposure16.
We implemented various procedures to check these assumptions. Pleiotropy, where a SNP affects multiple traits, can breach the independence and exclusivity assumptions, potentially leading to biased conclusions14.
Initially, we conducted an assessment of pleiotropy, defined as the potential for a single nucleotide polymorphism (SNP) to be associated with multiple phenotypic outcomes. The presence of pleiotropic SNPs can undermine the independence and exclusivity assumptions inherent to instrumental variables, which may consequently introduce bias into our analyses. To evaluate pleiotropy, we employed two established methods: the MR-Egger intercept test and the MR-PRESSO global test29,30. The MR-Egger intercept test evaluates the presence of pleiotropy by examining whether the intercept of the regression line deviates from zero. A significant deviation suggests that at least some of the SNPs may influence the outcome through pathways other than the exposure of interest, indicating potential pleiotropic effects. The MR-PRESSO global test identifies and adjusts for the presence of outliers that may indicate pleiotropic effects. This method assesses the overall impact of pleiotropic SNPs on the analysis. A P-value of less than 0.05 in either the MR-Egger intercept test or the MR-PRESSO global test is regarded as indicative of a pleiotropic effect.
Significant variation among SNPs could signal an invalid SNP, so we assessed heterogeneity using Cochran’s Q test in both IVW and MR-Egger analyses. Cochran’s Q test is a statistical method used to assess heterogeneity among effect estimates derived from different instrumental variables31. It quantifies the degree of variability in the estimates that cannot be attributed to sampling error alone. A significant Q statistic (p-value < 0.05) suggests the presence of heterogeneity, indicating that the causal effect may differ across the instrumental variables, potentially due to underlying biological differences or pleiotropic effects.
We employed the leave-one-out (LOO) method, which involves iteratively removing individual SNPs and re-evaluating their effects (Supplementary Fig. 2). This helps identify any SNPs that significantly impact the association, thereby validating the robustness of our results.
MR analysis
We used the inverse variance weighting (IVW) method with multiplicative random effects for the primary analysis due to its high statistical validity, provided the instrumental variables (IVs) meet the necessary assumptions32. To strengthen our results, we also applied MR Egger, weighted median, simple mode, and weighted mode methods33,34.
Results
Association between genetically predicted anti-VZV IgG levels and PD risk
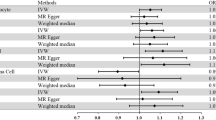
Our MR analysis revealed a significant association between increased anti-VZV IgG levels and higher risk of PD. The IVW model showed an odds ratio (OR) of 1.1388 (95% CI 1.0146–1.2781) per standard deviation (SD) increase in anti-VZV IgG levels (P = 0.0273) (Fig. 2). Sensitivity analyses using various MR models—MR-Egger, weighted median, simple mode, and weighted mode—yielded consistent results with the IVW model (Fig. 2 and Supplementary Table 2), confirming the robustness of this association. A scatter plot displaying the effect sizes of SNPs on anti-VZV IgG levels and PD risk is shown in Supplementary Fig. 1a. The leave-one-out (LOO) analysis did not identify any SNPs that significantly impacted the overall effect estimate (Supplementary Fig. 2a). Quality control checks for instrumental variables (IVs) indicated no evidence of heterogeneity or pleiotropy in the association between anti-VZV IgG levels and PD risk (Supplementary Table 2).
Association between genetically predicted anti-VZV IgG levels and PD progression
Our MR analysis also found significant associations between increased anti-VZV IgG levels and several aspects of PD progression. Specifically, higher anti-VZV IgG levels were associated with an increased risk of constipation (IVW OR = 1.668, 95% CI 1.147–2.425, P = 0.007, FDR-corrected P = 0.025) (Fig. 3a). Additionally, higher anti-VZV IgG levels correlated with more advanced Hoehn-Yahr stages (IVW OR = 3.044, 95% CI 1.691–5.479, P < 0.001, FDR-corrected P = 0.002) (Fig. 3a), increased risk of insomnia (IVW OR = 1.807, 95% CI 1.313–2.488, P < 0.001, FDR-corrected P = 0.002), and a greater likelihood of Restless Legs (IVW OR = 2.649, 95% CI 1.476–4.754, P = 0.0001, FDR-corrected P = 0.005) (Fig. 3a). Higher anti-VZV IgG levels were also linked to a lower UPDRS I (IVW Beta = 0.652, 95% CI 0.547–0.777, P < 0.001, FDR-corrected P = 0.005) (Fig. 3b). The scatter plots of SNP effects on exposure and PD outcomes are displayed in Supplementary Fig. 1b–f. LOO analysis did not reveal any SNPs with a significant effect on the overall estimates (Supplementary Fig. 2b–f).
Forest plot of Mendelian randomization results using the inverse-variance weighted method. This plot shows the inverse-variance weighted Mendelian randomization (MR) estimates of anti-VZV-IgG levels for (a) binary and (b) continuous outcomes related to Parkinson’s Disease (PD). It reveals a significant link between anti-VZV-IgG levels and risks of constipation, Hoehn-Yahr stage 3 or more, insomnia, restless legs, and UPDRS I in PD. Each square in the plot represents an estimate of the effect of anti-VZV-IgG levels on PD, with the horizontal line indicating the 95% confidence intervals (CI). For binary outcomes, MR estimates are presented as odds ratios (ORs) with 95% CIs. For continuous outcomes, MR estimates are presented as betas with 95% CIs. Abbreviations: FDR (False Discovery Rate), HY3 (Hoehn-Yahr Stage 3 or more), MMSE (Mini-Mental State Examination), MoCA (Montreal Cognitive Assessment), SEADL (Schwab and England Activities of Daily Living Scale).
In sensitivity analyses, the robust MR models aligned with the IVW results, confirming the robustness of the associations (Supplementary Table 2). However, quality control checks indicated potential issues with some IVs, as heterogeneity or pleiotropy analyses showed potential violations in the associations for Hoehn-Yahr stage 3 (Pleiotropy, P = 0.04) and UPDRS Part I score (Pleiotropy, P = 0.004; Heterogeneity, Q = 0.028, Supplementary Table 2).
Discussion
This study leveraged the largest available GWAS dataset to perform a comprehensive two-sample MR analysis, investigating the relationship between anti-VZV-IgG levels and PD (PD) risk and progression. Our findings suggest a significant association between elevated anti-VZV-IgG levels and an increased risk of both PD and its progression, providing genetic evidence consistent with a potential role of VZV infection in PD pathogenesis. However, the exact mechanisms (e.g., viral neuroinvasion, molecular mimicry, or neuroinflammation) remain to be elucidated.
PD risk
Our MR analysis indicated a significant association between increased anti-VZV-IgG levels and a higher risk of PD (IVW OR = 1.1388, 95% CI 1.0146–1.2781, P = 0.0273). This suggests that past exposure to varicella zoster virus may influence the risk of PD. Our findings align with meta-analytic evidence demonstrating a 20% increased risk of PD among individuals exposed to pathogens, including hepatitis C virus (HCV) and influenza A35. These pathogens share common mechanisms such as blood–brain barrier disruption, neuroinflammation, and direct neuronal damage. However, our study extends this literature by specifically implicating varicella-zoster virus (VZV) through elevated anti-VZV IgG levels as a potential risk modulator.
Notably, while Meng et al. focused on bacterial and viral infections broadly, our MR analysis provides genetic evidence for a direct link between VZV-specific immune responses and PD pathogenesis. Unlike HCV or influenza A, VZV exhibits unique latency-reactivation dynamics in sensory ganglia, which may lead to chronic antigenic stimulation and autoimmune cross-reactivity with nigral tissues—a mechanism not yet explored in prior studies.
While our MR analysis supports a causal association between anti-VZV IgG levels and PD, it does not directly establish a biological mechanism. Future studies should explore whether VZV reactivation triggers α-synuclein aggregation, disrupts dopaminergic neurotransmission, or induces chronic neuroinflammation—pathways implicated in other viral infections. Experimental models (e.g., VZV-challenged neuronal cultures or animal studies) are needed to validate these hypotheses. If VZV-specific mechanisms are confirmed, antiviral prophylaxis (e.g., zoster vaccination) could be prioritized in high-risk populations, paralleling strategies proposed for HCV-related PD risk reduction5.
Constipation in PD
Constipation is a common issue among patients with PD, with prevalence rates ranging from 24.6 to 63% and severity often correlating with PD progression36,37. Our study found a significant correlation between elevated anti-VZV-IgG levels and an increased risk of constipation in patients with PD. This finding is novel, as there are no previous reports linking anti-VZV-IgG levels to constipation in PD. Previous cases have suggested VZV reactivation can lead to conditions such as Ogilvie syndrome and Elsberg syndrome, which present with gastrointestinal symptoms, including constipation38,39,40. We hypothesize that latent VZV infection might damage sacral nerves, contributing to constipation in patients with PD. Further research using animal and cellular models is needed to elucidate the mechanisms underlying this association.
Insomnia in PD
Elevated anti-VZV-IgG levels were also significantly associated with an increased risk of insomnia in patients with PD. This finding supports previous observations of neuro-psychiatric symptoms following VZV infection, including cases of persistent CNS inflammation11. Our study suggests that anti-VZV-IgG levels may serve as a potential target for managing sleep disorders in PD. The association between VZV and sleep disturbances highlights the need for further investigation into the impact of VZV infection on PD-related sleep disorders.
Hoehn-Yahr stage and motor symptoms
Our MR analysis study observed a significant association between higher anti-VZV-IgG levels and increased Hoehn-Yahr (HY) stages, indicating more advanced PD. This discrepancy may suggest that anti-VZV-IgG levels could influence PD motor symptoms indirectly. Understanding the correlation between anti-VZV-IgG levels and motor symptoms could shed light on how viral infections might affect PD progression and help identify new therapeutic targets or preventive measures.
Limitations
Several limitations must be considered. First, while anti-VZV-IgG levels provide insight into past VZV exposure, they do not distinguish between the virus itself and the immune response it triggers. Therefore, it is unclear whether the VZV virus directly causes PD or if the immune response contributes to disease development. Second, the study lacked GWAS data for anti-VZV-IgM, which could have provided additional insights. Third, the statistical power to detect smaller effect size changes may be limited, and sample overlap between the GWAS datasets for anti-VZV-IgG and PD could introduce bias41. However, the high mean F-statistic of the SNP set suggests minimal impact from sample overlap42. Further studies in diverse populations are needed to confirm these findings and explore the causal relationship between anti-VZV-IgG levels and PD.
In conclusion, our two-sample MR study are the first to identify a significant association between elevated anti-VZV-IgG levels and increased PD risk and progression. These findings suggest that changes in anti-VZV-IgG levels could serve as a useful biomarker for monitoring PD risk and progression. Further research into the pathophysiological mechanisms underlying these associations may provide valuable insights into the role of VZV in PD and inform future therapeutic strategies.
Data availability
The datasets used and analysed during the current study available from thecorresponding author on reasonable request.
References
Tolosa, E., Garrido, A., Scholz, S. W. & Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 20, 385–397. https://doi.org/10.1016/S1474-4422(21)00030-2. (2021).
Kalia, L. V. & Lang, A. E. Parkinson’s disease. Lancet (London, England) 386, 896–912. https://doi.org/10.1016/S0140-6736(14)61393-3 (2015).
Smeyne, R. J., Noyce, A. J., Byrne, M., Savica, R. & Marras, C. Infection and risk of Parkinson’s disease. J. Parkinsons Dis. 11, 31–43. https://doi.org/10.3233/JPD-202279 (2021).
Hemling, N. et al. Herpesviruses in brains in Alzheimer’s and Parkinson’s diseases. Ann. Neurol. 54, 267–271 (2003).
Wu, W. Y. Y. et al. Hepatitis C virus infection: A risk factor for Parkinson’s disease. J. Viral Hepat. 22, 784–791. https://doi.org/10.1111/jvh.12392 (2015).
Takahashi, M. et al. The substantia nigra is a major target for neurovirulent influenza A virus. J. Exp. Med. 181, 2161–2169 (1995).
Hamza, T. H. et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat. Genet. 42, 781–785. https://doi.org/10.1038/ng.642 (2010).
Lema Tomé, C. M. et al. Inflammation and α-synuclein’s prion-like behavior in Parkinson’s disease–is there a link?. Mol. Neurobiol. 47, 561–574. https://doi.org/10.1007/s12035-012-8267-8 (2013).
Matsuo, K., Honda, M. & Shiraki, K. Role of neutralizing antibody in the pathogenesis of zoster and the correlation of severity with anti-gE: gi antibody response. J. Dermatol. 30, 109–115 (2003).
Giller, R. H., Winistorfer, S. & Grose, C. Cellular and humoral immunity to varicella zoster virus glycoproteins in immune and susceptible human subjects. J. Infect Dis. 160, 919–928 (1989).
Dahiya, D. et al. Case report: Varicella associated neuropsychiatric syndrome (VANS) in two pediatric cases. Brain Behav. Immun. Health 28, 100602. https://doi.org/10.1016/j.bbih.2023.100602 (2023).
Caggiu, E. et al. Humoral cross reactivity between α-synuclein and herpes simplex-1 epitope in Parkinson’s disease, a triggering role in the disease?. J. Neuroimmunol. 291, 110–114. https://doi.org/10.1016/j.jneuroim.2016.01.007 (2016).
Sekula, P., Del-Greco, M. F., Pattaro, C. & Köttgen, A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol 27, 3253–3265 (2016).
Davey Smith, G. & Hemani, G. Mendelian randomization Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23, 189–198. https://doi.org/10.1093/hmg/ddu328 (2014).
Zhou, H. et al. Mendelian randomization reveals association between retinal thickness and non-motor symptoms of Parkinson’s disease. Npj Parkinson’s Dis https://doi.org/10.1038/s41531-023-00611-z (2023).
Davies, N. M., Holmes, M. V. & Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 362, k601. https://doi.org/10.1136/bmj.k601 (2018).
Zeng, R. et al. Lack of causal associations of inflammatory bowel disease with parkinson’s disease and other neurodegenerative disorders. Mov Disord 38, 1082–1088. https://doi.org/10.1002/mds.29386 (2023).
Senkevich, K. et al. Potential protective link between type I diabetes and parkinson’s disease risk and progression. Mov Disord 38, 1350–1355. https://doi.org/10.1002/mds.29424 (2023).
Chohan, H. et al. Type 2 diabetes as a determinant of parkinson’s disease risk and progression. Mov Disord 36, 1420–1429. https://doi.org/10.1002/mds.28551 (2021).
Coneys, R., Storm, C. S., Kia, D. A., Almramhi, M. & Wood, N. W. Mendelian randomisation finds no causal association between urate and Parkinson’s disease progression. Mov Disord 36, 2182–2187. https://doi.org/10.1002/mds.28662 (2021).
Zhong, S. et al. Association between viral infections and glioma risk: A two-sample bidirectional Mendelian randomization analysis. BMC Med 21, 487. https://doi.org/10.1186/s12916-023-03142-9 (2023).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209. https://doi.org/10.1038/s41586-018-0579-z (2018).
Pritchard, J. K. & Przeworski, M. Linkage disequilibrium in humans: models and data. Am. J. Hum. Genet. 69(1), 1–14 (2001).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife https://doi.org/10.7554/eLife.34408 (2018).
Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102. https://doi.org/10.1016/S1474-4422(19)30320-5 (2019).
Elsworth, B. et al. The MRC IEU OpenGWAS data infrastructure. https://doi.org/10.1101/2020.08.10.244293 (2020).
Iwaki, H. et al. Genomewide association study of Parkinson’s disease clinical biomarkers in 12 longitudinal patients’ cohorts. Mov. Disord. 34, 1839–1850. https://doi.org/10.1002/mds.27845 (2019).
Zhou, H. et al. Mendelian randomization reveals association between retinal thickness and non-motor symptoms of Parkinson’s disease. NPJ Parkinsons Dis. 9, 163. https://doi.org/10.1038/s41531-023-00611-z (2023).
Haycock, P. C. et al. Best (but oft-forgotten) practices: the design, analysis, and interpretation of Mendelian randomization studies. Am. J. Clin. Nutr. 103, 965–978 (2016).
Verbanck, M., Chen, C.-Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698. https://doi.org/10.1038/s41588-018-0099-7 (2018).
Zhu, D. et al. Total brain volumetric measures and schizophrenia risk: A two-sample Mendelian randomization study. Front Genet. 13, 782476. https://doi.org/10.3389/fgene.2022.782476 (2022).
Burgess, S. et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res 4, 186. https://doi.org/10.12688/wellcomeopenres.15555.3 (2019).
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525. https://doi.org/10.1093/ije/dyv080 (2015).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314. https://doi.org/10.1002/gepi.21965 (2016).
Meng, L., Shen, L. & Ji, H.-F. Impact of infection on risk of Parkinson’s disease: A quantitative assessment of case-control and cohort studies. J. Neurovirol. 25, 221–228. https://doi.org/10.1007/s13365-018-0707-4 (2019).
Stocchi, F. & Torti, M. Constipation in Parkinson’s disease. Int. Rev. Neurobiol. 134, 811–826. https://doi.org/10.1016/bs.irn.2017.06.003 (2017).
Camacho, M. et al. Early constipation predicts faster dementia onset in Parkinson’s disease. NPJ Parkinson’s Dis. 7, 45. https://doi.org/10.1038/s41531-021-00191-w (2021).
Masood, I. et al. Herpes Zoster-induced Ogilvie’s syndrome. Case Rep. Surg. 2015, 563659. https://doi.org/10.1155/2015/563659 (2015).
Bhalerao, V. M., Gedam, B. S. & Mukharjee, A. K. Herpes Zoster infection presenting with urinary retention and constipation. J. Assoc. Phys. India 63, 63–65 (2015).
Lefeuvre, L., Schibler, M., Lalive, P. H. & Elsberg,. Syndrome secondary to cytomegalovirus infection in an immunocompetent patient: A case report. Neurol. Neuroimmunol. Neuroinflamm. https://doi.org/10.1212/NXI.0000000000200079 (2023).
Yang, G. & Schooling, C. M. Investigating sex-specific associations of lipid traits with type 2 diabetes, glycemic traits and sex hormones using Mendelian randomization. Cardiovasc. Diabetol. 22, 3. https://doi.org/10.1186/s12933-022-01714-2 (2023).
Minelli, C. et al. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int. J. Epidemiol. 50, 1651–1659. https://doi.org/10.1093/ije/dyab084 (2021).
Acknowledgements
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China [Grant No.: 81702469], Natural Science Foundation of Shandong Province [Grant No.: ZR2023MH321], the Key R&D Program of Shandong Province, China [Grant No.: 2022ZLGX03], and 2022 Industrial Technology Basic Public Service Platform Project under Grant No. 2022-189-181.
Author information
Authors and Affiliations
Contributions
C.Z. and W.L. performed the conceptualization; C.Z. performed the resource and funding acquisition; S.M., X.L. and J.S. performed the data analysis and investigation; Z.L., X.L and J.S. performed the formal analysis and data curation; H.W., Q.W., H.L, and G.Z. performed the validation; J.J. and J.S. performed the methodology; Q.Q.W. and X.L. performed the supervision; J.S. and Y.H. performed the visualization, software, project administration, and writing—original draft. All authors performed the writing—review & editing and approve of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Ethics Committee of Qilu Hospital, Shandong University, and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, J., Li, X., Ma, S. et al. Impact of anti-VZV IgG levels on Parkinson’s disease risk and progression: a Mendelian randomization analysis. Sci Rep 15, 11985 (2025). https://doi.org/10.1038/s41598-025-96382-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96382-z