Abstract
The distribution pattern of vascular bundles and microstructure characteristics significantly impact crop yield. Previous studies have primarily focused on investigating the micro-phenotypic characteristics and genetic regulation of individual internode, neglecting the exploration of the relationship between different internodes. This study, for the first time, comprehensively analyzed multi-scale phenotypic information of stem cross-sections, zones, and vascular bundles in three different internodes (basal third internode, ear internode and highest internode) of 268 inbred maize lines using Micro-computed tomography scanning. Key findings revealed that basal third internode and ear internode exhibited more stable microscopic characteristics than highest internode. Inbred lines with higher numbers of vascular bundle and well-developed inner zone in ear internode exhibited better yield characteristics, particularly in the kernel number per row. Genome-wide association analysis respectively identified 15, 1 and 1 putative candidate genes in basal third internode, ear internode and highest internode. These genes encode a variety of enzymes, such as oxidases, synthetases, ligase enzyme and protein kinases. Notably, Zm00001d042490 may be an important putative candidate gene for The number of vascular bundles in the periphery zone and corn grain traits. This study provides an important theoretical basis and genetic resources for accurately identifying different internode phenotypes of maize stalks, potentially advancing the selection of high-yielding, high-quality maize varieties.
Similar content being viewed by others
Introduction
In 1928, Mason and Maskell pioneered the “source-flow-sink” theory while investigating photosynthate distribution in cotton plants. This theory posits that optimal crop yield is achieved through a harmonious balance of three elements: an abundant source, a large sink (storage capacity), and smooth flow1,2. The flow component acts as a crucial intermediary, facilitating the transport of assimilates and biological signals from the source to the storage reservoir3. Central to this process are vascular bundles, which serve as the primary conduits of flow and provide essential structural support in vascular plants4,5. These specialized tissues play a vital role in transporting photosynthates, organic nutrients, and inorganic macromolecules, while simultaneously contributing to the plant’s mechanical strength and stability6. The intricate interplay of these elements underpins the physiological basis for high crop productivity7,8.
Traditional research on maize stems primarily relied on microscopic analysis of internal stem tissues, focusing on two major functions: mechanical support and substance transport. Studies identified that maize varieties with well-developed internal mechanical tissues exhibited characteristics such as thick stem epidermis, high peripheral density of vascular bundles, thick bundle sheaths, and numerous lignified thin-walled cells9,10,11. Interestingly, changes in internode cross-sectional area were found to be more accurate predictors of stalk strength compared to changes in internode material composition, with this predictive ability remaining consistent across varieties and planting densities12,13,14. Further research on maize varieties resistant to high planting densities revealed significant differences in the microstructure of vascular bundles in the third basal stem internode15. A notable positive correlation was observed between total stem flow and the area of large vascular bundles. The high-density-resistant Zhengdan 958 variety, even when densely planted, maintained superior vascular bundle structure, including higher numbers and areas of vascular bundles, resulting in enhanced stem flow rates. This advantage was considered a key factor in its high yield under high-density conditions15. Well-developed vascular bundles were found to provide strong mechanical support and establish more effective transport capabilities in plants7. The size, quantity, and capacity of vascular bundles significantly influenced the efficiency of photosynthate transport16,17. He et al. discovered positive correlations between maize shank internode characteristics (such as the number of large vascular bundles, their average area, and total area) and grain volume and spikelet count18.
The exploration of stem anatomical diversity has historically been hindered by traditional, labor-intensive microscopic measurements19. However, the widespread adoption of high-throughput technologies in botany has revolutionized this field, facilitating large-scale, multi-dimensional, and efficient micro-phenotypic studies of stems and vascular bundles20,21,22,23. Zhang et al. have developed sophisticated Micro-computed tomography (Micro-CT) data collection workflows and Micro-CT image processing software to address this challenge19,20,24. A notable advancement is the implementation of intelligent vascular bundle analysis software (VesselParser) based on “zones”, which categorizes stem cross-sections into three functional regions: the epidermal zone, periphery zone, and inner zone20. This approach identified 84 putative candidate genes associated with vascular bundle number (VB_N), vascular bundle area (VB_A) and vascular bundle distribution (VB_D). These putative candidate genes are linked to enzymes involved in cell wall metabolism regulation, transcription factors, protein kinases, and stress-related genes. Building on these findings, Zhao and Guo utilized Micro-CT scanning technology to obtain multiple vascular bundle phenotypes of ear internode and shank25,26. Their subsequent genome-wide association study (GWAS) analysis revealed that putative candidate genes in ear internodes are involved in biological processes such as organic matter transport, tissue development, and aminopolysaccharide catabolism. Furthermore, putative candidate genes related to the shank were found to primarily encode lignin metabolic regulatory enzymes, cellulose synthetase, transcription factors.
Extensive research has been conducted on the phenotypic characteristics and genetic performance of maize stem internodes, including basal third internode, ear internode and shank19,25,26. However, these studies have primarily focused on stem vascular bundles at a single internode, leaving gaps in our understanding of the relationship between microscopic traits of each internode and their effect on yield. Moreover, the complexity of interactions between different internodes in transport flow remains unclear. In this pioneering study, we employed Micro-CT scanning technology coupled with a deep learning-based stem vascular bundle phenotype analysis method to obtain and analyze multi-scale phenotypic information. For the first time, we examined cross-sections, zones and vascular bundles of different internodes (basal third internode, ear internode and highest internode) within the same plant. We further explored the correlation between microscopic phenotypes of different internodes and yield-related traits. Our primary objectives were to elucidate the relationship between internode traits and yield components such as kernel rows per ear, kernel number per row, hundred- kernel weight kernel weight per ear, and grain weight per ear Additionally, we aimed to identify putative candidate genes that may influence key traits through genome-wide association study (GWAS) analysis. The findings of this research establish an important theoretical foundation for accurately identifying stem vascular phenotypes of different maize internodes. This knowledge will contribute significantly to promoting the selection of new cultivars with enhanced yield and quality characteristics.
Materials and methods
Plant material and growth conditions
We used a maize association mapping population developed by Professor Jianbing Yan from Huazhong Agricultural University27. The population consisted of 268 maize inbred lines with genetic diversity, representing a wide genetic background of temperate, tropical, and subtropical maize germplasm. This provided an ideal material for studying stem microstructural traits and yield component. To evaluate the impact of the environment on stem microstructural traits, we conducted field experiments at two different locations. In 2018, 268 maize inbred lines were grown at Tongzhou Experimental Station of Beijing Academy of Agriculture and Foresting Science, in Beijing (116.68°E, 39.69°N). Sowing took place on 28 April 2018. Each inbred line was planted in four-row plot, with eight plants each row. In 2021, same maize inbred lines were grown at the experimental base of the Beijing Academy of Agriculture and Foresting Sciences at Sanya (109.19◦ E, 18.39◦ N). Sowing took place on 20 March 2021. Each row was 2.1 m long, and 60 cm between rows.
Sample pretreatment and preparation techniques
Sample collection
The basal third internode, the ear internode and the highest internode of the same plant were collected at silking stage (73 d after seeding) and immediately immersed in FAA solution (90:5:5 v/v/v, 70% ethanol :100% formaldehyde :100% acetic acid) in triplicate for each inbred line.
Dehydration
The test samples removed from the FAA fixative were dehydrated by ethanol gradient, and the specific process was as follows: The stem samples were dehydrated in 70% ethanol and 100% ethanol successively for 24 h each, and then underwent dehydration substitution with tert-Butanol. Specifically, the samples were soaked in 100% tert-Butanol for 24 h for the first time, and after replacing the 100% tert-Butanol with fresh solution, they were soaked again for another 24 h.
Ultra-low temperature drying
After completion of tertbutyl alcohol replacement, samples were removed into − 80-degree refrigerator and frozen for 48 h. The frozen samples were taken out and placed in the sample compartment of an ultra-low temperature freeze-drying instrument (LGJ-10E, China) and freeze-dried at -80 °C for 2 h.
Acquisition of microscopic phenotypes
The Micro-CT maize scanning technology system was used to quantitatively analyze the stem microstructure of 268 maize inbred lines in the maize association analysis population. The microscopic phenotype analysis of the basal third, ear position and top position of maize stem in multiple environments was completed.
Micro-CT scanning
The Skyscan 1172 X-ray computed tomography system (Bruker Corporation) scanned uniformly stained samples, 40 kV/250 mA, the sample was scanned by 180° rotations, and each scan took about 22 min. The specific parameters were referred to the Micro-CT scanning parameters set by Zhang28. The scanning resolution was 13.55 μm and the scanning mode was 2 K mode (2000 × 1332 pixels). Sections (cross-sectional images) of the stem samples were reconstructed as 8-bit BMP files.
Image reconstruction
Skyscan NRecon software (Bruker Corporation) was used to reconstruct the original image twice, and the Hounsfield unit (HU) was uniformly set from − 500 to 9240 to obtain a series of 8-bit corn stem cross section image file (BMP) format. It was used for later phenotypic feature extraction and analysis.
Microscopic traits extraction
We used the deep learning-based integrated phenotypic pipeline to automatically extract and quantify vascular bundles between different types of stem internodes20. With this software (VesselParser), we can simultaneously extract the number, size, shape, distribution of stem cross sections and their vascular bundles and the characteristics of the corresponding vascular bundles and functional areas from different perspectives.
Data analysis and visualization
Microsoft Excel 2016 and IBM SPSS Statistics 26 were used to organize and calculate the 20 microscopic indicators collected in the experiment. Statistical analysis, including variance analysis, correlation analysis, and cluster analysis, along with corresponding visual representations, were performed using Python 3.13, SigmaPlot 14.0, OriginPro 2024 and R 4.3.0.
To mitigate the impact of environmental factors on phenotypic data, the Best Linear Unbiased Predictor (BLUP) was calculated using the R language package lme4 for the data from Sanya and Beijing. The resulting BLUP values were used as the final phenotypic values for subsequent Genome-Wide Association Studies (GWAS). The BLUP calculation model is as follows:
where µ is the population mean. gi is the genotype effect. τk is the environmental effect. gτik is the interaction effect of genotype and environment. δ(k)m is the effect of the m-th repetition in the k-th environment. εikm is the random error effect. Genotype effects were fixed effects, and other factors were random effects.
In this study, Pearson correlation coefficient was adopted as the distance metric to systematically analyze the interrelationships among phenotypic traits. To evaluate phenotypic differences among different groups, analysis of variance (ANOVA) combined with Duncan’s multiple range test was employed, with the significance level set at p < 0.05. Hierarchical clustering analysis was performed using the linkage and dendrogram functions from the scipy library in Python 3.13, with the Ward clustering method and Euclidean distance applied to calculate phenotypic similarity. The Euclidean distance is calculated as follows:
where \({x}_{i}\), \({x}_{j}\) is any phenotypic data point points, \(p\) is the data dimension.
Genome-wide association study
Genotype data for inbred lines were generated in previous studies29. The genotype data are available online (http://www.maizego.org/Resources.html). We used PLINK software (PLINK 1.09) to control the minor allele frequency (MAF) of Marker trait associations (Marker trait associations with MAF < 0.05 and missingness > 0.1 were filtered out). The remaining effective Marker trait associations were used for subsequent population structure and kinship calculations. To reduce false positives, the first two principal components and the kinship matrix obtained from principal component analysis were included as covariates in the GWAS model30,31. Multivariate linear mixed models (LMMs) in GEMMA (version 0.98.1) software were used for GWAS analysis. The R package qqman was used to generate QQ plots and Manhattan plots. The significance threshold was used to identify significant Marker trait association. We appled the Bonferroni correction (0.05/N, N = 796,725, 0.05 for a 5% significance level) to minimize the false positives. ANNOVAR was used for gene annotation based on the corresponding assembly version. Functional annotation and analysis were performed using the maize B73 reference genome (B73RefGen_v4) from the MaizeGDB database (https://www.maizegdb.org/) and the NCBI database (https://www.ncbi.nlm.nih.gov/)32.
Results
Statistical analysis of microscopic phenotypes
It was observed that there were significant differences in the functional zones and vascular bundle characteristics at different internodes of corn stems (Fig. 1). Utilizing high-throughput stem vascular bundle phenotyping analysis software, quantitative analysis was performed on the Micro-CT images and 20 microscopic phenotypic traits were extracted from three stem internodes. The detailed list of phenotypic indicators can be found in Supplementary Tables 1 and Supplementary Table 2.
The change of internode microscopic character
As depicted in Fig. 1; Table 1, with the increase in internode position, the traits of the stem cross-section gradually decrease (including SZ_A, SZ_P, SZ_LA, and SZ_SA). Specifically, it is manifested as follows: the base third internode < ear internode < highest internode. Additionally, the ratio of the length of the major axis to the minor axis of the stem cross-section of basal third internode is the smallest. The vascular bundle area (VB_A) and the number of vascular bundles (VB_N) have always been important research targets. We found that VB_A and VB_N were consistent with the changing trend of the stem cross-section. However, the total vascular bundle area ratio (VB_AreaRatio) and the distribution of vascular bundles (VB_D) present different results: as the internode position ascends, the number of vascular bundles per unit area and the vascular bundle area per unit area increase (basal third internode < ear internode < highest internode) (Supplementary Figure S1).
Coefficient of variation comparison
Through further statistical analysis (Table 1), it was found that the microscopic phenotype of stems at different internodes had a wide range of variation at the population level. The variation of the ranged from 1.09 times of SZ_LWR to 2.98 times of PZ_VB_A at the population level. The variation range of ear internode was 1.20 times of SZ_LWR to 2.44 times of IZ_VB_A. The variation of highest internode ranged from 1.28 times of SZ_LWR to 3.58 times of IZ_A. The variable coefficient (CV) of microscopic phenotypes of the basal third internode, ear internode and highest internode were 1.50-16.25%, 4.01–14.25% and 4.12–20.72%. Compared with basal third internode and ear internode, the phenotypic CV of highest internode was generally higher than that of the other two internodes.
Cluster analysis
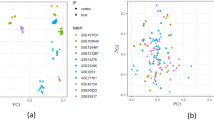
Based on Euclidean distance, we calculated the similarity among phenotypic data, constructing hierarchical clusters to elucidate the grouping of 268 maize inbred lines (Fig. 2). Through optimization of distance measurement methods, these inbred lines were divided into four distinct groups: Group 1 (44 inbred lines), Group 2 (103 inbred lines), Group 3 (56 inbred lines) and Group 4 (65 inbred lines). To better understand the clustering results, we reduced the data to two dimensions with different colors representing different groups (Supplementary Figure S2).
Morphological characterization revealed that: Group 1 (like Ry713, CIMBL106, CML479, 835b and chuan48-2) exhibited significantly larger stem cross-sections, well-developed periphery zone and inner zone, but relatively lower vascular bundle density. Group 2 (represented by GEMS63, Qi205 and LY) demonstrated higher numbers of vascular bundle and developed inner zone of the ear internode. Group 3 (represented by GEMS55, CIMBL146 and GEMS25) showed smaller stem cross-sections with reduced vascular bundle area and number, but higher vascular bundle density. Group 4 (represented by HYS, CIMBL96 and HSBN) was characterized by smaller base third internodes, reduced periphery zone and inner zone, smaller vascular bundle area, but higher vascular bundle density.
Correlation analysis
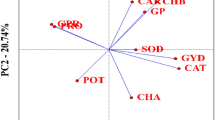
Correlation analysis (Supplementary Figure S3) revealed strong associations among microscopic phenotypes across different internodes (p < 0.01). As illustrated in Fig. 3, multiple phenotypes of basal third internode and ear internode demonstrated significant correlations with the kernel number per row (p < 0.05). Specifically, SZ_A, SZ_P, SZ_LA, SZ_SA, IZ_A, and IZ_T of both basal third internode and ear internode showed positive correlations with the kernel number per row, while IZ_VB_D exhibited a negative correlation. Furthermore, SZ_A, SZ_SA, IZ_A, and IZ_T of ear internode were positively correlated with both the kernel weight per ear and the grain weight per ear. Interestingly, SZ_LWR displayed significant but opposite correlations with the grain weight per ear at different internodes: negative at ear internode and positive at highest internode (Fig. 3A and B).
Further analysis of the differences in ear traits among the groups revealed that Group 2 significantly outperformed the other groups in the kernel rows per ear, the kernel number per row, the hundred-kernel weight, the kernel weight per ear and the grain weight per ear, particularly in the kernel number per row. As illustrated in Fig. 3C, Group 2 exhibited a statistically significant increase in kernel number per row compared to Group 3 and Group 4 (p < 0.05).
In conclusion, Group 2 with higher numbers of vascular bundle and well-developed inner zone in the ear internode exhibited significantly enhanced the kernel number per row. Notably, this structural characteristic showed significant associations with improved ear traits (ER_KW and ER_GW). The consistency between this study and comparative research strengthens the established relationship between maize stem architecture and yield performance, providing valuable insights for breeding strategies and yield improvement programs.
Correlation analysis between microscopic phenotypes and ear traits. (A) correlation analysis between the microscopic phenotypes ear traits, the base third internode phenotype is shown on the left and, the ear internode phenotype is shown on the right. (B) the correlation analysis of the highest internode and ear traits.C: comparison of ear traits based on cluster analysis. RW_N: the kernel number per row; ER_N: the kernel rows per ear; 100_KW: the hundred-kernel weight (g/100 kernels); ER_KW: the kernel weight per ear (g); ER_GW: the grain weight per ear (g). * Significant at 0.05 probability level.
Genome-wide association analysis and candidate gene screening
To elucidate the genetic mechanisms underlying stem tissue structure and vascular bundle phenotypes at different internodes in maize populations, we conducted a genome-wide association study (GWAS) focusing on microscopic traits of stem tissue structure and vascular bundles at basal third internode, ear internode, and highest internode. We employed the Linear Mixed Model (LMM) in GEMMA (Genome-wide Efficient Mixed Model Association) software for GWAS analysis, utilizing 796,725 effective Marker trait associations (P = 6.28E-08). For basal third internode, ear internode and highest internode, we respectively detected 15, 1 and 1 significant Marker trait association with 6, 1 and 1 traits (Fig. 4, Supplementary Table S3, Supplementary Figure S4).
Using the B73RefGen_v4 gene group as the reference genome, ANNOVAR annotation of the significant Marker trait association identified 8, 1, and 1 different putative candidate genes for basal third internode, ear internode, and highest internode. Functional annotation using the NCBI database revealed 6 genes with detailed functional descriptions. The proteins encoded by these genes covered synthetase, ligase, oxidase and protein kinase family proteins (Table 2).
The genes Zm00001d003511 and Zm00001d003512 are both located on chromosome 2 and significantly associated with SZ_P in basal third internode and Zm00001d003512 encodes zeaxanthin epoxidase. Zm00001d042143, located on chromosome 3, is encodes glucan endo-1,3-beta-glucosidase homolog 1. Significantly correlated with SZ_SA of basal internode (P = 4.20E-08). The gene Zm00001d035089 is located on chromosome 6 and is significantly associated with PZ_A in basal third internode. And Zm00001d036741 is significantly associated with VB_Aave in basal third internode. The gene Zm00001d023262 is located on chromosome 10 and is significantly associated with PZ_VB_D of basal third internode (P = 3.82E-08). Zm00001d005645 encodes holocarboxylase synthetase. Both this gene and Zm00001d005647 are located on chromosome 2 and are both significantly associated with IZ_VB_D of basal third internode. The putative protein kinase superfamily protein is encoded by Zm00001d005647. Zm00001d031075 is located on chromosome 1 and is significantly associated with VB_A of ear internode (P = 2.50E-08), but we have not found its specific function through literature review. The gene Zm00001d042490 is located on chromosome 3 and significantly correlated with PZ_VB_N of highest internode (P = 4.21E-08), which encodes glycosyltransferase.
Discussion
Stem growth and differentiation at different internodes occur sequentially, with varying environmental conditions at different developmental stages resulting in complex changes in internode traits throughout the growth process33,34. Our findings revealed extensive genetic variation in the microscopic phenotypes of different internodes at the population level, confirming these traits as typical quantitative characteristics. Consistent with the research of Zhao et al.35, we observed a gradual decrease in cross-sectional area, functional zone area and thickness, and vascular bundle area from basal third internode to ear internode and then to highest internode. Serving as a key supporting part for plant rooting and early growth36, basal third internode consistently exhibits relatively large values in terms of the traits of the stem cross-section. This ensures the structural stability, provides a solid foundation for the upright growth and nutrient transport of the plant in its initial stage, and effectively resists environmental challenges such as external mechanical pressure and wind impact36,37. As the “network pipeline” for material transport within the plant, the vascular bundles bear the important responsibility of precisely transporting nutrients from the roots and leaves to various parts of the plant38. The basal internode, as the starting point of the “network pipeline”, must ensure sufficient transport area and transport quantity to support the high-speed operation of the pipeline39. Due to the special position of highest internode, which is far away from the roots and leaves, the resources transported here are limited. Consequently, VB_A and VB_N of highest internode are much smaller than those in the other two internodes. However, since this part also undertakes the growth of the tassel, the effective transport efficiency is increased by increasing the vascular bundle area per unit area (VB_AreaRatio).
To assess trait stability, we utilized the CV across two internodes, with a lower CV indicating better stability for a cultivar in a particular environment40. As the internode position ascends, the CV of the stem cross - sectional traits and the phenotypes of functional areas increases. In contrast, the CV of vascular bundle traits first decreases and then increases (ear internode < basal third internode < highest internode). This indicates that the cross-sectional characters of basal third internode and vascular bundle traits of ear internode are relatively stable in response to environmental influences. Such stability ensures the efficient, precise, stable, and reliable pathways for the transport of substances to reproductive organs. Moreover, we have found that regardless of the position, stem cross-sectional phenotypes such as SZ_SA and SZ_P showed less phenotypic variation than vascular traits among inbred lines, and the CV of SZ_LWR was the smallest within each internode. This implies that to maintain the physical supportive function of the stem, the stem cross - sectional traits need to possess a certain degree of stability41.
The ideal vascular bundle phenotype is a crucial factor in ensuring water use efficiency, photosynthetic production capacity, and high crop yield42,43. Feng has shown a significant positive correlation between the number and area of basal third internode vascular bundles and grain filling, with the transport rate of assimilation products linked to the vascular bundle area in ear internodes15. Our study revealed positive correlations between stem zone attributes (SZ_A, SZ_P, SZ_LA, SZ_SA), inner zone characteristics (IZ_A, IZ_T), and the kernel number per row of basal third internode and ear internode. Conversely, IZ_VB_D negatively correlated with the kernel number per row. Basal third internode serves as a critical internode for transporting substances from the root system to the stalk19,44, while ear internode gathers photosynthetic products from the ear leaf and upstream nutrients, subsequently transporting them to the ear for storage via the shank6,25,26. Our findings indicate that inbred lines with higher numbers of vascular bundle and well-developed inner zone in ear internode exhibited better yield characteristics, particularly in the kernel number per row (p < 0.05). The inner zone, composed of parenchyma cells and large vascular bundles, benefits from a large area and thickness, promoting the formation of storage and channel tissues19,38. Vascular bundle number and area are primary factors affecting runoff15. A lower density of vascular bundles per unit area allows for larger individual bundles, maximizing the effective “flow” area within a unit cross-section.
GWAS identified several significant Marker trait associations and putative candidate genes related to stem tissue and vascular bundle characteristics. These genes encode a variety of enzymes, such as oxidases, synthetases, ligase enzyme and protein kinases. And studies have shown that these enzymes play an important role in plant growth and development. For example, Zm00001d003512, which is significantly associated with SZ_P in the basal segment, encodes zeaxanthin epoxidase. This enzyme catalyzes the conversion of zeaxanthin to epoxylated zeaxanthin, which in turn generates violaxanthin and participates in the lutein cycle, playing an important role in photoprotection and light adaptation45,46,47. Zm00001d005645 encodes holocarboxylase synthetase (HCS). Tissot et al. found from pea leaves that HSC is an enzyme that plays a key role in the biotin metabolic pathway48,49. Under drought stress, the activity and expression level of HCS changed, which could regulate the fatty acid synthesis pathway to change the lipid composition of cell membrane and enhance the tolerance to drought48. Therefore, putative candidate gene associated with IZ_VB_D at the basal internode may be related to the stress response of maize plants. Zm00001d03674 encodes isoleucine–tRNA ligase, cytoplasmic. This enzyme catalyzes the binding of isoleucine to its corresponding tRNA to form isoleucyl-trna, which ensures the accurate supply and incorporation of amino acids in protein synthesis50,51. Zm00001d005647 encodes putative protein kinase superfamily protein. This family of proteins is responsible for the phosphate transfer reaction, which can reversibly transfer the γ-phosphate group of ATP to the amino acid side chain of proteins, thereby changing the conformation and activity of proteins and regulating protein function52,53,54.
Moreover, Zm00001d042490 merits attention. This gene was significantly associated with the number of vascular bundles in periphery zone of highest internode. This gene encodes glycosyltransferase, which catalyzes the connection of activated sugars to different receptor molecules (proteins, lipids, nucleic acids) within living organisms55,56. This enzyme can glycosylate some small molecular substances, which might facilitate their loading into the phloem of the stem and long-distance transportation to other stems or sink organs56. In addition, the enzyme can also participate in the glycosylation of storage proteins and starches, promoting the accumulation and deposition of nutrients57. Glycosylation of secondary metabolites can also increase their stability and solubility, which is beneficial to their storage and utilization in sink organs58. Shang et al. has already confirmed that the zeatin O-glucosyltransferase OscZOG1 is preferentially expressed in the meristems and newly formed organs of stems and roots, regulating the development of rice roots and stems as well as the formation of other agronomic traits (such as tillering, panicle branching, number of grains per panicle, and grain size)59. This suggests that Zm00001d042490 may be an important putative candidate gene for PZ_VB_N and corn grain traits.
This paper mainly focuses on the relevant studies on the stem of the same plant at three internode positions in 2018 and 2021. However, considering that the climate conditions at these two locations are significantly different, it would be a better choice to conduct experiments over multiple years in each environment. In subsequent experiments, we will gradually supplement and improve the data of other years in each environment to obtain experimental data with a two-year, two-location, and multiple-replication design. Furthermore, we have not carried out specific experiments to verify the functions of genes. In future experiments, we will strengthen the integrated analysis with other omics methods such as genomics and transcriptomics, aiming to explore the biological mechanisms underlying the formation of maize stem traits at a deeper level, reveal the genetic relationship between stems and yields and thus provide a scientific basis for maize breeding and production.
Conclusion
The microscopic traits of the basal third internode and ear internode exhibit greater stability compared to those of highest internode. Several stem zone attributes and inner zone characteristics show significant positive correlations with kernel number per row, while IZ_VB_D correlates negatively with the kernel number per row. Based on stalk phenotypic indicators, 268 inbred lines can be accurately classified into four distinct categories (Group 1, Group 2, Group 3 Group 4). Ear traits of Group 2 significantly outperformed the other groups, especially the kernel number per row. GWAS and ANNOVAR annotation respectively identified 15,1 and 1 putative candidate genes in basal third internode, ear internode and highest internode. These genes encode a variety of enzymes, such as oxidases, synthetases, ligase enzyme and protein kinases. Notably, Zm00001d042490 may be an important putative candidate gene for PZ_VB_N and corn grain traits. This study provides a crucial theoretical foundation and genetic resources for accurately identifying phenotypes of different maize stalk internodes, potentially advancing the breeding of new high-yield, high-quality varieties.
Data availability
Genotypic data that support the findings of this research are open resource and can be downloaded from the website http://www.maizego.org/Resources.html, the genotypic data is available under “Here [1.25 M with 540 size]” in the Genotypic Data section. All other data are available in the manuscript.
References
Ross, S. M. & Tyree, M. T. Mason and Maskell’s Diffusion Analogue Reconciled with a Translocation Theory. Ann. Botany 44(5), 637–640 (1979).
Chang, T. G. & Zhu, X. G. Source-sink interaction: a century old concept under the light of modern molecular systems biology. J. Exp. Bot. 68(16), 4417–4431 (2017).
Ibañes, M., Fàbregas, N., Chory, J. & Caño-Delgado, A. I. Brassinosteroid signaling and auxin transport are required to establish the periodic pattern of Arabidopsis shoot vascular bundles. Proc. Natl. Acad. Sci. U.S.A. 106 (32), 13630–13635 (2009).
Nátrová, Z. & Nátr, L. Limitation of kernel yield by the size of conducting tissue in winter wheat varieties. Field Crops Res. 31(1), 121–130 (1993).
Tada, Y. & Shimizu, A. Vascular bundle cell-specific expression of a phosphate transporter improves phosphate use efficiency of Transgenic Arabidopsis without detrimental effects. Sci. Rep. 14(1) (2024).
Nguyen, H. L. et al. Identification and characterization of stable QTLs for vascular bundle number at the panicle neck in rice (Oryza sativa L). Breed. Sci. 73(4), 365–372 (2023).
Lucas, W. J. et al. The plant vascular system: evolution, development and functions. J. Integr. Plant Biol. 55(4), 294–388 (2013).
Ruonala, R., Ko, D. & Helariutta, Y. Genetic networks in plant vascular development. Annu. Rev. Genet. Evol. 51, 335–359 (2017).
Wang, Q. & Hu, C.H. Studies on the anatomical structures of the stalks of maize with different resistance to lodging. ACTA Agron. SINICA 17(01), 70–75 (1991).
Berger, M. et al. Darkfield and fluorescence macrovision of a series of large images to assess anatomical and chemical tissue variability in whole Cross-Sections of maize stems. Front. Plant Sci. 12 (2021).
Zhang, Y. et al. Phenotyping analysis of maize stem using micro-computed tomography at the elongation and tasseling stages. Plant. Methods 16(1). (2020).
Von Forell, G., Robertson, D., Lee, S. Y. & Cook, D. D. Preventing lodging in bioenergy crops: A biomechanical analysis of maize stalks suggests a new approach. J. Exp. Bot. 66(14), 4367–4371 (2015).
Stubbs, C. J. et al. Cross-sectional geometry predicts failure location in maize stalks. Plant. Methods 18(1) (2022).
Xie, L. Y., Wen, D. X., Wu, C. L. & Zhang, C. Q. Transcriptome analysis reveals the mechanism of internode development affecting maize stalk strength. BMC Plant Biol. 22(1) (2022).
Feng, H. et al. Effect of plant density on microstructure of stalk vascular bundle of summer maize (Zea Mays L.) and its characteristics of Sap flow. ACTA Agron. Sin. 40(08), 1435–1442 (2014).
Liao, S. Y. et al. Genetic basis of vascular bundle variations in rice revealed by genome-wide association study. Plant Sci. 302 (2021).
Chang, W. et al. Biomolecular strategies for vascular bundle development to improve crop yield. Biomolecules 12(12) (2022).
He, Q., Dong, P. & Gao, S. T. R, Q. Comparison of ear vascular bundles in different maize cultivar. ACTA Agron. Sin. 33(07), 1187–1196 (2007).
Zhang, Y. et al. Dissecting the phenotypic components and genetic architecture of maize stem vascular bundles using high-throughput phenotypic analysis. Plant Biotechnol. J. 19(1), 35–50 (2021).
Du, J. J. et al. A deep learning-integrated phenotyping pipeline for vascular bundle phenotypes and its application in evaluating Sap flow in the maize stem. Crop J. 10 (5), 1424–1434 (2022).
Oduntan, Y. A., Stubbs, C. J. & Robertson, D. J. High throughput phenotyping of cross-sectional morphology to assess stalk lodging resistance. Plant. Methods 18(1), 1 (2022).
Pan, X. D. et al. Three-dimensional reconstruction of maize roots and quantitative analysis of metaxylem vessels based on X-ray micro-computed tomography. Can. J. Plant Sci. 98(2), 457–466 (2018).
Heckwolf, S., Heckwolf, M., Kaeppler, S. M., de Leon, N. & Spalding, E. P. Image analysis of anatomical traits in stalk transections of maize and other grasses. Plant. Methods 11 (2015).
Zhang, Y. et al. High-throughput micro-phenotyping measurements applied to assess stalk lodging in maize (Zea Mays L). Biol. Res. 51 (2018).
Zhao, H. et al. Phenotype identification and genome-wide association study of ear-internode vascular bundles in maize (Zea Mays L). J. Plant. Res. 137 (6), 1073–1090 (2024).
Guo, S. J. et al. High-Throughput phenotyping accelerates the dissection of the phenotypic variation and genetic architecture of Shank vascular bundles in maize (Zea Mays L.) MT. Plants-Basel 11 (10), 1339–1339 (2022).
Yang, X. H. et al. Characterization of a global germplasm collection and its potential utilization for analysis of complex quantitative traits in maize. Mol. Breed. 28(4), 511–526 (2011).
Zhang, Y. et al. Phenotyping analysis of maize stem using micro-computed tomography at the elongation and tasseling stages. Plant. Methods 16(1), 2 (2020).
Liu, H. J. et al. Distant eQTLs and non-coding sequences play critical roles in regulating gene expression and quantitative trait variation in maize. Mol. Plant 10(3), 414–426 (2017).
Yu, J. M. et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 38(2), 203–208 (2006).
Sun, X. C. et al. Evaluation of maize root growth and genome-wide association studies of root traits in response to low nitrogen supply at seedling emergence. Crop J. 9(4), 794–804 (2021).
Jin, Y. et al. Differential analysis and genome-wide association analysis of stomata density of maize inbred lines leaves at ear position. Can. J. Plant Sci. 103(6), 529–540 (2023).
Le, L. et al. A Spatiotemporal transcriptomic network dynamically modulates stalk development in maize. Plant Biotechnol. J. 20(12), 2313–2331 (2022).
Wang, X. Q. et al. Stalk architecture, cell wall composition, and QTL underlying high stalk flexibility for improved lodging resistance in maize. BMC Plant Biol. 20(1). (2020).
Zhao, H. et al. High-throughput acquisition and accurate analysis of stem vascular bundle phenotypes in maize inbred lines based on Micro-CT. Jiangsu Agric. Sci. 50(12), 201–211 (2022).
Zhan, X. X. et al. Maize basal internode development significantly affects stalk lodging resistance. Field Crops Res. 286 (2022).
Cui, J. J. et al. Maize grain yield enhancement in modern hybrids associated with greater stalk lodging resistance at a high planting density: a case study in Northeast China. Sci. Rep. 12(1) (2022).
Zheng, Y. X. et al. Genome-Wide association study of vascular Bundle-Related traits in maize stalk. Front. Plant Sci. 12 (2021).
Xue, J. et al. How high plant density of maize affects basal internode development and strength formation. Crop Sci. 56 (6), 3295–3306 (2016).
Xiong, H. Z. et al. Genetic dissection of diverse seed coat patterns in cowpea through a comprehensive GWAS approach. Plants-Basel 13(9) (2024).
Fan, H. Z. et al. High-Throughput phenotyping of Cross-Sectional morphology to assess stalk mechanical properties in sorghum. Agriculture-Basel 12(10) (2022).
Liu, X. et al. Optimized irrigation practices with fertilizer utilization strategies to improve photo-fluorescence efficiency, vascular bundles and maize production in semi-arid regions. Heliyon 10(20), e39222 (2024).
Boch, S. et al. Effects of fertilization and irrigation on vascular plant species richness, functional composition and yield in mountain grasslands. J. Environ. Manag. 279 (2021).
Jing, L. L., Wei, X. H., Song, Q. & Wang, F. Study on the movement mechanism of rice stem under the action of canopy-opening device based on explicit dynamics simulation. Front. Plant Sci. 14 (2023).
Lukas, K., Rebecca, L., Christin, B., Stephanie, B. & Peter, J. The amount of zeaxanthin epoxidase but not the amount of violaxanthin de-epoxidase is a critical determinant of zeaxanthin accumulation in Arabidopsis Thaliana and Nicotiana tabacum. Plant Cell. Physiol. 64(10) (2023).
Verhoeven, A. S., Bugos, R. C. & Yamamoto, H. Y. Transgenic tobacco with suppressed zeaxanthin formation is susceptible to stress-induced photoinhibition. Photosynth. Res. 67(1–2), 27–39 (2001).
Zhang, R., Kramer, D. M., Cruz, J. A., Struck, K. R. & Sharkey, T. D. The effects of moderately high temperature on zeaxanthin accumulation and decay. Photosynth. Res. 108(2–3), 171–181 (2011).
Tissot, G., Douce, R. & Alban, C. Evidence for multiple forms of biotin holocarboxylase synthetase in pea (Pisum sativum) and in Arabidopsis Thaliana: Subcellular fractionation studies and isolation of a cDNA clone. Biochem. J. 323(Pt 1), 179–188 (1997).
Tissot, G., Job, D., Douce, R. & Alban, C. Protein biotinylation in higher plants: Characterization of biotin holocarboxylase synthetase activity from pea (Pisum sativum) leaves. Biochem. J. 314 (Pt 2), 391–395 (1996).
Allen, E. H., Glassman, E. & Schweet, R. S. Incorporation of amino acids into ribonucleic acid. I. The role of activating enzymes. J. Biol. Chem. 235, 1061–1067 (1960).
Berg, P., Bergmann, F., Ofengand, E. & Dieckmann, M. The enzymic synthesis of amino acid derivatives of ribonucleic acid. I. The mechanism of leucyl-, valyl-, isoleucyl-, and methionyl-ribonucleic acid formation. J. Biol. Chem. 236 (1961).
Lehti-Shiu, M. D. & Shiu, S. H. Diversity, classification and function of the plant protein kinase superfamily. Philos. Trans. R. Soc. B-Biol. Sci. 367(1602), 2619–2639 (2012).
Hegedus, D. D., Gerbrandt, K. & Coutu, C. The eukaryotic protein kinase superfamily of the necrotrophic fungal plant pathogen, sclerotinia sclerotiorum. Mol. Plant Pathol. 17(4), 634–647 (2016).
Hrabak, E. M. et al. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 132(2), 666–680 (2003).
He, B. et al. Glycosyltransferases: Mining, engineering and applications in biosynthesis of glycosylated plant natural products. Synth. Syst. Biotechnol. 7(1), 602–620 (2022).
Lim, E. K. Plant glycosyltransferases—their potential as novel biocatalysts. Chem.-A Eur. J. 11(19), 5486–5494 (2005).
Sirirungruang, S., Barnum, C. R., Tang, S. N. & Shih, P. M. Plant glycosyltransferases for expanding bioactive glycoside diversity. Nat. Prod. Rep. 40(7), 1170–1180 (2023).
Zhao, J. et al. A genome-wide association study reveals that the glucosyltransferase OsIAGLU regulates root growth in rice. J. Exp. Bot. 72(4), 1119–1134 (2021).
Shang, X. L., Xie, R. R., Tian, H., Wang, Q. L. & Guo, F. Q. Putative zeatin O-glucosyltransferase OscZOG1 regulates root and shoot development and formation of agronomic traits in rice. J. Integr. Plant Biol. 58(7), 627–641 (2016).
Acknowledgements
We thank the Maize Research Center department of the Beijing Academy of Agriculture and Forestry Sciences for preparing the seed and planting for the trial. We thank Pro. Jianbing Yan, from Huazhong Agricultural University, Pro. Xiaohong Yang, from the National Maize Improvement Center of China, China Agricultural University, for providing seeds of the maize inbred lines.
Funding
The project was supported by the National Nature Science Foundation of China (No. 32330075; No. U21A20205), the Construction of Collaborative Innovation Centre of Beijing Academy and Forestry Science (KJCX20240406), the Beijing Natural Science Foundation (JQ24037), the Science Technology Innovation Team of Maize Modern Seed Industry in Hebei (213263319D).
Author information
Authors and Affiliations
Contributions
Y.W. and Y.Z. carried out the experiments, drafted and revised the manuscript. C.Z. and X.G. conceived and designed the experiments, review and revised the manuscript. G.H., J.W., X.L., L.L., S.Z., M.Z., M.G., C.Z., and Q.M. participated in the sample collection, experimental data acquisition. Y.W. and Y.Z. analyzed data, made figures and tables. G.H. help to revise the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Zhang, Y., Huang, G. et al. Association analysis of maize stem vascular bundle micro-characteristics with yield components based on micro-CT and identification of related genes. Sci Rep 15, 13009 (2025). https://doi.org/10.1038/s41598-025-96518-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96518-1