Abstract
Since 2015, hydroxychloroquine (HCQ) has been widely used as a standard treatment for systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE) under the national health insurance system in Japan. However, the status of routine ophthalmological screening for retinopathy, which is a notable safety concern of HCQ, remains unclear. In this retrospective study using the nationwide health insurance claims database, we aimed to investigate the status of ophthalmological screening in routine clinical practice in Japan. A total of 2567 patients with SLE or CLE were included in the analysis. The proportion of ophthalmology visits was 88.0% at the initiation of HCQ prescription, decreased to 76.3% after one year, and then remained constant. Moreover, only 50% of the patients underwent spectral-domain optical coherence tomography (SD-OCT) and automated visual field (VF) tests, which are useful for the early detection of HCQ retinopathy-related changes. Importantly, the proportion of ophthalmology visits remained consistently high among patients who visited an ophthalmologist in the previous year, ranging from 77.3% to 90.2%. These findings highlight the need to improve screening adherence and promote regular ophthalmological evaluations, particularly for patients without prior ophthalmology visits, to enhance the early detection of HCQ retinopathy.
Similar content being viewed by others
Introduction
Hydroxychloroquine (HCQ) has been widely used as a standard treatment option for several rheumatic diseases, such as cutaneous lupus erythematosus (CLE) and systemic lupus erythematosus (SLE)1,2,3. The European Alliance of Associations for Rheumatology (EULAR) recommendations for the management of systemic lupus erythematosus (2023 update) state that HCQ is recommended for all patients with lupus with considerations of the individual risks for retinal toxicity3. In Japan, chloroquine was withdrawn from the market due to a series of cases of severe chloroquine retinopathy in the 1970s. In 2015, HCQ became available on the market for the treatment of CLE/SLE after regulatory approval by a clinical trial based on public initiatives to promote its introduction into clinical practice in the 2010s4.
Retinopathy due to HCQ use is the most concerning safety-related issue. The mechanism of retinal toxicity remains elusive; however, the risk of developing retinal damage depends on the dose and duration of administration (cumulative dose of HCQ)2. In addition, HCQ retinal toxicity is irreversible, and cellular damage may progress even after the drugs are discontinued; however, early detection through ophthalmological screening and discontinuation of HCQ administration may reduce the progression of visual deterioration2,5. Thus, the package insert of HCQ and various academic societies documented that the ophthalmological condition should be carefully monitored by routine ophthalmological screening6,7,8. The American Academy of Ophthalmology (AAO) Statement (2016 revision) recommends that automated visual fields (VF) plus spectral-domain optical coherence tomography (SD-OCT) be used as the primary screening examinations2. Similarly, the Royal Australian and New Zealand College of Ophthalmologists (RANZCO) advises SD-OCT, VF, and dilated fundus examination9, while Royal College of Ophthalmologists (RCOphth) endorses SD-OCT and fundus autofluorescence (FAF)10; annual screening after 5 years of prescriptions are recommended in these guidelines.
In Japan, safety management of HCQ has been very cautious because HCQ is a newly approved drug that has not existed on the market for a long time owing to previous chloroquine-related health problems. When approving HCQ as a new investigational drug, the Ministry of Health, Labor, and Welfare was the first among countries to request the inclusion of SD-OCT in the required ophthalmological tests on the package insert, in addition to visual acuity, intraocular pressure, slit-lamp microscopy, color vision tests, fundoscopy, and VF tests. These tests are required before treatment initiation and yearly or more frequently for patients with a cumulative dose > 200 g, liver or renal dysfunction, visual impairment, or older age7. As a result, rare cases of early onset (< 5 years of use) HCQ retinopathy has been detected in Japan11.
To date, some reports have been published on ophthalmological screening in clinical practice and adherence to ophthalmologic screening among patients undergoing long-term HCQ therapy varies across countries12,13,14,15. However, to the best of our knowledge, no comprehensive real-world data are available from Japan. This retrospective study aimed to investigate the status of ophthalmological screening and the factors that influence its implementation in patients with SLE/CLE prescribed HCQ in Japan. The findings of this study may provide insights into the improvement of strategies for preventing HCQ retinopathy.
Results
Study populations
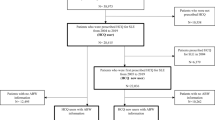
Of 14,828,101 patients with medical records between September 2015 and October 2022, 29,681 were diagnosed with SLE or CLE. Of those, 2567 patients (8.6%) met all inclusion criteria. The demographic and clinical characteristics of the patients are summarized in Table 1. The number of patients with SLE was 2487 (96.9%), mean age (± standard deviation) was 40.0 (± 13.0) years, and the most common age group at the initiation of HCQ prescription was 40–49 years (32.0%), followed by 30–39 years (22.0%) and 50–59 years (17.9%). The majority of patients were female (n = 2216, 86.3%). The distribution of mean daily dose was 46.1% for 200 mg/day, and 45.9% for > 200– < 400 mg/day in 2567 eligible patients. The proportion of patients who received a dose of ≤ 6.5 mg/kg/day per ideal body weight based on the package insert6 was 95.2% (n = 967) in 1016 patients with height data, and the proportion of patients who received a dose of ≤ 5 mg/kg/day per actual body weight as recommended by the AAO Statement (2016 revision)2 was 61.0% (n = 620) in 1016 patients with body weight data. The proportion of patients who received a cumulative dose of 200 g or more, one of the factors for HCQ retinopathy6, was 40.7% (n = 1045) (Table 2).
Ophthalmological screening
The number of patients prescribed HCQ decreased from 1680 at one year to 71 at six years and 16 at seven years (Supplementary Table S1). Because the number of patients who were prescribed HCQ at six and seven years was insufficient for analyzing the status of ophthalmology visits, we decided to use the data for up to five years for the analyses. As shown in Fig. 1, the proportion of ophthalmology visits was 88.0% (2258 of 2567 patients) at the initiation of HCQ prescription and decreased to 76.3% (1282 of 1680 patients) at one year; however, it remained in the 75–80% range thereafter. The ophthalmology visits from initiation to seven years are summarized in Supplementary Table S1.
The proportion of annual ophthalmology visits. Ophthalmology visits were defined as having a record of any of the six screening examinations (visual acuity test, intraocular pressure test, slit-lamp microscopy, spectral-domain optical coherence tomography, automated visual field test, or color vision test) stated in the hydroxychloroquine package insert.
SD-OCT was implemented in 70.4% of patients at initiation (1808 of 2567 patients), dropped to 58.7% at one year (986 of 1680 patients), and remained in the 60–70% range thereafter (Table 3 and Supplementary Table S1). The VF test was implemented in 56.5% of the patients at initiation (1450 of 2567 patients), dropped to 44.3% at one year (745 of 1680 patients), and remained at approximately 50–55% thereafter. The implementation rate of both SD-OCT and VF was 50.6% at initiation (1300 of 2567 patients), dropped to 39.3% at one year (661 of 1680 patients), and remained around 50% thereafter, whereas the implementation rate of either SD-OCT or VF was 76.3% at initiation (1958 of 2567 patients), dropped to 63.7% at one year (1070 of 1680 patients), and remained around 70% thereafter. The implementation rates of ophthalmological screening examinations performed routinely at ophthalmology visits, such as the visual acuity test, intraocular pressure test, and slit-lamp microscopy, were 82.0% (n = 2106), 85.6% (n = 2197), and 86.0% (n = 2207) of 2567 patients at initiation, respectively; however, it dropped to 70.2% (n = 1180), 73.4% (n = 1233), and 75.0% (n = 1260) of 1680 patients at one year, respectively, and remained above 70% thereafter. The implementation rate of other tests, such as the color vision test, FAF, and mfERG, also dropped from initiation to one year. The implementation rate of the color vision test was generally in the 30% range, that of FAF was generally in the 10% range, and that of mfERG was as low as 0.5% or less. When limited to patients who visited ophthalmologists, visual acuity test, intraocular pressure test, and slit-lamp microscopy were 100% at all time points, > 80% for SD-OCT, and 60–70% for VF. The implementation status of routine ophthalmological screening examinations from initiation to seven years is summarized in Supplementary Table S1.
Ophthalmology visits classified by HCQ dose per actual body weight were similar for ≤ 5 mg/kg/day and > 5 mg/kg/day: 86.1% (534 of 620 patients) and 87.9% (348 of 396 patients) at the initiation of HCQ prescription, 76.2% (292 of 493 patients) and 74.3% (182 of 245 patients) at one year, and 69.4% (43 of 62 patients) and 87.9% (29 of 33 patients) at five years, respectively (Fig. 2). A total of 1045 patients received a cumulative dose of HCQ ≥ 200 g by the end of the follow-up period, of whom 89.0% (n = 930) had ophthalmology visits at initiation (Supplementary Table S2). Among these patients, the proportion of those with ophthalmology visits who received a cumulative dose of < 200 g was 77.1% (806 of 1045 patients) at one year, 79.9% (789 of 988 patients) at two years, and 77.7% (317 of 408 patients) at three years. For patients receiving a cumulative dose of ≥ 200 g, the proportions were 80.8% (286 of 354 patients) at three years, 78.3% (354 of 452 patients) at four years, and 78.3% (198 of 253 patients) at five years. No significant differences were observed in the proportion of ophthalmology visits before and after reaching a cumulative HCQ dose of 200 g.
Factors associated with ophthalmology visits
The proportion of patients who visited ophthalmologists in the following year (at one year, two years, and six years) according to the presence or absence of ophthalmologist visits in the previous year (at initiation, one year, and five years) is shown in Fig. 3 and Supplementary Table S3. Focusing on the patients who visited ophthalmologists at initiation (n = 2258), at one year (n = 1282), and at five years (n = 201), the proportion of ophthalmology visits decreased modestly each following year: Of the 1494 patients who visited an ophthalmologist at the start of the study, 1155 (77.3%) of patients who visited ophthalmologists at initiation visited ophthalmologists at one year, and of the 897 patients who visited ophthalmologists at one year, 776 patients (86.5%) visited ophthalmologists at two years, and of the 51 patients who visited an ophthalmologist after 5 years, 46 patients (90.2%) visited ophthalmologists at six years. In contrast, patients who did not visit an ophthalmologist in the previous year had fewer follow-up visits than those who did. Patients who visited ophthalmologists at the initiation of the HCQ prescription had a significantly higher proportion of ophthalmology visits at one year than those who did not (p = 0.0063) (Supplementary Table S4). However, we observed no significant differences in the proportion of ophthalmology visits at five years between patients who visited ophthalmologists at the initiation of the HCQ prescription and those who did not. Conversely, patients who visited ophthalmologists at one year had a significantly higher proportion of ophthalmology visits at five years compared to those who did not (p < 0.0001).
The proportion of follow-up visits by ophthalmology visits status in the previous year. The table shows the number of patients who visited ophthalmologists at the initiation of HCQ prescription, at one year, and five years. The graph illustrates the proportion of ophthalmology visits in the subsequent year (i.e., one, two, and six years later) categorized by ophthalmology visit status in the previous year.
Regarding the association between demographic and clinical characteristics and ophthalmology visits at one year and five years, patients who visited institutions in the metropolitan area, where a large number of patients who were prescribed HCQ and institutions that they visited, had a significantly lower proportion of ophthalmology visits at one year (Odds ratio [OR], 0.66; 95% confidence interval [CI], 0.51–0.85; p = 0.0012). Visits to pediatric departments or ophthalmologists at the initiation of HCQ prescription were significantly associated with ophthalmology visits at one year (OR, 3.24; 95% CI, 1.33–7.87; p = 0.0096, and OR, 1.65; 95% CI, 1.18–2.33; p = 0.0037, respectively) (Supplementary Table S5). No factors influencing ophthalmology visits at five years were identified (Supplementary Table S6).
Discussion
In this retrospective observational cohort study, using a nationwide health insurance claims database provided by JMDC Inc., we aimed to determine the status of ophthalmological screening in patients with SLE and those with CLE who were prescribed HCQ in Japan. Our analysis revealed that 88.0% of patients visited ophthalmologists at the initiation of HCQ prescription, which decreased to 76.3% after one year. Moreover, the implementation rate of all six screening examinations stated in package insert6 was generally in the 20–30% range throughout the study period. Furthermore, the proportion of patients with ophthalmology visits decreased from the initiation of HCQ prescription to one year and remained stable thereafter. Visits to ophthalmologists at the initiation of HCQ prescription had an impact on ophthalmology visits at one year, and the status of ophthalmology visits at one year had an impact on ophthalmology visits at five years.
Our study showed that not all patients who were prescribed HCQ underwent ophthalmological screening examinations, and some patients were at risk of delayed recognition of HCQ retinopathy. In the post-marketing surveillance (PMS) of HCQ in Japan, conducted during the time overlapping with the current study period, one case of retinopathy, two cases of acquired pigmentary retinopathy, and two cases of abnormal visual field testing were reported over the five-year observation period16. Furthermore, at least three cases of HCQ retinopathy developing in the parafoveal or pericentral area within three years of HCQ treatment have been reported in Japan11. Considering our study results, SD-OCT, which is the most effective examination for early detection of retinopathy, was performed in only 60% of patients who were prescribed HCQ. If this implementation rate is extrapolated to the population for whom HCQ was prescribed during the PMS period, almost the same number of patients with potential side effects continued to receive HCQ without undergoing appropriate ophthalmological screening. Ophthalmological screening is the only way to detect HCQ retinopathy early and to limit its progression. The identification of retinal pigment epithelial damage is crucial for preventing vision loss. Therefore, proper ophthalmological screening is essential for prescribing HCQ.
Reports from other countries have indicated that the implementation rate of ophthalmological screening is insufficient. In the United States, of the patients who underwent ophthalmic screening examinations after 2016, 55.3% had been screened according to the AAO statement on screening for HCQ retinopathy, which was revised in 20162. Our study results were similar to that; the implementation rate of SD-OCT was generally in the 60–70% range, VF was generally in the 50–55% range. In South Korea, 31.6% of patients who had received HCQ for more than five years underwent at least one of the four screening examinations (i.e., SD-OCT, FAF, VF, and mfERG) after five years14. In Taiwan, only 1.2% of patients who had received HCQ for more than five years underwent at least one of the three screening examinations (i.e., SD-OCT, VF, and mfERG) after five years15. The lack of proper ophthalmological screening is a major concern, even on a global level. Considering these reports, we should actively promote awareness of ophthalmological screening in patients receiving HCQ, as well as in ophthalmologists and physicians who prescribe HCQ.
Our findings indicated that the implementation rate of all six ophthalmological screening examinations stated in the package insert of the HCQ5 remained as low as 20–30% by years. One factor influencing this is thought to be the low implementation rate of the color vision test, which was generally in the 30% range. Color vision loss associated with HCQ retinopathy occurs; however, AAO does not recommend color vision test for screening because they are not sensitive or specific to the retinal toxicity associated with HCQ2. Considering racial differences, recommendations outside of Japan may not be readily applicable without validations. The Japanese ophthalmological protocol is more comprehensive but potentially burdensome. If the number and types of examinations used in ophthalmological screening contribute to the lower implementation rate of ophthalmological screening in Japan, addressing screening methods based on the currently available evidence and appropriate medical judgment may be necessary.
Our findings revealed that visiting ophthalmologists at the initiation of HCQ prescription had an impact on ophthalmology visits at one year, and the status of ophthalmology visits at one year may have an impact on ophthalmology visits at five years. A South Korean study reported that screening at treatment initiation or within the first year increased the visit rate after five years14. In addition, the follow-up visits of patients who visited ophthalmologists at initiation, one year, and five years showed a decrease in the proportion of visits in the subsequent year. Although some patients did not visit ophthalmologists at initiation, one year, and five years but visited ophthalmologists the following year, their proportion of visits was lower than that of patients who had visited ophthalmologists in the previous year. These results indicate that encouraging patients to visit ophthalmologists at one year and continuously encouraging them to return to ophthalmologists every following year would be key to improving long-term ophthalmology visits. The location of the institution, department, and ophthalmology visits at the initiation of HCQ prescription were associated with ophthalmology visits at one year. In metropolitan areas, the availability of physicians is high and access to healthcare is generally considered good. However, a highly specialized and complexed healthcare system may lead to delayed initial ophthalmological visits, as patients prioritize other specialties. Additionally, a lack of awareness regarding the risk of HCQ retinopathy combined with a busy lifestyle may contribute to reduced adherence to regular ophthalmological screening. Collectively, these factors may influence screening practices in metropolitan populations. However, further research is needed to confirm these hypotheses. These results suggest that these factors should be considered when developing strategies to enhance follow-up adherence. A meta-analysis on factors influencing adherence to diabetic retinopathy screening, which also requires regular ophthalmologic examinations, identified patient-related factors such as socioeconomic status, family structure, disease duration, and eye care education as key determinants17. Understanding these factors provide valuable insights for patient groups that should focus on promoting ophthalmological screening.
Our study has several limitations. The JMDC database does not include all the patients with SLE and CLE in Japan. Most of the data are health insurance data for employees of large companies, and the dataset does not include data on patients who were covered by other health insurance, such as the National Health Insurance, National Health Insurance Association, Seamen’s Insurance, and Mutual Aid Association Health Insurance. Moreover, most insured people withdraw from health insurance when they retire; therefore, the percentage of the population aged over 65 years is significantly lower than that of the Japanese population. Therefore, this study may underestimate the number of elderly patients. However, since SLE primarily affects younger individuals, the generalizability of our findings is relatively preserved from this perspective. In contrast, since the database tracks patients as long as they remain employed and enrolled in associated medical care insurance, it is important to note that patients with severe diseases who were forced to leave their jobs may be underrepresented in this study. Regarding the study design, we followed up the patients for more than five years, but smaller sample sizes during long-term observation may have reduced the statistical power of the detailed analysis. Ophthalmology visits were defined based on the records of any of the six screening tests. However, some patients may have visited an ophthalmologist for reasons unrelated to HCQ retinopathy screening, and may not have undergone appropriate screening tests. Therefore, the true rate of adherence to screening may have been overestimated. Nonetheless, considering the general purpose of SD-OCD and VF tests, patients prescribed HCQ may visit ophthalmology for HCQ retinopathy screening, suggesting that the true adherence rate may not be substantially lower than the visits. Furthermore, the risk of incorrect coding is undeniable for studies using claims-based databases. Inaccuracies in diagnosis and procedure coding can lead to misclassification of adherence to ophthalmology visits and screenings. These limitations should be considered when interpreting the results. Despite these limitations, it is worthwhile to report the status of routine ophthalmological screening in patients with SLE and CLE prescribed HCQ in Japan.
Conclusions
Our study demonstrated that while approximately 80% of the patients prescribed HCQ underwent ophthalmological screening, only approximately 50% underwent SD-OCT and VF tests, which are essential tools for the early detection of retinopathy. Given that the risk of HCQ retinopathy increases significantly after 10 years, targeted strategies, such as enhancing patient awareness, standardizing screening protocols, and addressing structural barriers for both patients and healthcare providers, are believed to improve long-term adherence to ophthalmological screening and reduce preventable vision loss.
Methods
Study design/data source
In this retrospective observational cohort study, we used a nationwide health insurance claims database provided by JMDC Inc. (JMDC database)18,19. The JMDC database is a prominent healthcare claims database in Japan that has been widely used for health economics, epidemiology, and outcomes research. The dataset included healthcare insurance claims, prescriptions, diagnoses, procedures, and medical institution data. This database provides longitudinal data, and patients can be tracked even if they are transferred to different medical institutions. It is the largest anonymized database of accumulated claims data in Japan and consists of approximately 17 million insured Japanese people (as of March 2023).
Study population
HCQ became available on the market in September 2015, and we set the baseline period to six months prior to the initiation of HCQ prescription. Thus, patients diagnosed with SLE or CLE whose data were recorded between March 1, 2015 (six months before HCQ became available on the market) and March 31, 2023, were included in the study. Of these, patients who were prescribed HCQ between September 1, 2015, and October 31, 2022, were identified. Patients with SLE and those with CLE were identified using the standard disease codes M32 and L93, respectively (Supplementary Table S7). HCQ prescriptions were identified using the computerized receipt code YJ3999038F1029. Other inclusion criteria were an observation period of at least six months after the diagnosis of SLE or CLE, a diagnosis record of SLE or CLE within six months prior to the initiation of HCQ prescription, and an observation period of at least six months prior to the initiation of HCQ prescription.
Definition of variables and data handling with regard to the study period
For baseline demographic and clinical characteristic information, the following variables were assessed: disease name (SLE or CLE), age, gender, facility management entity, facility location (metropolitan, rural/urban, or depopulated areas), department, and physician specialty. For HCQ prescription status during the follow-up period, the mean daily dose, dose per ideal body weight, dose per actual body weight, and cumulative dose were evaluated. The mean daily dose was calculated from the total HCQ prescription dose on the HCQ prescription days. Renal function, a risk factor for retinopathy, was excluded from the assessment because renal function-related data were absent or normal in more than 96% of the study population. For the implementation status of routine ophthalmological screening, all ophthalmological screening examinations specified in the Japanese package insert (i.e., visual acuity test, intraocular pressure test, slit-lamp microscopy, ophthalmoscopy [SD-OCT], VF, and color vision test)6 and other screening examinations recommended by the AAO as additional useful screening examinations (i.e., FAF and multifocal electroretinogram [mfERG])2 were investigated at the initiation of HCQ prescription and annual screening thereafter. Screening examinations were identified using Japanese procedure codes (Supplementary Table S8). The package insert and guidelines state that baseline ophthalmological screening is recommended prior to the administration of HCQ6,7,8. Thus, the time window for screening at the initiation of HCQ prescription was set to 181 days prior to the HCQ prescription date. For the annual screening, the time window was set to 365 days from the day of HCQ prescription. Ophthalmology visits, defined as having a record of any of the six screening examinations (visual acuity, intraocular pressure, slit-lamp microscopy, SD-OCT, VF, and color vision test) stated in the package insert6, were also investigated. Patients were followed up for up to seven years; the maximum time period allowed for data collection was seven years because HCQ became available on the market in 2015, which was taken into account.
Regarding the location of the institution, a metropolitan area was defined as a population of more than 1 million or a population density of more than 2000 persons/km2, rural/urban areas were defined as a population of more than 200,000 or a population of more than 100,000 and a population density of more than 200 persons/km2, and underpopulated areas were defined as areas outside of metropolitan areas and rural or urban areas.
Data analysis
Categorical variables are presented as frequency and percentage, and continuous variables as mean ± standard deviation. The chi-squared test was used to compare categorical variables between the groups. To investigate the factors that influence ophthalmology visits, factors that have been considered from a medical perspective were collected with reference to previously reported items20. Gender, age, size of the facility, location of facility, department of the facility, and ophthalmology visits at the initiation of HCQ prescription were selected as the explanatory variables for the multivariable logistic regression analysis. We also investigated whether ophthalmology visits at the initiation of HCQ prescription affected ophthalmology visits at one year and five years. Univariable and multivariable logistic regression analyses were performed to identify factors affecting ophthalmological visits. Statistical significance was determined using a two-sided test with a significance level set at 0.05. All statistical analyses were performed using SAS, version 9.4 TS1M6 (SAS Institute Inc., Cary, NC, USA).
Data availability
The datasets generated and/or analyzed during the study are available from the corresponding author upon reasonable request.
References
Guidelines for referral and management of systemic lupus erythematosus in adults. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Arthritis Rheum. 42, 1785–1796 (1999).
Marmor, M. F., Kellner, U., Lai, T. Y., Melles, R. B. & Mieler, W. F.; American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology 123, 1386–1394 (2016).
Fanouriakis, A. et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann. Rheum. Dis. 83, 15–29 (2024).
Yokogawa, N. et al. Effects of hydroxychloroquine in patients with cutaneous lupus erythematosus: A multicenter, double-blind, randomized, parallel-group trial. Arthritis Rheumatol. 69, 791–799 (2017).
Marmor, M. F. & Hu, J. Effect of disease stage on progression of hydroxychloroquine retinopathy. JAMA Ophthalmol. 132, 1105–1112 (2014).
Sanofi K. K. & Asahi Kasei Pharma Corporation. Plaquenil® Tablets package insert (2024).
Kondo, M., Shinoda, K., Matsumoto, C. S., Yokogawa, N. & Terasaki, H. Guide for clinical use of hydroxychloroquine. Nihon Ganka Gakkai Zasshi 120, 419–428 (2016).
Japan College of Rheumatology & Japanese Dermatological Association. Guidance for Proper Use of Hydroxychloroquine (Simplified Version). https://www.ryumachi-jp.com/info/guideline_hcq.pdf. (in Japanese; accessed 7 October 2024).
The Royal Australian and New Zealand College of Ophthalmologists. Guidelines for Screening for Hydroxychloroquine Retinopathy. https://ranzco.edu/wp-content/uploads/2021/04/RANZCO-Guidelines-for-screening-for-HCQ-Retinopathy.pdf. (accessed 7 October 2024).
Yusuf, I. H., Foot, B. & Lotery, A. J. The Royal College of Ophthalmologists recommendations on monitoring for hydroxychloroquine and chloroquine users in the United Kingdom (2020 revision): executive summary. Eye 35, 1532–1537 (2021).
Ozawa, H. et al. Ocular findings in Japanese patients with hydroxychloroquine retinopathy developing within 3 years of treatment. Jpn. J. Ophthalmol. 65, 472–481 (2021).
Au, A. et al. Hydroxychloroquine screening practice patterns within a large multispecialty ophthalmic practice. Am. J. Ophthalmol. 160, 561-568.e2 (2015).
Islam, Y. F. K., Stroman, W. R. & Steigleman, W. A. Compliance with hydroxychloroquine screening guidelines at a large academic medical center. J. Vitreoretin. Dis. 6, 271–277 (2022).
Kim, J., Kim, K. E., Kim, J. H. & Ahn, S. J. Practice patterns of screening for hydroxychloroquine retinopathy in South Korea. JAMA Netw. Open 6, e2314816 (2023).
Yen, C. Y. et al. Current screening practice in patients under long-term hydroxychloroquine medication in Taiwan: A nationwide population-based cohort study. Medicine 98, e15122 (2019).
Tomura, A. & Usami, M. Safety and effectiveness of hydroxychloroquine in Japanese people with cutaneous lupus erythematosus and systemic lupus erythematosus: Results of a drug use–results survey. Ther. Res. 43, 817–842 (2022).
Rahmati, M. et al. Factors affecting global adherence for the uptake of diabetic retinopathy screening: A systematic review and meta-analysis. Am. J. Ophthalmol. 268, 94–107 (2024).
JMDC Inc. JMDC Claims Database. https://www.jmdc.co.jp/en/jmdc-claims-database/. (accessed 7 October 2024).
Nagai, K. et al. Data resource profile: JMDC claims database sourced from health insurance societies. J. Gen. Fam. Med. 22, 118–127 (2021).
Kim, J., Jeong, H. C., Kwon, H. Y., Kim, Y. H. & Ahn, S. J. Demographic and clinical characteristics associated with screening practices for hydroxychloroquine retinopathy. Sci. Rep. 14, 974 (2024).
Acknowledgements
The study was sponsored by the Asahi Kasei Pharma Corporation. The authors thank Sanofi for reviewing the manuscript and Drs. Shoichiro Inokuchi and Akiko Fujita of JMDC Inc. for their support with the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
M.K., K.K., and T. Takahashi: Study conceptualization and design; T. Tajima: Data collection, curation and analysis; K.K. and T. Takahashi: Preparation of the first draft; M.K., K.S., and N.Y.: Super vision of the study; All authors: Critical revision and approval of the final version.
Corresponding author
Ethics declarations
Competing interests
Kumiko Kato declares no competing interests; Mineo Kondo declares no competing interests; Kei Shinoda declares no competing interests; Naoto Yokogawa declares receiving speaker fees from Bristol-Myers Squibb Company, Chugai Pharmaceutical Co., Ltd., GlaxoSmithKline K.K., Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceutical Co., Ltd., and Asahi Kasei Pharma Corporation; Takumi Tajima is an employee of JMDC, Inc.; and Toshiya Takahashi is an employee of Asahi Kasei Pharma Corporation.
Ethical approval
The data source for this study was an anonymized, de-identified/de-linked secondary database that was used only for research purposes by the database vendor and was not linked to individual patient medical records. The requirement for informed consent was waived, and an opt-out approach was applied in compliance with local regulations. The study adhered to the principles of the Declaration of Helsinki. Ethical approval was not obtained because local regulations do not require it for studies utilizing pre-existing anonymized, de-identified/de-linked databases.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kato, K., Shinoda, K., Yokogawa, N. et al. A real-world descriptive study of screening practices for hydroxychloroquine retinopathy in Japan using an insurance claims database. Sci Rep 15, 12330 (2025). https://doi.org/10.1038/s41598-025-96579-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96579-2
Keywords
This article is cited by
-
Augenärztliche Screening-Untersuchungen bei Therapie mit Chloroquin oder Hydroxychloroquin
Die Ophthalmologie (2026)