Abstract
This study evaluated the prognostic value of the pan-immune-inflammation value (PIV) combined with the albumin-to-globulin ratio (AGR) for postoperative survival in colorectal cancer (CRC) patients and developed a nomogram for survival prediction. A total of 650 CRC patients who underwent radical surgery were included, with data from one institution used as the training set. The optimal cut-off values for PIV (426.8) and AGR (1.4) were determined using maximally selected rank statistics. Kaplan–Meier analysis showed that patients in the low-PIV group had significantly better 5-year overall survival (OS) compared to the high-PIV group, while those in the high-AGR group had better 5-year OS than those in the low-AGR group. Multivariate analysis identified age, N stage, degree of differentiation, PIV, and AGR as independent prognostic factors for OS. A nomogram for OS was developed and validated, demonstrating robust predictive performance. This study highlights the value of PIV and AGR as reliable indicators for predicting OS in CRC patients, with high PIV and low AGR associated with worse prognosis. Timely interventions may improve patient outcomes.
Similar content being viewed by others
Introduction
A serious global public health issue, cancer affects millions of individuals and causes a substantial amount of mortality and socioeconomic suffering. With 11% of all cancer diagnoses, colorectal cancer (CRC) is the third most frequent cancer in the world and has the second-highest death rate among cancer-related deaths worldwide1. It is estimated that the incidence of CRC is four times higher in developed countries than in developing countries. In China, CRC is the third most common cancer with the fifth highest mortality rate. The incidence of CRC in China is on a continuous upward trend2. Most CRC patients are diagnosed at an advanced stage and have a poor prognosis because there aren’t any early symptoms3. Even though patients with colorectal cancer have benefited from advancements in medical technology, the disease still has a high rate of metastasis and recurrence4. To enhance prognostic prediction and treatment efficacy evaluation in CRC patients, prospective biomarkers ought to be explored.
DNA damage, gene mutation, angiogenesis, tumor proliferation, invasion, and metastasis are all significantly impacted by tumor-associated inflammation5. The tumor microenvironment is determined not only by the tumor itself but also by the host’s systemic immune-inflammatory response. Inflammation alters the tumor microenvironment through multiple mechanisms, such as the production of cytokines and pro-inflammatory mediators, angiogenesis, and tissue remodeling6. One of the best examples of a tumor that is closely linked to chronic inflammation and can arise in the early stages of tumorigenesis is CRC7. An increasing amount of evidence suggests that inflammation associated with cancer has a significant impact on the prognosis and postoperative recovery of cancer patients8. Therefore, inflammation-based biomarkers are expected to be important predictors of surgery and long-term prognosis. In recent years, it has been reported that a novel biomarker Pan-immune-inflammation value (PIV) containing peripheral blood immune cell subpopulations (neutrophils, platelets, monocytes, and lymphocytes) has the potential to reflect the patient’s immune and systemic inflammation, and may become an effective predictor of long-term prognosis for tumor patients. This is because it accurately reflects the inflammatory and immune status of patients with malignant tumors9,10.
Numerous studies have demonstrated that nutritional status plays a significant role in the development and prognosis of tumors11. Numerous aspects of tumor development are linked to nutrition, which also has a significant impact on the course of treatment. Patients with colorectal cancer frequently suffer from malnutrition, which impairs immune function and lowers disease resistance12. Albumin and globulin are proteins that are abundant in human serum. As a major component of serum, albumin is commonly used to reflect the nutritional status of cancer patients13. Also, low albumin is used as a prognostic marker for human cancer. The albumin–globulin ratio (AGR), a serological measure of nutritional status, has been linked in recent years to the prognosis of several cancers12,14.
Currently, the majority of research focuses on examining the individual roles of PIV and AGR as biomarkers in various tumor types. However, compared to a single marker, the combination of these two indicators may provide more comprehensive and accurate prognostic information. This study aims to assess inflammation, immunity, and nutrition using the preoperative composite inflammatory marker PIV and the nutritional biomarker AGR. By integrating PIV and AGR, we seek to explore their relationship with CRC prognosis and develop a novel nomogram model to predict survival outcomes in CRC patients. This nomogram is designed to offer a more holistic and systematic approach to prognostic assessment, providing valuable support for clinical decision-making and improving patient management.
Materials and methods
Patients
This study retrospectively selected 470 primary CRC patients who underwent radical surgery at the Affiliated Cancer Hospital of Xinjiang Medical University from January 2016 to December 2018, and 180 primary CRC patients who underwent radical surgery at People’s Hospital of Bortala Mongolian Autonomous Prefecture. The inclusion criteria were: (1) Histopathologically confirmed stage I–III CRC; (2) Availability of a preoperative complete blood count; (3) Complete clinical and pathological data; (4) Availability of follow-up data. The exclusion criteria were: (1) Unresectable distant metastasis; (2) History of other malignancies or prior blood transfusion; (3) Autoimmune diseases or chronic inflammatory conditions; (4) Receipt of neoadjuvant therapy before surgery; (5) Hematological or autoimmune disorders. These exclusion criteria were applied to ensure the accuracy of the PIV and AGR in this study, minimizing potential confounders that could affect the reliability of the survival predictions.
The study was approved by the Ethics Committees of Xinjiang Medical University Cancer Hospital (K-2024056) and People’s Hospital of Bortala Mongolian Autonomous Prefecture (LLSH20241221) respectively, following a review for compliance with ethical standards. All procedures were conducted in accordance with the Declaration of Helsinki. Data collection and analysis were performed with strict adherence to ethical guidelines to ensure patient confidentiality. Written informed consent was obtained from all participants.
Follow-up
Patients were instructed to follow up every 3 months for the first 2 years, then every 6 months for the next 3–5 years, and finally once a year after that. Data collection also involves phone calls. Interval history, physical examination, blood tests, abdominal computed tomography, and fiberoptic colonoscopy were all part of the follow-up evaluation. The last follow-up took place in September 2023. OS was defined as the interval between surgery and death or the last follow-up.
Data collection
A list of patients attending our hospital for radical colorectal cancer surgery from January 2016 to December 2018 was collected from our information department. The laboratory data of the patients were then checked through the e-case system and recorded in detail in Excel. The following information was collected: basic patient information: gender, age, height, weight, body mass index (BMI), smoking and alcohol history. Laboratory results for the patient one week before surgery (during the initial consultation, when no treatment was administered): serum albumin, hemoglobin, platelets, lymphocytes, neutrophils, monocytes, and carcinoembryonic antigen (CEA). Clinicopathological parameters: Gross appearance, Histological type, pathological tumor stage (T stage), pathological node stage (N stage), TNM stage, Differentiated Degree, Nerve invasion, Intravascular tumor emboli. Follow-up information: survival outcome, survival time. Parameters were defined as follows: TNM staging was performed according to the Joint Committee on the American Classification of Cancers, 8th edition. CEA ≥ 5.00 ng/mL was considered high. PIV = [neutrophil count (109/L) × platelet count (109/L) × monocyte count (109/L)]/lymphocyte. AGR = albumin/globulin.
Statistical analysis
Data were analyzed using R software (version 4.2.3) and SPSS 25.0. The Shapiro–Wilk test was applied to assess the normality of continuous variables. Continuous variables are presented as mean ± standard deviation (SD) for normally distributed data, median (interquartile range, IQR) for non-normally distributed data, and counts (percentage, %) for categorical variables. Mann–Whitney U test or Student’s t-test was used for comparing continuous variables, while the Chi-square test or Fisher’s exact test was used for categorical variables. The optimal cut-off values were determined using the maximum selected rank statistic in the “survminer” R package. The cut-off values for PIV and AGR were 426.8 and 1.4, respectively. Patients were categorized into high and low PIV groups and high and low AGR groups based on these cut-off values. A Restrictive Cubic Spline (RCS) was employed to explore the non-linear relationship between PIV, AGR, and the risk of death in CRC patients. To compare the AUC values of combined biomarkers, we employed the DeLong test, a non-parametric approach utilized to evaluate the significance of differences between two or more ROC curves. Kaplan–Meier survival curves for OS were plotted and compared using the log-rank test. Univariate and multivariate analyses were performed using Cox proportional hazards regression models. Variables found to be statistically significant in the univariate analysis were included in the multivariate model to identify independent risk factors for OS. A nomogram was constructed based on these independent risk factors to predict the 1-year, 3-year, and 5-year OS after surgery. The discriminative ability and predictive performance of the nomogram were evaluated using receiver operating characteristic (ROC) curves and calibration curves. Decision curve analysis (DCA) was conducted to assess the net benefit of the prediction model. Additionally, external validation was performed using a cohort of 180 patients to assess the model’s performance. A two-tailed p value of less than 0.05 was considered statistically significant.
Results
The optimal cutoff value of PIV and AGR
The optimal cut-off value for PIV in the training cohort was 426.8, while the cut-off for AGR was 1.4, determined using maximally selected rank statistics. The high preoperative PIV group comprised 82 cases, and the low preoperative PIV group consisted of 388 cases (Fig. 1A). The high preoperative AGR group included 306 cases, while the low preoperative AGR group included 164 cases (Fig. 1B).
Baseline characteristics of patients
This study included 650 patients with CRC who underwent radical surgery, divided into a training group (n = 470) and a validation group (n = 180) (Table 1). The median age of the overall cohort was 61 years (interquartile range [IQR] 52–68), with males accounting for 57.8%. Baseline characteristics, including body mass index (BMI), smoking status, and alcohol consumption, were well balanced between the two groups (all p > 0.05). Tumors were predominantly staged as T3–T4 (77.1%) and N0–N1 (84.2%). A total of 60.8% of patients were classified as TNM stages I–II, while 39.2% were in stage III. Poorly differentiated or undifferentiated tumors accounted for 84.2%. Neural invasion and intravascular tumor thrombi were observed in 17.5% and 18.9% of patients, respectively. Elevated CEA levels were present in 36.0% of patients. Laboratory parameters, including median ALB levels (40.7 g/L), PLT (241 × 109/L), and lymphocyte count (1.81 × 109/L), were consistent across the cohorts. The training and validation groups showed high consistency in demographic, clinical, and laboratory characteristics, reinforcing the robustness of the validation group in model evaluation.
Clinical characteristics of CRC patients in the training cohort stratified by AGR and PNI cut-off values
A total of 470 CRC patients’ data were collected from the training cohort. The baseline characteristics table (Table 2) summarizes the relationship between PIV, AGR, and clinical pathological features. Briefly, the median age of the patients was 62 years, with 279 male patients (59.4%). Among the cases, 437 (93.0%) were adenocarcinomas, and 33 (7.0%) were of other pathological types. In terms of clinical staging, 284 (60.4%) patients were in stages I–II, while 186 (39.6%) were in stage III. Preoperative levels of albumin, platelet count, neutrophil count, monocyte count, and lymphocyte count were 40.6 g/L, 240 × 109/L, 3.62 × 109/L, 0.45 × 109/L, and 1.79 × 109/L, respectively. The median follow-up time was 75 months.
Prognostic value of PIV and AGR in I–III stage colorectal cancer
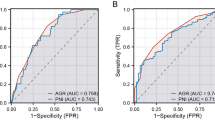
RCS revealed a nonlinear relationship between PIV and AGR and the hazard ratio (HR) of CRC patients. The results showed that HR gradually increased with the increase of PIV (Fig. 2A) and decreased with the increase of AGR (Fig. 2B). This suggests that PIV is a risk factor for death in CRC patients and AGR is a patient protective factor. Subsequently, we performed a Kaplan–Meier survival analysis. The results showed a significant difference in the 5-year OS rate between the different groups (p < 0.001). Compared with patients with high preoperative PIV, the 5-year OS rate was significantly higher in patients with low PIV levels (82.7% vs. 46.4%, p < 0.001 Fig. 3A). However, the opposite trend was observed concerning AGR, with patients with low preoperative AGR levels having a significantly lower 5-year OS rate compared with those with high preoperative levels (64.7% vs. 82.4%, p < 0.001; Fig. 3B). In subgroup analyses, we also obtained consistent results. Specifically, patients in the high PIV group (Fig. 4A) and the low AGR (Fig. 4B) group showed significantly reduced 5-year OS rates. We compared the prognostic performance of PIV and AGR with other inflammation- and immune-related markers, including the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII). PIV and AGR demonstrated superior predictive accuracy, with AUC values of 0.795 and 0.744, respectively (Fig. 5A). Furthermore, our analysis revealed that the combined use of PIV and AGR provided a significant predictive advantage over either marker alone, achieving a notably higher AUC of 0.859 compared to PIV (0.795) and AGR (0.744) (Fig. 5B).
Univariate and multivariate analyses of risk factors
In the training cohort, the Cox regression analysis model was applied to assess factors associated with OS. Results of the univariate analysis showed that Age (HR 1.02; 95% CI 1.01–1.03; p = 0.028), Gross appearance (HR 1.58; 95% CI 1.03–2.42; p = 0.037), T stage (HR 4.12; 95% CI 2.09–8.12; p < 0.001), the N stage (HR 2.40; 95% CI 1.61–3.58; p < 0.001), TNM stage (HR 2.09; 95% CI 1.46–2.98; p < 0.001), Differentiated degree (HR 0.38; 95% CI 0.26–0.56; p < 0.001), and Intravascular tumor emboli (HR 1.56; 95% CI 1.03–2.37; p = 0.036), CEA (HR 1.69; 95% CI 1.19–2.42; p = 0.004), PIV (HR 6.86; 95% CI 4.77–9.86; p < 0.001), AGR(HR 3.86; 95% CI 2.67–5.58); p < 0.001) were potential factors associated with OS. The above statistically significant factors were included in a multivariate Cox regression model and the analysis showed that Age (HR 1.02; 95% CI 1.01–1.03; p = 0.013), N stage (HR 1.97; 95% CI 1.20–3.22; p = 0.007), Differentiated degree (HR 0.58; 95% CI 0.38–0.90; p = 0.015), PIV (HR 7.42; 95% CI 5.06–10.89; p < 0.001), and AGR (HR 3.04; 95% CI 2.06–4.49; p < 0.001) were the independent risk factors for OS (Table 3).
Establishment and verification of nomogram
A nomogram model for predicting 1-, 3-, and 5-year OS was plotted based on statistically significant variables (Age, N stage, Differentiated degree, PIV, AGR) in multivariate Cox regression analysis. We used this model to assess individualized prognostic prediction in CRC patients. Nomograms can be interpreted by summing the points assigned to each variable, indicated at the top of the scale. The total points can be converted to a minimum scale predicting the probability of a patient’s death at 5 years (Fig. 6). The ROC curve analysis of the clinical model in the training cohort revealed AUC values of 86.9%, 90.2%, and 87.8% for 1-year, 3-year, and 5-year survival, respectively (Fig. 7A). In the external validation cohort, the AUC values for 1-year, 3-year, and 5-year survival were 93.4%, 90.8%, and 85.1%, respectively (Fig. 7B). These values demonstrate the strong discriminatory power of the predictive model. The calibration curves (Fig. 8A,B) showed good consistency between the predicted and actual complication rates, further confirming the model’s reliability. DCA curves illustrated the threshold probabilities of the predictive model in both the training cohorts (Fig. 9A–C) and validation cohorts (Fig. 9D–F), providing a comprehensive evaluation of the nomogram’s clinical utility. These findings underscore the model’s superior predictive performance and highlight its potential for clinical application.
Discussion
CRC is a current public health concern due to its high degree of malignancy, rapid progression, and high rates of disability and mortality. It ranks third among malignant tumors worldwide15. Numerous systemic inflammatory factors may be potential indicators for predicting the oncological prognosis of human cancers, according to an expanding body of research. By more effectively matching patient needs with available treatments, prognostic factors can impact clinical decision-making and enhance patient health. The longer overall survival and better prognosis in colorectal cancer may be due to the creation of customized treatment plans16. Numerous studies have demonstrated that the inflammatory response is crucial to the development of the tumor microenvironment, that specific alterations in inflammatory cells may serve as prognostic indicators, and that variations in the immune cell composition of peripheral venous blood may indicate the tumor’s inflammatory state and, consequently, the prognosis for survival17. This study retrospectively included a total of 650 patients with stage I–III colorectal cancer from two dependent institutions, all of whom underwent radical surgery between January 2016 and December 2018. Kaplan–Meier survival analysis revealed that patients with high preoperative PIV had significantly better 5-year OS rates compared to those with low PIV levels. However, with regard to AGR, patients with low preoperative AGR levels had significantly lower 5-year OS rates than those with high preoperative AGR levels. Subgroup analysis showed that PIV demonstrated strong prognostic value in multiple subgroups, particularly in patients with T3–T4 and N2–N3 stage colorectal cancer, suggesting that PIV is an important marker of colorectal cancer progression. AGR also exhibited significant prognostic value in several subgroups, particularly in patients with high BMI, T3–T4 stage, and poorly differentiated tumors, highlighting the impact of nutritional and immune status on prognosis. In Cox multivariate analysis, both PIV and AGR were identified as independent risk factors for predicting the prognosis of colorectal cancer patients. Moreover, based on these independent risk factors, we constructed nomogram models to predict 1-, 3-, and 5-year OS. PIV and AGR play critical roles in prognostic assessment for colorectal cancer, and their combined use shows significant advantages, offering higher accuracy and reliability. Finally, comprehensive testing of the nomogram demonstrated strong discriminatory and predictive abilities. Therefore, PIV and AGR can serve as biomarkers to identify poor prognosis in colorectal cancer patients. Our findings are consistent with previous studies that suggest PIV and AGR as valuable prognostic biomarkers in cancer, particularly in colorectal cancer. However, our study adds novelty by combining these biomarkers and demonstrating their complementary role in predicting patient outcomes. Unlike some previous studies that focus on single biomarkers, we show that PIV and AGR together may offer superior prognostic accuracy. This is in line with recent research suggesting that combined biomarkers offer more reliable predictions compared to individual markers.
PIV and AGR offer unique advantages in reflecting the prognosis of CRC patients. Traditional biomarkers, such as CEA18, are primarily used for monitoring tumor burden and recurrence, while PIV and AGR focus on the host’s immune-inflammatory response and nutritional status, providing a more comprehensive disease assessment. A higher PIV indicates excessive inflammation activation, which may lead to an imbalance in the immune microenvironment, promoting tumor growth and metastasis. On the other hand, a lower AGR, often accompanied by decreased albumin and increased globulin, suggests malnutrition and a chronic inflammatory state, factors that may accelerate disease progression. Therefore, incorporating PIV and AGR into the risk assessment system for CRC may provide a new perspective for clinical decision-making.
Simple graphical depictions of statistical predictive models that produce numerical probabilities of clinical events are called nomograms. In many cancers, nomograms are comparable to the conventional TNM staging system; as a result, they are recognized as a substitute or even a new standard19. By using nomograms to create personalized predictions, doctors can find and categorize patients who are a good fit for clinical trial participation.
Tumor genesis, progression, and metastasis are all significantly influenced by systemic inflammation20. Inflammation plays a crucial role in initiating tumorigenesis by damaging specific tissues21, and neutrophils are an important part of this process. The significance of neutrophils in tumor initiation has been demonstrated by chemical carcinogen-induced inflammation-induced cancer models, including the oxidized azomethane-dextran sodium sulfate-associated colon cancer model and the DMBA-TPA skin cancer model22. The release of prostaglandin E2 by neutrophils intensifies the inflammatory response and generates a tumor microenvironment that encourages the development of colon tumors. Signals that drive neutrophil polarization and activation cause them to be functionally perturbed and show high levels of plasticity in the tumor microenvironment23. Both direct and indirect mechanisms of cancer promotion are fueled by this. Neutrophils contribute to tumor progression through the secretion of vascular endothelial growth factor (VEGF), a key angiogenic factor that promotes endothelial cell proliferation and new blood vessel formation24. The release of neutrophil-derived VEGF enhances vascular permeability and supports the hypoxic adaptation of tumor cells, thereby facilitating tumor growth and metastasis25. Additionally, neutrophils produce matrix metalloproteinases (MMP-9 in particular)26, which degrade the extracellular matrix (ECM) and enable tumor cell invasion. MMPs also contribute to the activation of latent growth factors, further amplifying tumor-promoting signals. Neutrophils secrete matrix metalloproteinases and interleukin-1β, which increase tumor cell exudation and inhibit natural killer cell activity. By adhering to tumor vessels and aggregating at the primary tumor site, platelets affect tumor growth and metastasis27. Growth factors are stored in large quantities in the alpha granules of platelets. They can come into direct contact with cancerous cells and are found in the extravascular tumor microenvironment. Platelets, on the other hand, store and release VEGF from their alpha granules upon activation, reinforcing tumor vascularization and supporting the survival of circulating tumor cells. By forming platelet-tumor cell aggregates, platelets shield tumor cells from immune surveillance, particularly from natural killer cell-mediated lysis28. Furthermore, platelets secrete MMPs, which facilitate ECM degradation and enhance metastatic potential by promoting the extravasation of tumor cells into distant tissues29. Platelets can also protect tumor cells that are circulating in the periphery, prevent natural killer cells from identifying tumor cells, and increase the likelihood that the tumor cells will spread30. The interplay between neutrophils, platelets, and the TME highlights the importance of these cellular components in tumor progression through VEGF-driven angiogenesis and MMP-mediated matrix remodeling. The functions of various monocyte subpopulations during cancer include phagocytosis, secretion of tumor-killing mediators, promotion of angiogenesis, extracellular matrix remodeling, lymphocyte recruitment, and differentiation into tumor-associated macrophages and dendritic cells. These functions all help to promote anti-tumor immunity. Monocytes are a primary source of dendritic cells and long-lived tumor-associated macrophages that help to form the tumor microenvironment31. Monocytes generate antitumor responses, activate antigen-presenting cells to exert antitumor effects, induce immune tolerance, and improve tumor cell dissemination, all of which have a differential impact on the tumor microenvironment32. Tumor-associated immunity is significantly influenced by lymphocytes. It can stop the growth of many tumors and has a potent anti-tumor immune effect. However, lymphocyte activity and function are changed in the inflammatory state33, including T-cell subpopulation imbalance and immune regulation dysfunction. This can result in impaired immune surveillance and enable tumor cells to elude immune attack34. PIV allows for a combined assessment of systemic inflammatory status by combining neutrophils, platelets, monocytes, and lymphocytes35. Our study demonstrated that patients with low PIV levels had a high 5-year OS rate compared with CRC patients with high preoperative PIV levels, suggesting that PIV is an independent risk factor for poor prognosis in CRC patients.
There are many immunoinflammatory biomarkers (IIBs) developed in previous studies, such as NLR, PLR, lymphocyte/monocyte (MLR), SII and Systemic Inflammatory Response Index (SIRI) among others. However, they are all composed of two or three peripheral blood cell components. Thus, the inclusion of four peripheral blood cell immunoinflammatory biomarkers PIV in one can capture the complexity of the immune environment more comprehensively. In a study by Fucà et al.36, PIV was strongly associated with survival in patients with metastatic CRC and was superior to other IIBs (NLR, PLR, SII) in predicting survival in metastatic colorectal cancer. Similarly, in a study of human epidermal growth factor receptor 2-positive patients with advanced breast cancer37, PIV is superior to NLR, PLR, and MLR in predicting OS. In addition, PIV has been shown in several meta-analyses and retrospective studies to predict prognosis in patients with a variety of solid tumors10,38,39,40,41,42,43. Our findings are consistent with those of the above studies in that both the high PIV group had higher tumor stage and worse survival outcomes than the low PIV group.
According to earlier research44, clinical outcomes and the nutritional status of cancer patients are correlated, and patients with different cancers have a lower survival rate the worse their nutritional status. Malnutrition is a major problem in patients with CRC45, especially after surgery where restricted feeding is a major cause of undernutrition. Clinical results are improved, surgical complications are decreased, and survival is increased when malnourished patients are identified early. The two primary components of serum proteins are globulin (GLB) and serum albumin.
It is known that serum albumin is a sensitive indicator for determining nutritional status46. Serum albumin levels rapidly drop when malignant cells are present because they cause malnourishment and a generalized inflammatory response that prevents albumin synthesis in tumor patients. Low albumin levels in a variety of malignant tumors are a poor indicator of the clinical prognosis47. Furthermore, systemic inflammation causes cytokines to decrease albumin synthesis, and hypoalbuminemia is a major factor in weakening the immune response and accelerating the growth of cancer48. But unlike albumin, globulins are pro-inflammatory proteins that contain complex elements like immunoglobulins, interleukins, acute phase proteins, and tumor markers49. It is considered that distant metastasis, immune escape, and tumor growth are all linked to high GLB expression50. Albumin, as a negative acute-phase protein, reflects the systemic nutritional and inflammatory status of cancer patients. Malignant cells and the associated inflammatory microenvironment downregulate albumin synthesis through cytokine-mediated pathways, particularly via interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). Low albumin levels weaken immune surveillance by impairing lymphocyte proliferation, antigen presentation, and cytokine production, thereby facilitating immune evasion. Additionally, hypoalbuminemia is associated with increased oxidative stress and endothelial dysfunction, which further promote tumor growth and metastasis. Conversely, globulins, particularly immunoglobulins, acute-phase proteins, and inflammatory cytokines, actively contribute to pro-tumorigenic inflammation. Elevated globulin levels are associated with chronic inflammation, immune escape, and tumor progression. For instance, high globulin expression correlates with increased levels of interleukin-1β (IL-1β) and IL-651, which promote tumor cell survival, angiogenesis, and resistance to apoptosis. Furthermore, tumor cells exploit immunoglobulin-dependent mechanisms to evade immune destruction, as certain globulin subfractions suppress T-cell activity and promote regulatory T-cell (Treg) expansion, facilitating immune tolerance52. Combining albumin and globulin, AGR reduces the susceptibility of its expression to confounding variables like fluid retention or dehydration. AGR might be a more accurate predictor than just albumin or globulin. Emerging evidence suggests that AGR may serve as a useful predictive tool for cancer diagnosis and prognosis. Several studies have reported that low serum AGR is associated with poor prognosis in several cancers, including gastric, lung, and breast cancers. Low preoperative AGR was associated with poor prognosis in patients in our study. In addition, AGR was also an independent risk factor for poor prognosis in patients in a multifactorial analysis.
However, our study has some limitations. First, this study was a retrospective study with a relatively small sample size that may include case selection bias. Therefore, prospective studies with large sample sizes are needed for further validation. In addition, we only collected patients with stage I–III CRC and excluded some vulnerable populations, which may limit the generalizability of the results. Therefore, future studies urgently require multicenter, large-scale prospective research supplemented with external validation to enhance the reliability and scientific validity of the findings. Finally, the integration of PIV and AGR into routine clinical practice still requires further clinical validation and standardization efforts to ensure their reliable application in different diseases and populations. In our study, we focused primarily on the prognostic value of PIV and AGR as independent biomarkers in CRC patients after radical surgery. However, the effect of different treatment modalities on the prognostic value of PIV and AGR was not explored in detail. We acknowledge that various post-operative treatment strategies, such as adjuvant chemotherapy, targeted therapy, and immunotherapy, may influence the inflammatory response and nutritional status, potentially affecting the prognostic value of these biomarkers. Future studies should investigate the interaction between treatment strategies and the prognostic significance of PIV and AGR, as this may enhance our ability to stratify patients based on treatment and prognosis more accurately. In future studies, it would be valuable to stratify CRC patients based on treatment modalities (e.g., adjuvant chemotherapy, targeted therapy, etc.) and assess the prognostic value of PIV and AGR within these subgroups. This approach could help determine whether these biomarkers maintain their prognostic utility across different treatment regimens or whether their predictive power is influenced by specific therapeutic interventions. In this study, we recognize that certain confounding factors, such as chronic diseases (e.g., diabetes, COPD) and preoperative nutritional interventions, were not fully controlled for, which could introduce bias into the results. Diabetes is often associated with a chronic inflammatory state, which may affect the levels of inflammatory markers. Similarly, COPD patients may exhibit different immune responses compared to healthy individuals. Furthermore, preoperative nutritional interventions are known to enhance immune function and modulate inflammatory responses, which could also impact markers like PIV and AGR.
Thus, we suggest that future research should consider controlling for these variables more comprehensively. Prospective studies should collect detailed data on chronic diseases and preoperative nutritional interventions, allowing for more precise adjustment and stratification. These factors should be accounted for in future studies to strengthen the validity and generalizability of the findings. Nonetheless, we also confirmed by our analysis that PIV and AGR are independent risk factors for poor prognosis in CRC patients. We developed a simple predictive model to identify the inflammatory status of patients before surgery. This helps to provide preventive measures and personalized treatment to patients promptly.
Conclusion
In conclusion, to the best of our knowledge, this is the first study to correlate the interrelationship between PIV and AGR in clinical outcomes in CRC patients. We demonstrated that PIV and AGR are independent predictors of prognosis in patients with stage I–III CRC. Column line plots based on PIV and AGR can provide intuitive and accurate prognostic predictions for individual patients. In conclusion, as a low-cost, noninvasive, easily assessable, and reproducible prognostic parameter, PIV and AGR can be simple and powerful indicators to identify different prognoses in patients with stage I–III CRC.
Data availability
All data generated or analysed during this study are included in this published article.
References
Siegel, R. L., Kratzer, T. B., Giaquinto, A. N., Sung, H. & Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 75, 10–45. https://doi.org/10.3322/caac.21871 (2025).
Cao, M., Li, H., Sun, D. & Chen, W. Cancer burden of major cancers in China: A need for sustainable actions. Cancer Commun. (Lond.) 40, 205–210. https://doi.org/10.1002/cac2.12025 (2020).
Morris, V. K. et al. Treatment of metastatic colorectal cancer: ASCO guideline. J. Clin. Oncol. 41, 678–700. https://doi.org/10.1200/JCO.22.01690 (2023).
Cervantes, A. et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 34, 10–32. https://doi.org/10.1016/j.annonc.2022.10.003 (2022).
Wang, Q. et al. IL1RN and PRRX1 as a prognostic biomarker correlated with immune infiltrates in colorectal cancer: Evidence from bioinformatic analysis. Int. J. Genom. 2022, 2723264. https://doi.org/10.1155/2022/2723264 (2022).
Greten, F. R. & Grivennikov, S. I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 51, 27–41. https://doi.org/10.1016/j.immuni.2019.06.025 (2019).
Shen, G. et al. Bridging chronic inflammation and digestive cancer: The critical role of innate lymphoid cells in tumor microenvironments. Int. J. Biol. Sci. 20, 4799–4818. https://doi.org/10.7150/ijbs.96338 (2024).
Chen, Y. et al. Prognostic value of preoperative modified Glasgow prognostic score in predicting overall survival in breast cancer patients: A retrospective cohort study. Oncol. Lett. 29, 180. https://doi.org/10.3892/ol.2025.14926 (2025).
Şahin, A. B. et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci. Rep. 11, 14662. https://doi.org/10.1038/s41598-021-94184-7 (2021).
Sato, S. et al. The preoperative pan-immune-inflammation value is a novel prognostic predictor for with stage I–III colorectal cancer patients undergoing surgery. Surg. Today 52, 1160–1169. https://doi.org/10.1007/s00595-021-02448-6 (2022).
Deng, Y. et al. Global, regional, and national disease burden and prediction analysis of colorectal cancer attributable to tobacco, alcohol, and obesity from 1990 to 2030. Front. Oncol. 15, 1524308. https://doi.org/10.3389/fonc.2025.1524308 (2025).
de Sousa, I. M., Silva, F. M., de Carvalho, A. L. M., da Rocha, I. M. G. & Fayh, A. P. T. Accuracy of isolated nutrition indicators in diagnosing malnutrition and their prognostic value to predict death in patients with gastric and colorectal cancer: A prospective study. JPEN J. Parenter. Enteral Nutr. 46, 508–516. https://doi.org/10.1002/jpen.2199 (2022).
Shayimu, P. et al. Serum nutritional predictive biomarkers and risk assessment for anastomotic leakage after laparoscopic surgery in rectal cancer patients. World J. Gastrointest. Surg. 16, 3142–3154. https://doi.org/10.4240/wjgs.v16.i10.3142 (2024).
Quan, L., Jiang, X., Jia, X. & Cheng, F. Prognostic value of the albumin-to-globulin ratio in patients with colorectal cancer: A meta-analysis. Nutr. Cancer 74, 3329–3339. https://doi.org/10.1080/01635581.2022.2076890 (2022).
Benson, A. B. et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 19, 329–359. https://doi.org/10.6004/jnccn.2021.0012 (2021).
Lee, S. & Song, M. Adjuvant aspirin therapy and colorectal cancer survival. Lancet Gastroenterol. Hepatol. 10, 184–185. https://doi.org/10.1016/S2468-1253(24)00393-5 (2025).
Michal, M. et al. Inflammatory leiomyosarcoma shows frequent co-expression of smooth and skeletal muscle markers supporting a primitive myogenic phenotype: A report of 9 cases with a proposal for reclassification as low-grade inflammatory myogenic tumor. Virchows Arch. 477, 219–230. https://doi.org/10.1007/s00428-020-02774-z (2020).
Hai-Jing, Y., Shan, R. & Jie-Qiong, X. Prognostic significance of the pretreatment pan-immune-inflammation value in cancer patients: An updated meta-analysis of 30 studies. Front. Nutr. 10, 1259929. https://doi.org/10.3389/fnut.2023.1259929 (2023).
Xu, Z. et al. Stemness-related lncRNAs signature as a biologic prognostic model for head and neck squamous cell carcinoma. Apoptosis 28, 860–880. https://doi.org/10.1007/s10495-023-01832-6 (2023).
Li, Z., Li, J., Bai, X., Huang, X. & Wang, Q. Tumor microenvironment as a complex milieu driving cancer progression: A mini review. Clin. Transl. Oncol. https://doi.org/10.1007/s12094-024-03697-w (2024).
Sionov, R. V. Leveling up the controversial role of neutrophils in cancer: When the complexity becomes entangled. Cells 10, 2486. https://doi.org/10.3390/cells10092486 (2021).
Li, J. et al. GNL3L exhibits pro-tumor activities via NF-κB pathway as a poor prognostic factor in acute myeloid leukemia. J. Cancer 15, 4072–4080. https://doi.org/10.7150/jca.95339 (2024).
Wu, G. et al. Neutrophils’ dual role in cancer: From tumor progression to immunotherapeutic potential. Int. Immunopharmacol. 140, 112788. https://doi.org/10.1016/j.intimp.2024.112788 (2024).
Pérez-Gutiérrez, L. & Ferrara, N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat. Rev. Mol. Cell Biol. 24, 816–834. https://doi.org/10.1038/s41580-023-00631-w (2023).
Sreesada, P. et al. Matrix metalloproteinases: Master regulators of tissue morphogenesis. Gene 933, 148990. https://doi.org/10.1016/j.gene.2024.148990 (2025).
Josefsson, E. C. Platelets and megakaryocytes in cancer. J. Thromb. Haemost. 23, 804–816. https://doi.org/10.1016/j.jtha.2024.12.016 (2025).
Sun, Y. et al. Platelet-mediated circulating tumor cell evasion from natural killer cell killing through immune checkpoint CD155-TIGIT. Hepatology 81, 791–807. https://doi.org/10.1097/HEP.0000000000000934 (2025).
Huang, Z. et al. Prognostic significance of platelet-to-albumin ratio in patients with esophageal squamous cell carcinoma receiving definitive radiotherapy. Sci. Rep. 12, 3535. https://doi.org/10.1038/s41598-022-07546-0 (2022).
Bhatia, D., Dolcetti, R. & Mazzieri, R. Are monocytes a preferable option to develop myeloid cell-based therapies for solid tumors?. J. Exp. Clin. Cancer Res. 44, 98. https://doi.org/10.1186/s13046-025-03359-x (2025).
Wang, S. et al. Perspectives of tumor-infiltrating lymphocyte treatment in solid tumors. BMC Med. 19, 140. https://doi.org/10.1186/s12916-021-02006-4 (2021).
Zhang, H. et al. KIF18A inactivates hepatic stellate cells and alleviates liver fibrosis through the TTC3/Akt/mTOR pathway. Cell. Mol. Life Sci. 81, 96. https://doi.org/10.1007/s00018-024-05114-5 (2024).
Wang, K. et al. Prognostic value of combined NP and LHb index with absolute monocyte count in colorectal cancer patients. Sci. Rep. 15, 8902. https://doi.org/10.1038/s41598-025-94126-7 (2025).
Shapaer, T. et al. Elevated BEAN1 expression correlates with poor prognosis, immune evasion, and chemotherapy resistance in rectal adenocarcinoma. Discov. Oncol. 15, 446. https://doi.org/10.1007/s12672-024-01321-5 (2024).
Turcotte, S. et al. Art of TIL immunotherapy: SITC’s perspective on demystifying a complex treatment. J. Immunother. Cancer 13, e010207. https://doi.org/10.1136/jitc-2024-010207 (2025).
Wu, Z. et al. Mitochondrial-related drug resistance lncRNAs as prognostic biomarkers in laryngeal squamous cell carcinoma. Discov. Oncol. 15, 785. https://doi.org/10.1007/s12672-024-01690-x (2024).
Fucà, G. et al. The pan-immune-inflammation value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer 123, 403–409. https://doi.org/10.1038/s41416-020-0894-7 (2020).
Ligorio, F. et al. The pan-immune-inflammation-value predicts the survival of patients with human epidermal growth factor receptor 2 (HER2)-positive advanced breast cancer treated with first-line taxane-trastuzumab-pertuzumab. Cancers (Basel) 13, 2021. https://doi.org/10.3390/cancers13081964 (1964).
Li, K. et al. Pan-immune-inflammatory values predict survival in patients after radical surgery for non-metastatic colorectal cancer: A retrospective study. Oncol. Lett. 29, 197. https://doi.org/10.3892/ol.2025.14943 (2024).
Turan, Y. B. The prognostic importance of the pan-immune-inflammation value in patients with septic shock. BMC Infect. Dis. 24, 69. https://doi.org/10.1186/s12879-023-08963-w (2024).
Yang, X. C. et al. Prognostic value of pan-immune-inflammation value in colorectal cancer patients: A systematic review and meta-analysis. Front. Oncol. 12, 1036890. https://doi.org/10.3389/fonc.2022.1036890 (2022).
Wu, Z., Chen, Y., Yu, G. & Ma, Y. Research trends and hotspots in surgical treatment of recurrent nasopharyngeal carcinoma: A bibliometric analysis from 2000 to 2023. Asian J. Surg. 47, 2939–2941. https://doi.org/10.1016/j.asjsur.2024.02.106 (2024).
Shi, J., Liu, C., Yang, N. & Qiu, C. Pan-immune-inflammation value: A new prognostic index in operative laryngeal and pharyngeal carcinomas. Clin. Transl. Oncol. 27, 151–159. https://doi.org/10.1007/s12094-024-03558-6 (2025).
Yeh, C. C. et al. Discovering the clinical and prognostic role of pan-immune-inflammation values on oral cavity squamous cell carcinoma. Cancers (Basel) 15, 322. https://doi.org/10.3390/cancers15010322 (2023).
Almasaudi, A. S., McSorley, S. T., Dolan, R. D., Edwards, C. A. & McMillan, D. C. The relation between malnutrition universal screening tool (MUST), computed tomography-derived body composition, systemic inflammation, and clinical outcomes in patients undergoing surgery for colorectal cancer. Am. J. Clin. Nutr. 110, 1327–1334. https://doi.org/10.1093/ajcn/nqz230 (2019).
Zeng, X. et al. Effects of micronutrients and macronutrients on risk of allergic disease in the European population: A Mendelian randomization study. Food Agric. Immunol. 13, 35. https://doi.org/10.1080/09540105.2024.2442369 (2024).
Rinninella, E. et al. Effects of nutritional interventions on nutritional status in patients with gastric cancer: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 38, 28–42. https://doi.org/10.1016/j.clnesp.2020.05.007 (2020).
Kang, S. C., Kim, H. I. & Kim, M. G. Low serum albumin level, male sex, and total gastrectomy are risk factors of severe postoperative complications in elderly gastric cancer patients. J. Gastr. Cancer 16, 43–50. https://doi.org/10.5230/jgc.2016.16.1.43 (2016).
Xu, S. S. et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J. Gastroenterol. 26, 828–838. https://doi.org/10.3748/wjg.v26.i8.828 (2020).
Chang, W. J., Du, Y., Zhao, X., Ma, L. Y. & Cao, G. W. Inflammation-related factors predicting prognosis of gastric cancer. World J. Gastroenterol. 20, 4586–4596. https://doi.org/10.3748/wjg.v20.i16.4586 (2014).
Li, J. et al. Preoperative albumin-to-globulin ratio and prognostic nutritional index predict the prognosis of colorectal cancer: A retrospective study. Sci. Rep. 13, 17272. https://doi.org/10.1038/s41598-023-43391-5 (2023).
Silva, A. D., Hwang, J., Marciel, M. P. & Bellis, S. L. The pro-inflammatory cytokines IL-1β and IL-6 promote upregulation of the ST6GAL1 sialyltransferase in pancreatic cancer cells. J. Biol. Chem. 300, 107752. https://doi.org/10.1016/j.jbc.2024.107752 (2024).
Elewaut, A. et al. Cancer cells impair monocyte-mediated T cell stimulation to evade immunity. Nature 637, 8716–8725. https://doi.org/10.1038/s41586-024-08257-4 (2025).
Funding
This study was financially supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region (Project No. 2022D01C297).
Author information
Authors and Affiliations
Contributions
Zeliang Zhao contributed to study concept and design. Kejin Li, Yi Chen, Ziyi Zhang, Kuan Wang, Subinur Sulayman, Xiangyue Zeng, Saibihutula Ababaike, and Junmin Guan collected clinical data. Kejin Li, Yi Chen, Ziyi Zhang, and Kuan Wang contributed to analyze the data. Kejin Li, Yi Chen and Zeliang Zhao contributed to prepare the manuscript. Zeliang Zhao provided critical feedback on methods, supervised the study. All authors approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Ethics Committee of Xinjiang Medical University Cancer Hospital (Approval No. K-2024056) and the Ethics Committee of the People’s Hospital of Bortala Mongolian Autonomous Prefecture (Approval No. LLSH20241221) in accordance with the Declaration of Helsinki, and written informed consent was obtained from all participants or their legal guardians.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, K., Chen, Y., Zhang, Z. et al. Preoperative pan-immuno-inflammatory values and albumin-to-globulin ratio predict the prognosis of stage I–III colorectal cancer. Sci Rep 15, 11517 (2025). https://doi.org/10.1038/s41598-025-96592-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96592-5
Keywords
This article is cited by
-
Integrated analysis reveals GSTA1 as a prognostic biomarker in stage I lung adenocarcinoma
BMC Cancer (2026)
-
Integration of circulating biomarkers and clinical factors: construction and validation of a prediction model for lung cancer metastasis
BMC Pulmonary Medicine (2025)
-
Preoperative hyperglycemia as an independent predictor of reduced long-term survival after gastric cancer surgery
European Journal of Medical Research (2025)
-
Development of a novel nomogram to predict the prognosis of acute pancreatitis in pregnancy
BMC Gastroenterology (2025)
-
Characteristics and risk factors for thrombocytopenia in malignancy patients with pulmonary embolism: a case control study
BMC Pulmonary Medicine (2025)