Abstract
Neurohormonal benefits of Chronic Chagas’ Cardiomyopathy (CCC) remain controversial. This study aimed to assess therapeutic interventions on CCC evolution in T. cruzi-infected hamsters at pre and post-treatment (2 months with beta-blocker (CH + BB) or ACE inhibitor (CH + ACEI)). Echocardiography was performed through evolution and compared to histopathological myocardial analysis. At post-treatment, a significant reduction of LV global systolic function and segmental function was observed between the control group and all Chagas’ groups. Compared to the Control, a reduction in LV regional strain was observed in three LV segments, regardless of treatment. No differences were observed in apoptosis, myocardial fibrosis, and the number of inflammatory cells among the groups. In an experimental model of CCC, LV global and regional function is compromised, and the treatment with ACEi and BB did not change LV remodeling. Regional LV function was slightly better in animals treated with BB, and this difference was not observed in the CH + ACEi group.
Similar content being viewed by others
Introduction
Chagas disease (CD) persists as a neglected chronic infectious disease, imposing a significant healthcare burden1,2,3. The typical progression of CD after an initial acute phase, which may have non-specific symptoms or even be silent, may be followed by a quiescent phase which can last decades (Indeterminate phase). A proportion of 20–30% of infected individuals may develop chronic Chagas’ cardiomyopathy (CCC) 20–30 years after the initial infection4. CCC is still a significant cause of heart failure and sudden death5. However, our understanding of the mechanisms underlying the progression of myocardial injury, cardiac remodeling, and functional ventricular changes remains limited.
The left ventricular (LV) remodeling process appears to be related to several different etiopathogenic mechanisms (chronic incessant inflammation, myocardial apoptosis, and fibrosis replacement, added to dysautonomia and neurohormonal activation). There are no proven effects of an etiological treatment benefit at late chronic phases of CD6. Therefore, the pharmacological treatment for CCC is non-specific, and the use of neurohormonal drugs to prevent remodeling and fibrosis is extrapolated from other etiologies of heart failure7. Specific results of the benefits of neurohormonal treatments in CD still need to be clarified in clinical studies and animal experimental disease models8.
New translational medicine techniques, such as small animal-dedicated high-resolution echocardiography, with advanced tools to explore myocardial function, such as Speckle Tracking, open a window to explore mechanisms of myocardial global and regional dysfunction related to signs of myocardial tissue damage. This study was designed to test the effects of therapeutic intervention with a beta-blocker (BB) or an angiotensin-converting enzyme inhibitor (ACEi) in LV remodeling and dysfunction progression through CCC in Syrian hamsters infected with T. cruzi.
Methods
Experimental animals
Twelve-week-old female hamsters (Mesocricetus auratus) (Anilab – Animais de Laboratório Criação e Comércio Ltda, Paulínia/SP, Brasil) were maintained in a controlled environment with a 12-hour light/dark cycle and provided ad libitum access to food and standard chow. All experimental procedures and protocols were approved by the Animal Research Ethics Committee of the Institution (CEUA FMRP-USP: Ethical Committee in animal use of Ribeirão Preto Medical School- Sao Paulo University- Protocol: 223/2018).
Experimental protocol
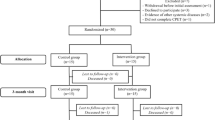
The animals from the T. cruzi-infected groups were intraperitoneally injected with 3.5 × 10^4 trypomastigote forms of the Y strain of T. cruzi. The control group (uninfected) was inoculated intraperitoneally with the same volume of saline solution. At 6 months post-infection (baseline condition - pre-treatment), the animals were distributed into the following groups: (1) Uninfected control (Control); (2) Untreated Chagas (Chagas); (3) Chagas treated with beta-blocker (CH + BB); (4) Chagas treated with ACE inhibitor (CH + ACEI).
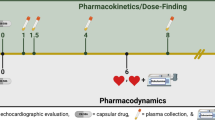
Animals in the groups treated with BB (carvedilol) or ACEi (enalapril) received the medications orally, diluted in drinking water, based on a spontaneous demand, through two months after the sixth month of infection. The doses were based on interspecies extrapolation, with 10 mg/kg/day of carvedilol and 20 mg/kg/day of enalapril (Sigma-Aldrich Inc., San Louis/MO – USA), dissolved in dimethyl sulfoxide (Synth, Diadema/SP – Brazil)9 at 1.0% of the water volume per bottle. The therapeutic doses were adjusted after four weeks of the treatment, considering the variation in the animals’ weight throughout the protocol. Animals assigned to the untreated Chagas group also received drinking water orally, based on spontaneous demand. The animals underwent echocardiographic study 6 months post-infection (baseline - pre-treatment) and eight months after infection (post-treatment). At the end of the experimental protocol, the animals were euthanized for organ and tissue removal for histopathological study. Geometric and left ventricular function parameters, myocardial strain analysis, and segmental mobility analysis were obtained from echocardiographic images.
All methods were carried out in accordance with relevant guidelines and regulations.
Echocardiographic assessment of left ventricular structure and function
Following sedation with ketamine and xylazine (100 and 10 mg/kg, respectively)10, bidimensional echocardiography was performed using a high-resolution ultrasound system (Vevo 2100, Visual, Toronto, ON, Canada) equipped with a 30 MHz high-frequency linear transducer. The video sequences were acquired with at least three cardiac cycles in the following projections: parasternal long-axis and parasternal short-axis, obtained at two cardiac levels: basal level, at the level of the mitral valve, and papillary muscle level, corresponding to the mid-ventricular level at the papillary muscles. M-mode images were acquired, guided by the two-dimensional image in the parasternal long-axis window, to measure the thickness of the interventricular septum and the posterior wall of the left ventricle and their systolic and diastolic dimensions. The diastolic dimension of the left ventricle (LVEDD) was measured at maximum ventricular diastolic dimension. The LV end-systolic dimension (LVESD) was obtained during maximum anterior movement of the posterior wall, following the guidelines for chamber quantification from the American Society of Echocardiography and the European Society of Cardiovascular Imaging11,12. LV fractional shortening (FS) and LV mass were also quantified, and LV mass was compared to the animal’s weight. LV ejection fraction was calculated using three different techniques:1 M-Mode;2 bidimensional images with the modified Simpsons rule adapted to parasternal long axis image; and3 speckle tracking technique. The obtained images were recorded for “off-line” data analysis at the end of the study. Conventional echocardiographic ventricular geometry and function measurements were performed on a Vevo® Lab workstation (VisualSonics Inc, Toronto, Canada). Similar to human protocols, although considering 13 segments, the left ventricle segments were individually analyzed for segmental motion if normal1, hypokinetic2, akinetic3, or dyskinetic4 and the sum of scores was divided by the number of segments to generate the wall motion score index (WMSi), determining the regional systolic function13.
The LV systolic deformation analysis was performed using a speckle tracking technique on a dedicated workstation based on a protocol previously described for the same animal population14. Using the longitudinal parasternal projection, the beginning and end of systole were identified, and the endocardial borders were manually delineated by the examiner, with the sequential placement of 8 to 12 marking points along the LV cavity. Papillary muscles were excluded from the endocardial tracing. To better define the region of interest (ROI), the epicardial to endocardial tracing was manually adjusted according to the myocardial thickness, avoiding reaching the most reflective areas representative of the pericardium. Myocardial strain analysis was performed using specific software (Vevo Strain), and the quality of point tracking throughout systole was assessed visually by the examiner. Peak systolic strain values were then obtained for each of the six segments of the longitudinal parasternal projection to determine regional longitudinal strain (RegLS), and the average value of these segments was defined as the global longitudinal myocardial strain (GLS).
Euthanasia procedures
After the reevaluation, the animals were euthanized following the protocol approved by the institution’s Animal Experimentation Ethics Committee. Initially, the animals were submitted to analgesia and deep anesthesia using a combination of ketamine and xylazine at a dose three times higher than that used for imaging procedures (300 mg/kg and 30 mg/kg, respectively), administered intraperitoneally, ensuring deep anesthesia and rapid induction of unconsciousness. After anesthesia, the loss of the paw withdrawal reflex and the absence of response to painful stimuli were confirmed before proceeding with euthanasia. Euthanasia was performed by thoracotomy and rapid excision of the heart, which was immediately washed in saline solution. The major vessels at the base and the atria were discarded, and the left ventricle was sectioned into three levels (basal, mid-ventricular, and apical) for histopathological analysis.
Histopathology
After experimental time, animals were euthanasied, the hearts were removed, and fixed in neutral buffered 10% formaldehyde in PBS. For the histopathological study, the samples were dehydrated, clarified, embedded in paraffin, stained with hematoxylin and eosin, and picrosirius red, and examined by light microscopy. The tissue sections stained with hematoxylin and eosin were used to evaluate the intensity of inflammation. The slides stained with picrosirius red were used to assess fibrosis through collagen quantification. The Leica QWin software (Leica Imaging Systems Ltd., Cambridge, England) was used for morphometric analysis with a Leica microscope, video camera, and an online computer. Inflammation was scored by counting the total number of inflammatory cells (mononuclear rounded interstitial cells) in 10 microscope fields (x200) per animal from each group in a blinded manner. To estimate the volume fraction (%) of fibrosis in picrosirius red-stained sections, 10 microscope fields (400X magnifications) were measured under polarized light with the QWin software. Measurements were made by a skilled observer blinded to the groups.
Analysis of cardiomyocyte apoptosis
Apoptosis was assessed in situ using the TUNEL (Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling) technique, employing the “Cell Death Detection Kit, POD” (Roche Applied Science, Germany). The slides were deparaffinized in xylene baths at room temperature and washed with water. Permeabilization was performed using proteinase K (2.5 µg/ml) in 10 mM Tris-HCl pH 7.4 solution, immersed in a water bath at 37 °C for three and a half minutes. Subsequently, endogenous peroxidase was blocked with 6% hydrogen peroxide for 10 min, followed by thorough rinsing with running water. Finally, the slides were immersed in PBS and incubated with the TUNEL reagent for 60 min at 40 °C. Thirty microliters of TUNEL reaction were added to each section, and then the slides were washed in PBS three times for 5 min each, under constant agitation. Next, POD was added, and the incubation protocol was followed in a humid chamber for 30 min at 37 °C. Finally, the slides were washed three times in PBS and developed with DAB. Hematoxylin was used for counterstaining. The slides were scanned and analyzed using a 20X magnification Scan Scope device (Aperio, USA-Vista, CA) and Image Scope software.
Statistical analysis
Continuous variable data are reported as mean ± standard deviation, and nominal variables are reported as absolute (n) and relative (%) frequency.
The Shapiro-Wilk test was used to determine the Gaussian distribution of variables.
Ordinary one-way ANOVA was used to compare inflammation, and Kruskal-Wallis was used simultaneously to compare fibrosis and TUNEL quantification of apoptotic cells. The Mann-Whitney test was used to compare two independent variables simultaneously.
A mixed-effect model ANOVA for repeated measures was used to assess the interaction (main effect) between the experimental groups (between-subject effect) and time (within-subject effect) on echocardiography variables. When a statistically significant interaction was found, simple effect tests were performed to evaluate differences between pre-and post-treatment values in each experimental group (Dunn’s multiple comparisons test or Šídák’s multiple comparisons test). The significance level was set at p < 0.05, two-tailed in all analyses. Statistical analysis was performed using GraphPad Prism, version 9.1.2 (GraphPad Software, San Diego, CA, USA).
The study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Results
Forty-seven animals were studied, initially divided into Control and Chagas groups. The results obtained in the baseline evaluation are presented in Table 1. At pre-treatment (6 months post-infection with T. cruzi), no significant differences were observed between the body weight and heart rate values of the control and Chagas´groups. A significant difference was observed between the two groups in LVEDD (p = 0.02) and LV mass (p = 0.0001), where the Chagas groups presented higher LVEDD and indexed LV mass.
The temporal evolution of echocardiographic parameters of the animals and their respective groups at pre- (6 months after infection) and post-treatment (8 months post-infection and treatment) are presented in Table 2. No significant differences were observed between the studied groups in echocardiographic measurements at pre-treatment. At the post-treatment time (8 months), 50% of the Chagas group presented any clinical signs of infection, such as weight loss, lesions on the mucosa and skin, hair loss, lethargy, or cachexia. At that time, a significant reduction in LVEF (from M-Mode and STE techniques) and LV FS was observed in all Chagas´ groups compared to Control, regardless of the intervention. Compared with baseline, WMSi was also worse at post-treatment time (8 months post-infection) in all Chagas´ groups. At post-treatment time, the Chagas + BB group presented a better WMSi than the Chagas´ (Untreated) group, and no difference was observed between the Chagas + ACEi and Chagas´ (Untreated) group.
In the comparative analysis from baseline to post-treatment, a slight increase in LVEDD and LVESD was observed in all studied groups, although with no statistical significance. A tendency to decrease in bidimensional LVEF was observed during follow-up, but the values did not show statistical significance between the groups (p = 0.5). Mixed-model ANOVA showed a significant reduction of LVEF (M-Mode) and FS from baseline to post-treatment in the CH + IECA group. Furthermore, a substantial decrease in LVEF by the GLS method was observed in animals from the CH + BB and CH + IECA groups.
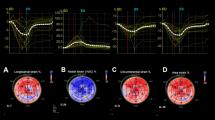
At post-treatment time, the heart rate of animals was not statistically different (p = 0.2), consecutively: 146.4 ± 22.3 (Control), 163.3 ± 15.8 (Chagas), 167.3 ± 23.4 (CH + BB) and 178.9 ± 48.3 (CH + ACEi). At this time, a significant increase in WMSi was observed in animals from the Chagas (Untreated) group. Animals treated with BB (CH + BB) presented a significantly preserved WMSi compared to the Chagas (Untreated) group, and this difference was not observed in the CH + ACEi group (Fig. 1). Also, LV RegLS was reduced in some LV segments such as Post_med (p = 0.03), Post_apex (p = 0.03), and Ant_apex segments (p = 0.01) in all Chagas´ groups regardless of treatment, compared to Control (Table 3), with no significant difference between Chagas´ treated groups (Fig. 2).
– Evolution of LV geometrical and functional parameters through intervention groups from pre-treatment (6 months after infection) and post-treatment (8 months after infection). LVEDD: left ventricle end-diastolic dimension (A); LVESD: left ventricle end-systolic dimension (B); LVEF- 2D: left ventricle ejection fraction from bidimensional (C); LVEF-MMode: left ventricle ejection fraction from MMode (D); GLS: global longitudinal strain (E); WMSi: wall motion score index (F)..mm = millimeters; %=percentage. p = mixed_ANOVA.
Regional Longitudinal Strain measurements (RegLS) from each segment in the different groups at the post-treatment time (8 months). Panel a represents one example of an animal from the Ch-BB group, with RegLS reduced in post-med, post-apex, and ant-apex segments. *p < 0.05 vs. Control. No significant difference was observed between each Chagas group.
The histopathological data are presented in Table 4. No difference in apoptosis was signaled by the TUNEL method in all Chagas´groups. Myocardial fibrosis was less than 5% in the experimental model, and there was no difference in the percentage of fibrosis among the studied groups. There was also no significant difference in the number of inflammatory cells between the studied groups.
Discussion
The experimental animal model of CD in Syrian hamsters simulates CCC with the same characteristics as human disease. Left ventricle remodeling and myocardial dysfunction can be assessed by echocardiography dedicated to small animal models, revealing not only global LV dilatation and global myocardial dysfunction, but also the regional myocardial dysfunction, when evaluated with both techniques, the WMSi and Speckle Tracking regional strain (Reg LS). This is the first study to quantify myocardial LV regional dysfunction in an animal model of CD and to show that mainly compromised LV segments reproduce human disease, such as the posterior wall and apex. Treatment of this specific kind of heart failure with neurohormonal substances potentially preventing myocardial remodeling, such as beta-blockers (BB) and ACEi, had no significant effect on the global LV functional indexes of CCC in this experimental model study. Treatment with BB resulted in less regional myocardial dysfunction compared to non-treated Chagas animals when evaluated by the WMSi.
Neurohormonal treatment of heart failure and depressed ventricular function in CCC follows evidence-based knowledge from other non-ischemic causes of cardiomyopathy. The benefit of therapy with ACEi and BB to prevent ventricular remodeling, inhibit myocardial tissue fibrosis substitution, and avoid hard clinical outcomes has already been proven in several other non-ischemic causes of cardiomyopathy. When represented in clinical trial cohorts of heart failure, Chagas´ patients accounted for about 8% of cases15. These patients are usually younger and present a higher prevalence of bradycardia and conduction disturbances, as well as a higher use of amiodarone. They also present worse renal function, lower levels of arterial blood pressure, and higher rates of mortality15,16. These characteristics may represent a challenge to using ACEi and BB in efficient dosages. Studies evaluating the effects of ACEi and BB in restricted populations of CCC patients are scarce and with a low number of individuals. So, neurohormonal therapies are extrapolated for this group of patients7.
Previous studies conducted before the availability of cardiac imaging in rodents showed a beneficial effect of Captopril in acute myocarditis related to CCC17. Captopril ameliorates myocarditis in acute experimental Chagas disease17. In rodent models of myocardial infarct, BB such as Carvedilol was previously proven to avoid myocardial fibrosis substitution in late phases of infarct, even better than Enalapril18. In other animal experimental models of non–ischemic cardiomyopathy, new vasodilators, such as the combination of Sacubitril-Valsartan, have already impacted LV remodeling and function19. However, conflicting results exist regarding the effect of BB in Chagas’ animal experimental models. Tested before in the model of Syrian hamsters with CCC, Carvedilol did not attenuate myocardial collagen deposition20.
The difficulty in reproducing the chronic phase of Chagas´ disease in rodents per se may represent one of the challenges in comparing articles that tried to prove therapeutic effects in myocardial damage evolution. Animal experimental models using rats have failed to reproduce CCC, with a high mortality and excessive individual variability in surviving T. cruzi infection. When studied from a translational point-of-view, using cardiac images dedicated to rodents such as echocardiography, and not only with myocardial tissue histopathologic analysis, we have also shown that the Syrian hamster model of CCC can reproduce acute infection in the first month, with an expressive LV systolic dysfunction and a high mortality rate21. Prospectively, survival animals evolve to an apparent quiescent phase, with a still preserved LVEF, although with indices of global myocardial deformation by Speckle tracking echocardiography already reduced14,22. This period, which lasts until 6 months after infection, is similar to the Indeterminate phase of disease described in humans7. From 6 months on, there is already evidence of LV remodeling, such as the increase in LVEDD, which will be significantly higher than controls at 8 months after infection, where LVEF will be reduced14. Even in this long-lasting evolution, myocardial fibrosis in this model will be only discrete at 8 months, with an expected amount of myocardial fibrosis of less than 3%. Therefore, proving neurohormonal treatment effects in LV remodeling by only quantifying tissue fibrosis is obviously limiting. After acquiring knowledge about CD’s evolution phases in the hamster animal model, we could hypothesize that the best period to test treatments that could impact cardiac remodeling and function should be from 6 months onwards.
CCC is characteristically an LV regional myocardial disease. Therefore, techniques that measure global LVEF based on geometrical assumptions from only one linear diastolic dimension, such as FS and LVEF derived from the MMode technique, are theoretically limited. Novel bidimensional methods derived from echocardiography images, such as LVEF from bidimensional images and the Speckle Tracking technique, are advantageous. This was not possible when human clinical machines were used to image rodent hearts, and the resolution was only good using MMode with linear transducers, but now it is feasible with dedicated small animal high-resolution machines12,23. Also, with high-resolution images, regional myocardial function can be studied with similar techniques applied to infarct disease models in humans, such as WMSi24. Chagas´ animals studied here presented reduced LVEF through time compared to Controls when analyzed by myocardial Strain and linear methods (LVEF-MMode and FS). We have previously reported that, in this model, LV regional myocardial motion abnormalities were also related to myocardial inflammation better than to fibrosis, suggesting that these could represent a time of early myocardial damage in probably a reversible time of disease. Consequently, this time could be sensitive to some treatments related to the different physiopathologic processes of the disease25. Previously, when we treated CCC with pentoxifylline, pointing to the myocardial perfusion damage, although treatment has significantly reduced myocardial inflammatory cells, it did not change echocardiographic parameters of LV remodeling or global LV function evaluated by echocardiography. Similarly, our study’s neurohormonal treatments could not change LV geometrical or global functional parameters assessed by echocardiography. However, the group treated with BB presented fewer wall regional motion abnormalities when measured by WMSi than non-treated Chagas animals.
Based on the previous exposure, we then studied myocardial systolic regional deformation in each LV segment using RegLS from Speckle tracking. To the best of our knowledge, this is the first study to demonstrate reduced Reg LS in an experimental model of CD. Chagas´ animals differ from Controls by a reduced Reg LS in three segments of the LV (postero-medial, postero-apex, and antero-apex), reproducing the same regional pattern observed in human disease. However, contrary to previous results of our group demonstrated in this animal model, we could not show a significant reduction in global indices of LV deformation as GLS trough time (from 6 to 8 months), probably because of a higher dispersion of values. In humans, CCC also presents a compromise of LV global deformation indices such as global longitudinal (GLS) and circumferential strain (GCS)26. Still, previous studies also demonstrated some regional myocardial disturbances when patients were evaluated at the Indeterminate phase of the disease26,27. The mainly compromised segments in humans’ Indeterminate form of Chagas disease were infero-septal and infero-lateral. Indeed, patients who presented these alterations did not show significant myocardial fibrosis when evaluated by non-invasive techniques such as cardiac magnetic resonance. Therefore, our animal experimental model reproduces myocardial regional systolic dysfunction similarly to human disease. Although this seems to be an excellent time to initiate medical interventions, probably before the irreversible myocardial damage, we could not demonstrate significant effects of neurohormonal treatments in reverse or protect regional myocardial function in our study.
Focusing on a tool able to detect the pathological myocardial incipient damage in CD, we studied not only myocardial fibrosis substitution but also a measure of myocardial cell apoptosis with the TUNEL technique. CCC in the model of the Syrian hamster presents detectable myocardial apoptosis at the acute phase28; however, previous studies failed to show statistical differences at the end of the chronic phase at 10 months of evolution28. Neurohormonal drugs such as Sacubitril-Valsartan19, Bisoprolol, and Perindopril reduced apoptosis in other animal experimental models of non-ischemic heart failure in rats29. Our data failed to demonstrate a statistically significant difference in apoptotic myocardial cells between the studied groups. We interpret the high dispersion of data and their no-normal distribution as a limitation to demonstrate apoptosis. Unlike previous studies using the same animal model30, a similar limitation seems to happen when fibrosis and myocardial inflammatory cells were compared between the four studied groups in our study.
Although our study did not show benefit of neurohormonal substances such as ACEi to prevent myocardial remodeling, future perspectives of the use of this rodent animal model of Chagas disease to test myocardial remodeling with other drugs such as sacubitril-valsartan and GLT2-inhibitors must be considered.
Limitations
Our study presents several limitations. As a long-time evolution animal experimental model, chronic treatment of animals would not be feasible through gavage, and it was done by water dilution of substances and natural ingest. We are aware of the problems of reproducibility of the oral administration of drugs may present, as also as some minor uncontrolled effects in our data. However, our hamster model of Chagas´ disease trough 8–10 months, have been proved to better represent the human disease in a specie of rodent which is big enough to examinate with very human-similar methods such as echocardiography (applied here) or others such as perfusion myocardial scintigraphy in previous published data. Also, based on previous published data using Strain Echocardiography we concluded that this animal model represent the acute phase of Chagas disease through first month, with a very high mortality rate, and present sights of cardiomyopathy from 6 months on, pronounced from 8 to 10 months. So we opted to treat with remodeling drugs before the phase of major cardiac damage (from the sixth to eight month), in a model of high mortality was safer trough oral via.
Although Speckle-Tracking echocardiography presents a robust ability to diagnose myocardial dysfunction in several cardiovascular diseases and different etiologies of non-ischemic heart failure, besides its evolution to be applied to high-resolution echocardiography dedicated to rodents, it still presents some limitations. RegLS is still subject to some variability. LV segments, which are in the side lobes of the ultrasound beam, as those presented in parasternal projections, as they are in rodents, may be more sensitive to variability and explain the lack of statistical significance between groups.
We did not perform other measurements of myocardial apoptosis, such as the dosage of Caspase3, to add value to the TUNEL technique because of costs. We also lost some samples of myocardial tissue analysis similarly in each group because of quality control of staining and the incubation technique with the TUNEL reagent.
In a Syriam hamster experimental model of CCC, LV regional function is early compromised and can be quantified by echocardiography conventional methods such as WMSi and advanced techniques such as RegLS (Strain). Treatment at the early chronic phase with classic neurohormonal drugs, ACEi, and BB (Enalapril and Carvedilol) did not change LV remodeling when evaluated by high-resolution echocardiography or LV global function. Regional LV function was slightly better in animals treated with BB but was not different in those treated with ACEi.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. I can answer a solicitation by email, minna@fmrp.usp.br.
Abbreviations
- CCC:
-
chronic Chagas’ cardiomyopathy
- CD:
-
Chagas´ disease
- LV:
-
left ventricle
- ACEi:
-
angiotensin-converting enzyme inhibitor
- BB:
-
beta-blocker
- LVEDD:
-
left ventricle end-diastolic diameter
- LVESD:
-
left ventricle end-systolic diameter
- FS:
-
fractional shortening
- WMSi:
-
wall motion score index
- RegLS:
-
regional longitudinal strain
- GLS:
-
global longitudinal myocardial strain
- STE:
-
speckle tracking echocardiography
References
Bern, C., Messenger, L. A., Whitman, J. D. & Maguire, J. H. Chagas disease in the united States: a public health approach. Clin. Microbiol. Rev. ;33(1). (2019).
Disease, G. B. D., Injury, I. & Prevalence, C. Global, regional, and National incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet 388 (10053), 1545–1602 (2016).
Monge-Maillo, B. & Lopez-Velez, R. Challenges in the management of Chagas disease in Latin-American migrants in Europe. Clin. Microbiol. Infect. 23 (5), 290–295 (2017).
Pérez-Molina, J. A. & Molina, I. Chagas disease. Lancet 391 (10115), 82–94 (2018).
Bocchi, E. A., Bestetti, R. B., Scanavacca, M. I., Cunha Neto, E. & Issa, V. S. Chronic Chagas heart disease management: from etiology to cardiomyopathy treatment. J. Am. Coll. Cardiol. 70 (12), 1510–1524 (2017).
Schmidt, A. et al. Effects of trypanocidal treatment on echocardiographic parameters in Chagas cardiomyopathy and prognostic value of wall motion score index: A BENEFIT trial echocardiographic substudy. J. Am. Soc. Echocardiogr. 32 (2), 286–295 (2019). e3.
Marin-Neto, J. A. et al. SBC guideline on the diagnosis and treatment of patients with cardiomyopathy of Chagas disease – 2023. Arq. Bras. Cardiol. 120 (6), e20230269 (2023).
Pimentel Wde, S. et al. The effect of beta-blockade on myocardial remodelling in Chagas’ cardiomyopathy. Clin. (Sao Paulo). 67 (9), 1063–1069 (2012).
Wang, L. et al. Carvedilol attenuates 6-hydroxydopamine-induced cell death in PC12 cells: involvement of Akt and Nrf2/ARE pathways. Neurochem Res. 39 (9), 1733–1740 (2014).
Tanaka, D. M. et al. Effect of different anesthetic agents on left ventricular systolic function assessed by echocardiography in hamsters. Braz J. Med. Biol. Res. 49 (10), e5294 (2016).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging. 16 (3), 233–270 (2015).
Barros Filho, A. C. L. et al. Feasibility and reference intervals assessed by conventional and speckle-tracking echocardiography in normal hamsters. Physiological Rep. 9 (5), e14776 (2021).
Morgan, E. E. et al. Validation of echocardiographic methods for assessing left ventricular dysfunction in rats with myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 287 (5), H2049–H2053 (2004).
Ribeiro, F. F. F. et al. Prospective analysis of myocardial strain through the evolution of Chagas disease in the hamster animal model. Int. J. Cardiovasc. Imaging. 38 (1), 117–129 (2022).
Shen, L. et al. Contemporary characteristics and outcomes in Chagasic heart failure compared with other nonischemic and ischemic cardiomyopathy. Circ. Heart Fail. ;10(11). (2017).
Tanowitz, H. B. et al. Developments in the management of Chagas cardiomyopathy. Expert Rev. Cardiovasc. Ther. 13 (12), 1393–1409 (2015).
Leon, J. S., Wang, K. & Engman, D. M. Captopril ameliorates myocarditis in acute experimental Chagas disease. Circulation 107 (17), 2264–2269 (2003).
Wei, S., Chow, L. T. & Sanderson, J. E. Effect of carvedilol in comparison with Metoprolol on myocardial collagen postinfarction. J. Am. Coll. Cardiol. 36 (1), 276–281 (2000).
Jeremic, J. et al. Sacubitril/valsartan reverses cardiac structure and function in experimental model of hypertension-induced hypertrophic cardiomyopathy. Mol. Cell. Biochem. 478 (12), 2645–2656 (2023).
Pimentel, W. S. et al. The effect of beta-blockade on myocardial remodelling in Chagas’ cardiomyopathy. Clinics 67 (9), 1063–1069 (2012).
Vargas, A. L. et al. Prospective study of ventricular function and myocardial deformation related to survival in acute Chagas disease: an experimental animal model. Rev. Inst. Med. Trop. Sao Paulo. 63, e61 (2021).
Oliveira, L. F. L. et al. (eds) Regional myocardial perfusion disturbance in experimental model of chronic Chagas cardiomyopathy. In European Heart Journal European Society of Cardiology Congress; 26–30 August 2017 Barcelona, Spain. (2017).
Ram, R., Mickelsen, D. M., Theodoropoulos, C. & Blaxall, B. C. New approaches in small animal echocardiography: imaging the sounds of silence. Am. J. Physiol. Heart Circ. Physiol. 301 (5), H1765–H1780 (2011).
Kan, G., Visser, C. A., Koolen, J. J. & Dunning, A. J. Short and long term predictive value of admission wall motion score in acute myocardial infarction. A cross-sectional echocardiographic study of 345 patients. Br. Heart J. 56 (5), 422–427 (1986).
Tanaka, D. M. et al. Pentoxifylline reduces inflammation and prevents myocardial perfusion derangements in experimental chronic Chagas’ cardiomyopathy. J. Nucl. Cardiol. 30 (6), 2327–2337 (2023).
Romano, M. M. D. et al. Early impairment of myocardial deformation assessed by regional speckle-tracking echocardiography in the indeterminate form of Chagas disease without fibrosis detected by cardiac magnetic resonance. PLoS Negl. Trop. Dis. 14 (11), e0008795 (2020).
Gomes, V. A. et al. Analysis of regional left ventricular strain in patients with Chagas disease and normal left ventricular systolic function. J. Am. Soc. Echocardiogr. 29 (7), 679–688 (2016).
Fonseca, K. C. B. et al. Air pollution’s impact on cardiac remodeling in an experimental model of Chagas cardiomyopathy. Front. Cell. Infect. Microbiol. 12, 830761 (2022).
Lodi, M. et al. Advantages of prophylactic versus conventionally scheduled heart failure therapy in an experimental model of doxorubicin-induced cardiomyopathy. J. Transl Med. 17 (1), 229 (2019).
Lemos de Oliveira, L. F. et al. Regional myocardial perfusion disturbance in experimental chronic Chagas cardiomyopathy. J. Nucl. Med. 59 (9), 1430–1436 (2018).
Acknowledgements
This research was only possible without the exceptional support of the Multi-user ultrasound and echocardiography laboratory for small animals of Medical School of Ribeirão Preto-USP, handled by the technician Vanessa de Souza Nakagi.
Author information
Authors and Affiliations
Contributions
DMT and BPD developed the practical aspects of research, including experimentation. DMT was responsible for the writting and performed statistical analysis.AS and ALV helped with experimentation.ACLBF performed experimental echocardiography and its interpretation.KCBF developed animal experimentation actions as physiology testingMTO and JSS were responsible for the animals Chagas infection and confirmation diagnosisMLH, MVS and FJAR helped to conceive experimental hypothesis and review the textCMP was responsible for the anatomopathologic analysis.JAMN reviewed the written paper and was reponsible for funding.MMDR developed the project, was responsible for the practical implementation, rational conception of research, guided PhD students, and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Sources of funding
This study was supported by research grants from Fundação de Apoio à Pesquisa do Estado de São Paulo (FAPESP – Process: 2009/54010 - 1, 2017/16450 - 6, 2016/25403 - 9 and 2019/21250 - 1) and Fundação de Apoio ao Ensino Pesquisa e Assistência do Hospital das Clínicas (FAEPA).
Disclosures
The authors declare that they have no conflict of interest.
Ethical consideration
No human studies were carried out by the authors for this article. The work was conducted with the approval and in accordance with the guidelines of the institution where it was performed. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tanaka, D.M., de Paula Dias Zara, B., Sapalo, A.T. et al. Effect of neurohormonal therapeutic in left ventricle global and regional function in Chagas cardiomyopathy in a translational animal experimental model. Sci Rep 15, 12595 (2025). https://doi.org/10.1038/s41598-025-96676-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96676-2