Abstract
To investigate the levels and diagnostic significance of carbonic anhydrase I (CAI) and II (CAII) autoantibodies and oxidative stress status in fibromyalgia syndrome (FMS) patients. A total of 59 FMS patients and 53 healthy controls were included. CAI and CAII autoantibody levels were measured using a manual ELISA protocol. Serum malondialdehyde (MDA), serum total oxidant status (TOS), serum total antioxidant status (TAS), and oxidative stress index (OSI) were also analyzed. The mean CAI and CAII autoantibody levels were significantly higher in the FMS group compared to the control group (p < 0.000 and p < 0.003, respectively). FMS patients had significantly higher MDA, TOS and OSI levels(p < 0.000 for all comparisons), and lower TAS levels compared to controls but no significant differences (p > 0,705). Elevated CAI and CAII autoantibodies and altered oxidative stress markers in FMS patients suggest autoimmune processes and oxidative stress involvement in the pathogenesis of FMS, providing new insights into potential diagnostic and therapeutic approaches.
Similar content being viewed by others
Introduction
FMS is a chronic disorder with an unclear etiology, characterized by widespread musculoskeletal pain, fatigue, sleep disturbances, anxiety, depression, cognitive dysfunctions, headaches, and gastrointestinal problems. Despite its high prevalence, especially among women aged 40–60 years, the multifactorial nature of FMS suggests the involvement of genetic, immunological, and neuroinflammatory mechanisms. Research has increasingly pointed to the significance of oxidative stress and specific enzyme systems in the pathogenesis of FMS1,2. CAI and CAII are zinc-containing metalloenzymes that catalyze the reversible hydration of carbon dioxide. These enzymes are crucial in maintaining acid-base balance and facilitating CO2 transport3,4. Recent studies have proposed that alterations in CA activity may be linke to various pathophysiological conditions, including autoimmune and inflammatory diseases5. In the context of FMS, there is no evidence suggesting that dysregulation of CAI and CAII may contribute to the disease process.
Oxidative stress, defined as an imbalance between the production of reactive oxygen species (ROS) and the body’s ability to detoxify them, has been implicated in the development and progression of many chronic conditions, including FMS. Elevated levels of oxidative stress markers and decreased antioxidant defenses have been reported in FMS patients, indicating a potential role for oxidative damage in the disorder6. The interaction between oxidative stress and CA enzymes is of particular interest; oxidative modifications can impair the function of CAI and CAII, potentially exacerbating inflammatory and neuroinflammatory responses7. Furthermore, FMS shares several clinical and pathophysiological features with other autoimmune rheumatologic diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). For example, increased oxidative stress and altered CA activity have been implicated in the pathogenesis and severity of rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE)8,9. Studies have demonstrated that autoantibodies against carbonic anhydrase isoenzymes, particularly CAI and CAII, are associated with autoimmune diseases such as Sjögren’s syndrome and idiopathic chronic pancreatitis10. These autoantibodies have been implicated in tissue damage and inflammatory responses through mechanisms involving oxidative stress and immune dysregulation. Moreover, in rheumatoid arthritis, elevated levels of CAII autoantibodies have been reported, correlating with disease severity and suggesting their potential role in exacerbating inflammatory pathways8. These parallels suggest that similar mechanisms may be at work in fibromyalgia syndrome (FMS), a condition sharing overlapping clinical features with autoimmune and rheumatologic disorders. The interplay between oxidative stress, autoantibody production, and altered enzyme activity could contribute to the complex symptomatology and chronic nature of FMS. By investigating CAI and CAII autoantibodies in the context of FMS, this study aims to fill a critical gap in understanding their potential role in its pathogenesis and explore their utility as diagnostic biomarkers.
This study aims to investigate the role of CAI and CAII isoenzymes and oxidative status in the pathogenesis and diagnosis of FMS. The primary outcome of study is the CAI and CAII autoantibody levels in FMS patients compared to healthy controls.
Results
In this study, while the mean age of the participants in the control group was 40.75 ± 10.44, this value was determined as 46.31 ± 9.82 in the FMS group. No significant difference was found between the two groups in terms of age (p > 0.05). When FSDC values were analyzed, the mean FSDC value in the control group was 5.20 ± 2.46, while this value was 19.81 ± 3.10 in the FMS group. A significant difference was found between the two groups in terms of FSDC values (p = 0.000). Serum glucose, creatinine, aspartate transaminase (AST), alanine transaminase (ALT), total protein, albumin, magnesium, C-reactive protein (CRP), hemoglobin and platelet values were not significantly different between the control and FMS groups (p > 0.05)(no shown data).
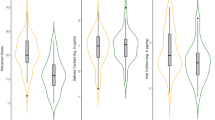
CAI autoantibody levels were 0.21 ± 0.07 units in the control group and 0.33 ± 0.30 units in the FMS group (Fig. 1) (Table 1). CAII autoantibody levels were 0.28 ± 0.05 units in the control group and 0.42 ± 0.33 units in the FMS group. The difference between the groups was statistically significant (p = 0.003) (Fig. 1) (Table 1).
MDA levels were 5 ± 0.69 units in the control group and 5.76 ± 1.04 units in the FMS group. The difference between the two groups was statistically significant (p = 0,000) (Fig. 2) (Table 1). TAS values were 1.07 ± 0.14 in the control group and 1.03 ± 0.16 in the FMS group. There was no statistically significant difference between the two groups (p = 0.705) (Fig. 2) (Table 1). TOS values were 22 ± 7.97 in the control group and 35.86 ± 13.26 in the FMS group. The difference between the groups was statistically significant (p = 0,000) (Fig. 2) (Table 1). OSI values were 2.09 ± 0.88 in the control group and 3.63 ± 1.81 in the FMS group. The difference between the two groups was statistically significant (p = 0,000) (Fig. 2) (Table 1).
The performance of CAI autoantibody and CAII autoantibody tests in discriminating between positive and negative true state groups was rigorously evaluated using Receiver Operating Characteristic (ROC) curve analysis. The Area Under the Curve (AUC) for the CAI autoantibody test was determined to be 0.704 (Standard Error: 0.049; 95% Confidence Interval: 0.608–0.799; p < 0.001). Similarly, the AUC for the CAII autoantibody test was found to be 0.664 (Standard Error: 0.051; 95% Confidence Interval: 0.564–0.763; p = 0.003) (Fig. 3).
Discussion
FMS is a chronic disease characterized by widespread musculoskeletal pain, fatigue, and localized tenderness. This condition negatively affects the quality of life of patients and presents challenges in diagnosis and treatment due to its complex and poorly understood pathogenesis. Recent studies have highlighted potential roles for autoimmunity and oxidative stress in FMS, yet their exact contributions require further clarification7,8,9,10. In this study, we investigated the levels of CAI and CAII autoantibodies and oxidative stress parameters in patients with fibromyalgia syndrome (FMS). Our results showed significant differences between FMS patients and healthy controls, with markedly elevated levels of CAI and CAII autoantibodies in the patient group. This finding suggests that autoimmune processes may play a role in the pathogenesis of FMS and may contribute to its characteristic symptoms. In support of this idea, previous research showed that IgG-type autoantibodies from FMS patients increased pain sensitivity when injected into mice. These autoantibodies were shown to accumulate in the dorsal root ganglia (DRG), suggesting their role in peripheral nociceptive afferent activation and the hypersensitivity seen in FMS11. Such findings highlight the potential for therapeutic approaches targeting IgG autoantibodies, such as reducing their levels or inhibiting their activity, as promising strategies for the treatment of FMS. The clinical utility of CAI and CAII autoantibodies was also evident in our study. Monitoring CAI and CAII autoantibody levels and correlating them with clinical symptoms during patient follow-up may improve our understanding of their role in the pathogenesis of FMS. ROC analysis further supported the diagnostic potential of CAI and CAII autoantibodies. The area under the curve (AUC) values showed that the diagnostic accuracy of CAI autoantibody levels was superior to that of CAII autoantibodies (0.704 vs. 0.664, respectively).This finding suggests that CAI autoantibodies may serve as a more robust biomarker for distinguishing FMS patients from healthy individuals. However, it is important to recognise that the distribution and classification of true positive and true negative cases may introduce some bias into the statistical results, potentially affecting the interpretation of the diagnostic performance of CAI and CAII autoantibodies. Despite this limitation, CAI and CAII autoantibodies may serve as potential biomarkers to provide a laboratory-based diagnostic tool for FMS, a disease currently diagnosed solely by clinical criteria. The inclusion of objective biomarkers such as CAI and CAII autoantibodies could significantly improve diagnostic accuracy and reduce reliance on subjective assessment. Our study, focusing on newly diagnosed FMS patients, makes a unique contribution to the literature by investigating the potential diagnostic value of these biomarkers at an early stage of the disease. Although CAI and CAII autoantibodies have diagnostic potential, they are unlikely to serve as biomarkers on their own. However, their clinical significance increases and overall diagnostic accuracy improves when evaluated in combination with other diagnostic approaches and complementary biomarkers. The ROC curves illustrate the sensitivity and specificity of these biomarkers, supporting their potential role in clinical practice. Our findings pave the way for future studies to validate the use of CAI and CAII autoantibodies in larger and more diverse cohorts. In addition, longitudinal studies investigating the utility of these biomarkers in monitoring disease progression and response to treatment may further elucidate their role in the management of FMS.
Significant changes in oxidative stress parameters were observed in FMS patients, and oxidative stress emerged as a key factor in our study. Elevated serum levels of malondialdehyde (MDA), total oxidant status (TOS) and oxidative stress index (OSI) in the patient group highlight an increased oxidative burden. Although the differences in total antioxidant status (TAS) levels were not statistically significant, TAS levels were consistently lower in FMS patients compared to healthy controls. This imbalance suggests that oxidative stress, characterised by an overwhelmed antioxidant defence system, plays a key role in the pathogenesis of FMS. Our findings are consistent with previous research. Fidan et al. and Karataş et al. reported increased natural thiol levels and decreased disulfide levels in FMS patients, reflecting a shift towards increased thiol-disulfide conversion and insufficient disulfide oxidation. These findings highlight a potential disturbance in thiol-disulfide homeostasis, a critical component of the body’s antioxidant defence mechanism12,13. However, a notable limitation of these studies is the lack of differentiation between newly diagnosed and treated patients, which could influence the dynamics of oxidative stress. By focusing specifically on newly diagnosed FMS patients, our study provides novel insights into the oxidative stress profile of this subgroup, minimising the confounding effects of treatment interventions. Research has consistently shown increased oxidative stress in RA patients, with elevated levels of ROS correlating with disease activity and tissue damage14. In addition, studies indicate that markers of oxidative stress, such as malondialdehyde and 8-isoprostane, are significantly elevated in RA compared to healthy controls15, providing a clear pathological link between ROS and the mechanisms of RA. Furthermore, Wruck et al. have shown that activation of the Nrf2 pathway, a key regulator of the cellular response to oxidative stress, is impaired in RA, resulting in reduced expression of protective antioxidants. This impairment highlights the link between chronic oxidative stress and the joint inflammation typical of RA16. In SLE, oxidative stress also plays a key role in disease exacerbation. Elevated levels of oxidative markers and decreased antioxidant capacity are frequently reported in SLE patients, correlating with disease manifestations such as nephritis and skin lesions. Previous studies have shown that oxidative damage is closely related to the presence of autoantibodies, further complicating the disease landscape17. Importantly, the presence of ROS has been linked to the activation of pro-inflammatory pathways, thereby enhancing the chronic inflammation that characterises SLE18. Beyond SLE and RA, diseases such as ankylosing spondylitis (AS) and fibromyalgia also demonstrate the role of oxidative stress in their pathology. Öztürk et al. point out that dynamic thiol-disulfide homeostasis, an important marker of oxidative stress, is disturbed in AS patients, suggesting a systemic oxidative imbalance that may contribute to disease symptoms19.
Thiols, such as glutathione, act as primary antioxidants, protecting cellular components from oxidative damage. Erel et al. further demonstrated significant alterations in natural thiol levels in FMS patients, although total thiol levels remained unchanged. In our study, TAS - a comprehensive indicator including enzymatic antioxidants (e.g. superoxide dismutase, catalase, glutathione peroxidase) and non-enzymatic antioxidants (e.g. vitamins C, E, A, coenzyme Q10, polyphenols) - showed no significant differences between groups19. This finding may be due to possible deficiencies of specific non-enzymatic antioxidants within the patient group, which warrants further investigation. Taken together, these observations suggest that oxidative stress, mediated by an imbalance between pro-oxidant and antioxidant systems, contributes to the pathophysiology of FMS20. Future research should investigate the longitudinal effects of treatment on oxidative stress parameters and consider lifestyle interventions, such as an antioxidant-rich diet and exercise, as potential strategies to mitigate oxidative damage in FMS patients.
In conclusion, this study highlights the interplay between autoimmune processes and oxidative stress in the pathogenesis of FMS and provides new insights into its complex mechanisms. The significant elevation of CAI and CAII autoantibodies, together with the imbalance in oxidative stress markers, highlights potential diagnostic and therapeutic targets. In particular, CAI and CAII autoantibodies are promising biomarkers that could complement clinical criteria in the diagnosis and monitoring of FMS. In addition, the observed oxidative stress parameters reinforce the need to explore antioxidant-based interventions as adjunctive strategies in the management of FMS. Further longitudinal and interventional studies are essential to validate these findings and pave the way for more precise diagnostic tools and personalised treatment approaches for FMS patients.
This study has several limitations that should be considered. First, the case-control design with a cross-sectional approach does not allow to establish causal relationships between oxidative stress, autoantibodies and FMS pathogenesis. Longitudinal studies are needed to explore the dynamic interactions between these factors over time. Second, the sample size, although sufficient for initial comparisons, may limit the generalisability of the findings to larger and more diverse FMS populations. Multicentre studies with larger cohorts are recommended to validate these findings. Third, the study focused exclusively on newly diagnosed FMS patients, which reduces confounding by treatment but does not provide insight into how oxidative stress and autoantibody levels may change with therapeutic interventions. Finally, although our findings highlight the potential diagnostic utility of CAI and CAII autoantibodies, their functional role in the pathogenesis of FMS was not investigated in detail. As this is one of the first studies to explore the relationship between these autoantibodies and FMS, our work may serve as a foundation for future mechanistic research aimed at uncovering their role in disease development and progression.
Methods
Study design and participants
This study was designed as a case-control study with a cross-sectional approach. Fibromyalgia patients and healthy controls were recruited over a one-year period, and data collection, including oxidative stress markers and autoantibody levels, was conducted at a single time point for each participant. The study included 59 FMS patients new diagnosed according to the American College of Rheumatology (ACR) 2016 criteria and 53 healthy controls matched for age and gender. The sample size for this study was determined using a power analysis based on prior studies investigating autoantibody levels in similar populations. A minimum detectable effect size (Cohen’s d) of 0.7 was assumed, with a power of 80% (1-β = 0.80) and a significance level of 5% (α = 0.05). Participants were recruited from Recep Tayyip Erdoğan University Training and Research Hospital, Physical Therapy and Rehabilitation Clinic between 01.07.2023–31.03.2024. Written informed consent was obtained from all participants prior to their inclusion in the study. Inclusion criteria for patients were a fibromyalgia survey diagnostic criteria and severity scale (FSDC) of 12 or higher according to the ACR 2010/2011 criteria revised in 2016. To ensure the reliability and reproducibility of the study results, a comprehensive set of exclusion criteria was applied to maintain a homogeneous study population and minimise potential confounding factors. Patients with any of the following conditions or circumstances were excluded from the study: First, individuals with rheumatological diseases such as rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis or scleroderma were excluded to avoid overlap with other autoimmune diseases. Second, patients diagnosed with major depressive disorder, bipolar disorder, schizophrenia or severe anxiety disorder were not included because these conditions could confound the assessment of fibromyalgia-related symptoms. Major chronic illnesses, including cancer, end-stage renal disease, cirrhosis and congestive heart failure, were also exclusion criteria because of their potential impact on oxidative stress and immune function. People with neurological conditions, such as multiple sclerosis, Parkinson’s disease and epilepsy, or chronic infections, including chronic hepatitis B or C and HIV/AIDS, were also excluded to eliminate confounding by these conditions. Patients with chronic pain syndromes with significant organic causes, such as chronic migraine, chronic low back pain and myofascial pain syndrome, were also excluded to ensure a clear focus on fibromyalgia. Patients were also excluded if they were continuously taking painkillers or anti-inflammatory drugs, or if they were receiving steroid or immunosuppressive therapy, to prevent medication effects from influencing the study results. Pregnant or lactating women were not included to eliminate potential physiological variations associated with these conditions. Finally, individuals who were unable to give informed consent were excluded. This study received ethical approval from the Recep Tayyip Erdoğan University Clinical Research Ethics Committee (Decision no: 2023/173). All procedures were conducted in strict accordance with relevant guidelines and regulations. Furthermore, informed consent was obtained from all participants after they were fully informed about the nature and purpose of the study. The primary outcome of this study is the CAI and CAII autoantibody levels in fibromyalgia syndrome (FMS) patients compared to healthy controls. The main exposure in this study is fibromyalgia syndrome. In addition, oxidative stress markers (MDA, TOS, OSI, and TAS) were examined as potential contributors to disease pathology. We identified age, sex, comorbidities, medication use and lifestyle factors as potential confounders. These were adjusted for in the statistical analyses to minimise their effect on the results.
Potential sources of bias: Selection bias may be present, as participants were recruited from a hospital-based setting, which may not fully represent the general population of FMS patients. Recall bias may also be a factor, as subjective self-reported symptom severity may be influenced by recall bias.
Sample collection
Blood samples were collected from all participants after obtaining informed consent. Serum was separated and stored at -80 °C until analysis. Patient and sample collection completed in 6 months.
CAI and CAII autoantibody measurement
Autoantibodies determination was performed manually by enzyme-linked immunosorbent assay (ELISA) method. The method developed by Kino-Ohsaki was used in this study21,22,23,24,25,26. Measurement bias was minimised by the implementation of intra-assay and inter-assay variability checks in the ELISA assay, ensuring the reliability of the biomarker measurements. The intra-assay and inter-assay variations were 2.7 and 2.9% respectively(Tablo S1).
Oxidative status analysis
Serum MDA levels were measured by the method developed by Yagi27. TOS and TAS were measured in serum samples. OSI was calculated as the ratio of TOS to TAS. TOS was measured (mikromol/L) using a Rel Assay Diagnostics kit (Gaziantep, Turkey). The intra-assay and inter-assay variations were 3.2 and 3.9%respectively. TAS was measured (mmol/L)using another Rel Assay kit; the intra-assay and inter-assay variations were 2.8 and 3.3% respectively. TOS to TAS ratio served as the OSI, calculated as follows: [(TOS)/(TAS)/100].
Statistical analysis
Data were analyzed using statistical methods. Shapiro-Wilk was applied as the normal distribution test of the variables. Comparisons between groups were made using the student’s t-test for parametric variables and the nonparametric variables were evaluated using the Mann-Whitney U test. A p-value of < 0.05 was considered statistically significant.
Data availability
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
References
Wolfe, F. & Editorial The status of fibromyalgia criteria. Arthritis Rheumatol. 67, 330–333 (2015).
Maffei, M. E. & Fibromyalgia recent advances in diagnosis, classification, pharmacotherapy and alternative remedies. Int. J. Mol. Sci. 21, 7877 (2020).
Supuran, C. T. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin. Ther. Pat. 28, 709–712 (2018).
Ruusuvuori, E. & Kaila, K. Carbonic anhydrases and brain pH in the control of neuronal excitability. in 271–290 (2014). https://doi.org/10.1007/978-94-007-7359-2_14
Ono, M. et al. A study of anti-carbonic anhydrase II antibodies in rheumatic autoimmune diseases. J. Dermatol. Sci. 21, 183–186 (1999).
Assavarittirong, C. & Samborski, W. & Grygiel-Górniak, B. Oxidative stress in fibromyalgia: from pathology to treatment. Oxid. Med. Cell Longev. 1–11 (2022). (2022).
Biswas, S. K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox?? Oxid. Med. Cell. Longev. 2016, 1–9 (2016).
Alver, A. et al. Carbonic anhydrase II autoantibody and oxidative stress in rheumatoid arthritis. Clin. Biochem. 44, 1385–1389 (2011).
Liu, C. et al. Carbonic anhydrases III and IV autoantibodies in rheumatoid arthritis, systemic lupus erythematosus, diabetes, hypertensive renal disease, and heart failure. Clinical & developmental immunology (2012). (2012).
Häuser, W. et al. Fibromyalgia. Nat. Rev. Dis. Primers. 1, 15022 (2015).
Goebel, A. et al. Passive transfer of fibromyalgia symptoms from patients to mice. J. Clin. Invest. Vol. 131, 13 (2021).
Fidan, F. Dynamic thiol/disulphide homeostasis in patients with fibromyalgia. Arch. Rheumatol. 32, 112–117 (2017).
Karatas, G. et al. Dynamic thiol and disulphide homoeostasis in fibromyalgia. Archives Med. Sci. 16, 597–602 (2020).
Shakeri, F. et al. The relation between biochemical parameters and rheumatoid arthritis disease. J. Adv. Biology Biotechnol., 2018 19,3(2018).
Łuczaj, W. et al. The onset of lipid peroxidation in rheumatoid arthritis: consequences and monitoring. Free radical research. 50 – 3,304 – 13. (2016).
Wruck, C. J. et al. Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Ann. Rheum. Dis. 70 (5), 844–850 (2011).
Yan, Z. et al. Oxidative stress contributes to inflammatory and cellular damage in systemic lupus erythematosus: cellular markers and molecular mechanism. J. Inflamm. Res. 16, 453–465 (2023).
Tsai, C. Y. et al. Cross-Talk between mitochondrial dysfunction-provoked oxidative stress and aberrant noncoding RNA expression in the pathogenesis and pathophysiology of SLE. Int. J. Mol. Sci. 20, 5183 (2019).
Öztürk, C. et al. Dynamic thiol-disulfide homeostasis as an oxidative stress marker in ankylosing spondylitis and undifferentiated spondyloarthropathy. Turk. J. Med. Sci., 53,5,1387–139420239 .
Hajam, Y. A. et al. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells 11, 552 (2022).
Kino-Ohsaki, J. et al. Serum antibodies to carbonic anhydrase I and II in patients with idiopathic chronic pancreatitis and Sjogren’s syndrome. Gastroenterology 110, 1579–1586 (1996).
Mentese, A. et al. Serum carbonic anhydrase I and II autoantibodies in patients with chronic lymphocytic leukaemia. Cent. Eur. J. Immunol. 43 (3), 276–280 (2018).
Pinar, K. Carbonic anhydrase I and II autoantibody levels in primary hypertension: our preliminary results. Eur. Rev. Med. Pharmacol. Sci. 24 (24), 12821–12826 (2020).
Frulloni, L. et al. May. Elevated serum levels of aAntibodies to carbonic anhydrase I and II in patients with chronic pancreatitis. Pancreas 20 (4) 382–388, (2000).
Kiechle, F. L., Quattrociocchi-Longe, T. M. & Brinton, D. A. Carbonic anhydrase antibody in Sera from patients with endometriosis. Am. J. Clin. Pathol. 101 (5), 611–615 (1994).
Andoh, A. et al. Elevated serum anti-carbonic anhydrase II antibodies in patients with ulcerative colitis. Int. J. Mol. Med. 9 (5), 499–502 (2002).
Yagi, K. assay for blood plasma or serum. Methods Enzymol. 105 (4), 328–331. https://doi.org/10.1016/S0076-6879(84)05042-4 (1984).
Acknowledgements
We would like to thank Köksal Karaca for his management in collecting serum samples for this study.
Funding
This study was supported by Recep Tayyip Erdoğan University Scientific Research Projects Co-ordinatorship. Project code: TSA-2023-1582. This study has been supported by the Recep Tayyip Erdoğan University Development Foundation (Grant number: 02025004008400).
Author information
Authors and Affiliations
Contributions
H.K. designed the study, analyzed the data, and wrote the manuscript. N.H. was responsible for sample collection, conducted the laboratory experiments and assisted in manuscript writing. M.S.T. managed patient recruitment. E.Ş. , N.S. , N.T.A. and A.M. conducted the laboratory experiments. A.M. reviewed the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by Recep Tayyip Erdoğan University Clinical Research Ethics Committee (Decision no: 2023/173). Informed consent was obtained from all participants after they were fully informed about the nature and purpose of the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kılıç, H., Hasanova, N., Topaloğlu, M.S. et al. A case-control study on the role of carbonic anhydrase autoantibodies in the pathogenesis and diagnosis of fibromyalgia. Sci Rep 15, 13158 (2025). https://doi.org/10.1038/s41598-025-96677-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96677-1