Abstract
The pharmacokinetics of LXT-101 sustained-release suspension, an anti-prostate cancer polypeptide, were studied on beagle dogs using a high-performance liquid chromatography-tandem mass spectrometry (LC–MS/MS) method. Samples were prepared by the protein precipitation, evaporation and reconstitution. Chromatographic separation was performed using a Hypersil GOLD C18 column (50 mm × 2.1 mm, I.D. 5 μm). The mobile phase consisted of acetonitrile, water and formic acid (20:80:0.1, v/v/v) at a flow rate of 0.3 mL/min. The acquisition was carried out in selected reaction monitoring (SRM). The method was validated in terms of selectivity, linearity, precision and accuracy, extraction recovery and matrix effect, and stability. It showed good linearity over the range of 2–600 ng/mL (R2 = 0.9977). The intra- and inter-batch accuracy were within 93.36–93.94% and 95.61–99.27%, while the intra- and inter-batch precision were in the range of 3.23–14.26% and 5.03–11.10%, respectively. The extraction recovery and matrix effect data for LXT-101 in beagle dog plasma ranged from 75.90–126.40% and 83.13–95.50%, respectively. The stability results proved that the storage conditions, disposal, intermittent analysis and analysis techniques were valid and reliable for LXT-101 in beagle dog plasma. In the single-dose groups (20 mg/kg and 40 mg/kg), the values of AUC0-t (588.09 ± 137.79 ng/mL·d vs. 1203.62 ± 877.42 ng/mL·d) and AUC0-∞ (592.89 ± 134.21 ng/mL·d vs. 1209.97 ± 873.78 ng/mL·d) were observed increasing proportionately with the increasing dose of LXT-101 sustained-release suspension. The results in the repeated-dose group suggested the possibility of accumulation in beagle dogs.
Similar content being viewed by others
Introduction
Prostate cancer is a complex disease, which affects millions of men worldwide1,2. This disease is the second largest male cancer after lung cancer, accounting for 7% of newly diagnosed male cancers worldwide (15% in developed regions)2. In addition, there are over 1.2 million newly confirmed cases every year, and the global number of prostate cancer related deaths exceeds 0.35 million, making it one of the main causes of male cancer related deaths2,3,4.
Most current research focuses on improving prostate cancer detection, management, and outcomes, including understanding the basic biology of various stages of the disease. Current treatment options include radical prostatectomy (RP), external beam radiotherapy (EBRT), chemotherapy, neoadjuvant hormonal therapy (NHT) and so on5,6.
As an endocrine therapy drug, gonadotropin-releasing hormone (GnRH) analogs, including agonists and antagonists, are synthetic analogs of GnRH that act mainly on the human pituitary gland7. GnRH antagonists, which are developed in the recent years, inhibit hormone production by counteracting natural GnRH via binding to its pituitary receptors. Compared to other hormone therapy drugs, GnRH antagonists are more potent and have fewer side effects8,9.
LXT-101(Ac-D-Nal1-D-Phe(4-Cl)2-D-Phe3-Ser4-Arg5-D-Pal6-Leu7-Arg8-Pro9-DAla10-NH2) is a novel GnRH analog developed by our institute (Fig. 1)10,11. Experimentally, it has showed an excellent character of chemical castration immediately after administration. The development of sustained-release formulations, such as microspheres and suspension, would be advantageous to the clinical application of LXT-10112,13,14.
Up to present, a limited number of studies have explored the pharmacokinetics of novel GnRH antagonists like LXT-101 in pre-clinical models. This study aimed to bridge this gap by developing a validated high performance liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS) method for quantifying LXT-101 in beagle dog plasma. Meanwhile, there are currently no reports about the pharmacokinetics of LXT-101 sustained-release suspension in the beagle dogs by using LC–MS/MS method. The main purpose of this study was to develop and validate a sensitive, robust and specific LC–MS/MS method for the determination of LXT-101 in beagle dog plasma. The full validated assay was successfully applied to the pre-clinical pharmacokinetics research of LXT-101 sustained-release suspension after intramuscular injection administration to beagle dogs. This research would provide necessary theoretical basis and methodological innovation for the potential development and utilization of LXT-101 sustained-release suspension.
Experimental
Chemical reagent
LXT-101 (purity: 95%, LOT: 050503) and 127I-LXT-101 (IS, purity: ≥ 99%, LOT: 050307) were obtained from Beijing Institute of Pharmacology and Toxicology (Beijing, China). Methanol, acetonitrile and formic acid, which were of the HPLC-grade, were all supplied by Sigma Company (Sigma, St. Louis, Mo, USA). All other chemicals and solvents used were at least of analytic grade.
Animal experiments
Twelve male beagle dogs weighing 10 ± 2 kg were supplied by the Laboratory Animal Center of Beijing Institute of Pharmacology and Toxicology with the Certificate Number SCXK (Beijing) 2002-001. The beagle dogs housed in the room, which was monitored continuously with controlled temperature (20–25 °C), relative humidity (40–70%), air-change rates (10–15 air changes per hour) and 12-h day-night cycle. All the beagle dogs were allowed free access to standard laboratory dog food and water ad libitum for at least 7 days before the experiment. All the animal protocols for experiments were approved by the Institutional Animal Care and Use Committee of the Beijing Institute of Pharmacology and Toxicology (nos 11400700031606) and strictly in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International (National Research Council of USA, 1996).
Instruments and analytical conditions
The LC–MS/MS system consisted of a triple-stage quadrupole mass spectrometer (TSQ Quantum Discovery) equipped with an electrospray ionization (ESI) interface (Thermo Electron, San Jose, CA, USA) coupled with a Finnigan Surveyor HPLC system consisting of a Finnigan Surveyor LC pump and a Surveyor AS autosampler (Thermo Electron San Jose, CA, USA). The method was performed on a Hypersil GOLD C18 column (50 mm × 2.1 mm, I.D. 5 μm, Thermo Fisher Scientific, USA) maintained at 25 °C. The mobile phase consisted of acetonitrile, water and formic acid (20:80:0.1, v/v/v). The flow rate was set at 0.3 mL/min. The source parameters of the select reaction monitoring (SRM) conditions were defined as follows: spray voltage 4800 V, TEM 300 °C, sheath gas N2 10 psi, auxiliary gas N2 1 psi, CID Ar 1.5 mTorr, Source CID 15 V, CE 43 eV and scan time 0.5 s. The SRM mode of m/z 472.13+ → 587.8 (quantification) and m/z 472.13+ → 170.0 (qualitation) for LXT-101 and m/z 502.83+ → 633.8 for 127I-LXT-101 at positive ionization mode were used as quantitative analysis. The operations and acquiring during the analysis process were controlled using Xcalibur software (version 1.4, Thermo Finnigan, USA) and the experimental data was analysed by Lcquan software (version 2.0, Thermo Finnigan, USA).
Preparation of standard solutions, calibration samples, and quality control samples
Standard stock solutions of LXT-101 and 127I-LXT-101 (IS) were both prepared in the mobile phase (acetonitrile: water: formic acid, 20:80:0.1, v/v/v) at 1 mg/mL. Then the LXT-101 stock solution was serially diluted with the mobile phase to obtain the working solutions with the appropriate extractions. The 127I-LXT-101 (IS) stock solution was further diluted with methanol solution to obtain the IS working solution at 1000 ng/mL. All the standard solutions were stored at 4 °C before sample analysis.
The diluted LXT-101 standard solutions were mixed with blank beagle dog plasma at final concentrations of 2, 10, 40, 200, 400 and 600 ng/mL. The quality control (QC) samples were prepared using the same method as calibration samples. There were three different concentration levels of LXT-101 in blank beagle dog plasma, low QC (5 ng/mL), medium QC (50 ng/mL), and high QC (500 ng/mL).
Sample preparation
The frozen beagle dog plasma samples were thawed at room temperature and vortexed for at least 60 s. Then 300 μL of beagle dog plasma, 50 μL of IS working solution (1000 ng/mL) and 600 μL of acetonitrile solution were combined and vortex-mixed for at least 3 min. After centrifuging at 12,000 rpm for 10 min at 4 °C, the supernatant were transferred into clean Ependorf tubes and evaporated to dryness with a gentle nitrogen stream at 37 °C. The residues were reconstituted in 200 μL of mobile phase and carried out by vortex-mixing for 3 min and centrifuging at 12,000 rpm for 10 min at 4 °C. 20 μL of the supernatant was injected onto the LC–MS/MS system for analysis.
Method validation
The selectivity, linearity, precision, accuracy, extraction recovery, matrix effect, and stability of the method were validated for the determination of LXT-101 in beagle dog plasma according to the the international guidelines for bioanalytical method validation set by the US Food and Drug Administration (US FDA)15,16,17,18,19.
Pharmacokinetic study
The twelve beagle dogs fast for at least 12 h with water ad libitum and were divided randomly into two groups. Group 1 (n = 3) were administered with LXT-101 sustained-release suspension by intramuscular injection with a dose of 20 mg/kg and the blood samples were withdrawn and placed into clean heparinized tubes from the hind leg vein before and at 3 min, 2 h, 4 h, 8 h, 12 h, 1 d, 2 d, 3 d, 4 d, 5 d, 6 d, 7 d, 8 d, 9 d, 10 d, 11 d, 12 d, 13 d, 14 d, 15 d, 16 d, 17 d and 18 d min after the single administration. Nine beagle dogs in group 2 were given an intramuscular injection dose of 40 mg/kg of LXT-101 sustained-release suspension and the blood samples, about 2 mL each collected through hind leg vein, were placed in heparinized tubes pre-dose and at 30 min, 2 h, 4 h, 8 h, 12 h, 1 d, 2 d, 3 d, 4 d, 5 d, 6 d, 7 d, 8 d, 9 d, 10 d, 11 d, 12 d, 13 d, 14 d, 15 d, 16 d, 17 d, 18 d, 19 d, 20 d, 21 d, 22 d, 23 d, 24 d, 25 d, 26 d, 27 d, 28 d, 29 d, 30 d, 31 d, 32 d, 33 d and 34 d post-dose. In addition, the repeated administration of LXT-101 sustained-release suspension at the same dose and route of administration were given to dog 1, 2 and 3 in group 2 at 12 d post the first dose. The separation of plasma samples was immediately centrifuged at 2000 g for 10 min at 4 °C and stored below − 20 °C until LC–MS/MS analysis. The main pharmacokinetic parameters of LXT-101, including AUC(0-t), AUC(0-∞), MRT, T1/2Z, Tmax, Cmax, Vd/F, and CLz/F, were calculated by Drug and Statistics software version 2.0 (DAS 2.0, Mathematical Pharmacology Professional Committee of China, Shanghai, China) using a non-compartmental method20,21,22,23,24.
Results and discussion
Mass spectrometry method development
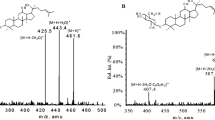
LXT-101 is a small chain peptide compound with 10 amino acids. Under ESI conditions, a full scan in flow injection mode could obtain the spectra with multiple charges, namely [M + 3H]3+ (m/z 472.1) and [M + 2H]2+ (m/z 707.7). The m/z of three charges for LXT-101 could be calculated theoretically as (1413 + 3)/3 = 472.0, meanwhile the m/z of two charges for LXT-101 could be calculated theoretically to be (1413 + 2)/2 = 707.5. The theoretical values were completely consistent with the measured value. IS compound could be similarly calculated. Due to the stronger and stabler response signal of [M + 3H]3+compared to the signal of [M + 2H]2+, the [M + 3H]3+ was selected as the parent ion. Under optimized CID conditions, the parent ion could be bombarded to produce the following sub ions: m/z 170.0, m/z 153.0, and m/z 587.8. There was no interference from endogenous substances in blank plasma, so the SRM mode of m/z 472.1 → 587.8 was chosen for quantitative analysis of LXT-101 (Fig. 2).
Method Validation
Selectivity
The selectivity of the method toward endogenous biological matrix was assessed with blank beagle dog plasma from six beagle dogs. The retention times of LXT-101 and IS were both detected at 4.45 min. No unacceptable interferences from endogenous substances were observed in the retention regions of either LXT-101 or IS (Fig. 3).
SRM chromatograms for LXT-101 and 127I-LXT-101 (IS) in beagle dog plasma. Chromatographic profile of (I) a blank plasma sample spiked with IS, (II) a sample of plasma spiked with LXT-101 (2 ng/mL) and IS, and (III) a sample of plasma at 30 min after the single administration of LXT-101. (A) LXT-101; (B) 127I-LXT-101 (IS).
Linearity and LLOQ
The linear regressions of LXT-101 in the beagle dog plasma exhibited good linear relationships in the range from 2 to 600 ng/mL (R2 = 0.9977). The mean value of regression equation for LXT-101 in the beagle dog plasma was: y = − 2.1 × 10–3 x + 2.0 × 10–3 with the weighting factor of 1/x.
The LLOQ for LXT-101 under present LC–MS/MS conditions was found to be 2 ng/mL with the signal-to-noise ratio being at least 10:1. The precision (within ± 20%) and accuracy (< 20%) of LLOQ samples both met the requirements.
Precision and accuracy.
The intra-batch and inter-batch precision and accuracy date for LXT-101 in beagle dog plasma are shown in Table 1. The QC samples at three concentration levels (5 ng/mL, 50 ng/mL and 500 ng/mL) were selected and analyzed to evaluate the precision and accuracy during intra-batch and inter-batch on three consecutive batches (n = 5). An accuracy within ± 15% and a precision of < 15% were accepted. The intra-batch and inter-batch accuracy (%) were within 93.36–93.94% and 95.61–99.27%, while the intra-batch and inter-batch precision (RSD, %) were in the range of 3.23–14.26% and 5.03–11.10%, respectively.
Extraction recovery and matrix effect
The ratio of the area of QC samples at low QC, medium QC and high QC concentration levels (n = 3) with known amounts of LXT-101 to the area of compound added at the same concentrations to blank beagle dog plasma after protein precipitation reflected the extraction recovery. The matrix effect of LXT-101 and IS was determined by the ratio of the amounts of analyte dissolved in the blank sample against those of neat standards. IS-normalized matrix factor was calculated by dividing the matrix effect of LXT-101 by that of IS. The extraction recovery and matrix effect data for LXT-101 in beagle dog plasma ranged from 86.67–108.06% and 88.58–108.50%, respectively (shown in Table 2).
Stability
The stability of LXT-101 in beagle dog plasma was evaluated under the following temperature and timing conditions: 2 h and 8 h at ambient temperature, 3 d, 7 d and 15 d at − 20 °C, freeze–thaw for 2 cycles (− 20 °C to room temperature), and 24 h post-preparative stability in the auto sampler. The measured concentrations (QC samples) of LXT-101 in beagle dog plasma were all within acceptable limits (± 15%) during the entire validation (shown in Table 3).
Pharmacokinetics study
The validated method was successfully applied to assay the plasma concentrations of LXT-101 in beagle dogs after intramuscular injection administration of LXT-101 sustained-release suspension.
In the single-dose groups (20 mg/kg and 40 mg/kg), the values of AUC0-t (588.09 ± 137.79 ng/mL·d vs. 1203.62 ± 877.42 ng/mL·d) and AUC0-∞ (592.89 ± 134.21 ng/mL·d vs. 1209.97 ± 873.78 ng/mL·d) were observed increasing proportionately with the increasing dose of LXT-101 sustained-release suspension. Moreover, no apparent differences for the pharmacokinetic parameter values including MRT, T1/2z, Tmax, Vz/F and Clz/F were observed regardless of increasing dose. Compared with the single-dose group (40 mg/kg), the values of AUC0-t and AUC0-∞ showed a significant increase in the repeated-dose group (40 mg/kg), which suggested the possibility of accumulation in beagle dogs. In addition, repeated-dose of LXT-101 sustained-release suspension increased the values of MRT and T1/2z, meanwhile the value of Clz/F was reduced. The plasma concentration–time profiles of LXT-101 following intramuscular injection administration in beagle dogs are showed in Fig. 4. The main pharmacokinetic parameters of h LXT-101 are presented in Table 4.
Conclusions
Prostate cancer represents as usual a significant health burden, being the most aggressive cancer and the second most frequent cause of cancer-related deaths among men all over the world25,26. The research and development of GnRH antagonists, such as cetrorelix, abarelix, degarelix, ganirelix and ozarelix, might provide an important advance for patients with prostate cancer. However, it still could not meet the requirement of market27,28.
Research indicated that LXT-101 sustained-release suspension showed in vivo dose-dependent and could exert the efficacy to suppress the testosterone level to castration with little toxicity11.
For further development and utilization, a sensitive, robust and specific LC–MS/MS method for the determination of LXT-101 in beagle dog plasma was developed and validated in this research. The fully validated LC–MS/MS assay was successfully applied to study the pre-clinical pharmacokinetics of LXT-101 sustained-release suspension in beagle dogs after intramuscular injection administration. The beagle dogs were excellent non-clinical models used in preclinical pharmacokinetics experiments. A large number of diseases seen in the beagle dogs closely depict the phenotype of human diseases29. Consequently, this assay methodology of LXT-101 and the application to pharmacokinetic characteristics of its sustained-release formulation in beagle dogs could afford theoretical basis and methodological innovation for the pharmacological mechanism and potential development and rational use of LXT-101 sustained-release suspension.
In conclusion, a LC–MS/MS method for the determination of LXT-101 in dog plasma was developed and applied to pharmacokinetics study of LXT-101 sustained-release suspension in beagle dogs.
Data availability
If you want to request the the experimental information and data presented from this study, please contact the corresponding author.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics. CA Cancer J. Clin. 68(1), 7–30 (2018).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2018).
Dy, G. W., Gore, J. L., Forouzanfar, M. H., Naghavi, M. & Fitzmaurice, C. Global burden of urologic cancers, 1990–2013. Eur. Urol. 71(3), 437–446 (2017).
Wong, M. C. et al. Global incidence and mortality for prostate cancer: Analysis of temporal patterns and trends in 36 countries. Eur. Urol. 70(5), 862–874 (2016).
Rebello, R. J. et al. Prostate cancer. Nat. Rev. Dis. Primers. 7(1), 9 (2021).
Wang, G., Zhao, D., Spring, D. J. & DePinho, R. A. Genetics and biology of prostate cancer. Genes Dev. 32(17–18), 1105–1140 (2018).
Crawford, E. D. Hormonal therapy of prostatic carcinoma: Defining the challeng. Cancer 66(5 Suppl), 1035–1038 (1990).
Guess, H. A., GormLey, G. J., Stoner, E. & Oesterling, J. E. The effect of finasteridem on prostate specifica antigen: Review of available data. J. Urol. 155(1), 3–9 (1996).
Scher, H. I. & Kelly, W. K. Flutamide withdrawal syndrome: Its impact on clinical trials in hormone-refractory prostate cancer. J. Clin. Oncol. 11(8), 1566–1572 (1993).
Chi, X., Zhou, W., Cheng, J., Zhang, Y. & Liu, K. In vivo characterization of a novel GnRH (gonadotropin-releasing hormone) antagonist, LXT-101, in normal male rats. Regul. Pept. 136(1–3), 122–129 (2006).
Du, L. et al. Biodegradable PLGA microspheres as a sustained release system for a new luteinizing hormone-releasing hormone (LHRH) antagonist. Chem. Pharm. Bull. (Tokyo). 54(9), 1259–1265 (2006).
Wang, T., Gao, L. & Quan, D. Multivesicular liposome (MVL) sustained delivery of a novel synthetic cationic GnRH antagonist for prostate cancer treatment. J. Pharm. Pharmacol. 63(7), 904–910 (2011).
Zhang, G., Wang, T., Gao, L. & Quan, D. Oral delivery of oil-based formulation for a novel synthetic cationic peptide of GnRH (gonadotropin-releasing hormone) antagonist for prostate cancer treatment. Int. J. Pharm. 450(1–2), 138–144 (2013).
Zhang, G., Li, J., Gao, L., Wang, T. & Quan, D. Morphology of nanostructures and their long-acting properties in vivo for a novel synthetic peptide of gonadotropin-releasing hormone antagonist. J. Pharm. Pharmacol. 66(8), 1077–1081 (2014).
US-FDA. Bioanalytical Method Validation Guidance for Industry, US-FDA, Silver Spring, MD, USA, 2018. https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugsgen/documents/document/ucm070107.pdf.
Xu, R. A. et al. UPLC-MS/MS method for the simultaneous determination of imatinib, voriconazole and their metabolites concentrations in rat plasma. J. Pharm. Biomed. Anal. 166, 6–12 (2019).
Tang, C. et al. In vivo Pharmacokinetic drug-drug interaction studies between fedratinib and antifungal agents based on a newly developed and validated UPLC/MS-MS method. Front. Pharmacol. 11, 626897 (2020).
Feng, G. et al. Effect of Rhei Radix et Rhizoma and Eupolyphaga Steleophaga on liver protection mechanism based on pharmacokinetics and metabonomics. Chin. Herb Med. 16(1), 121–131 (2024).
Zhang, Y., Liu, Y. N., Xie, S., Xu, X. & Xu, R. A. Evaluation of the inhibitory effect of quercetin on the pharmacokinetics of tucatinib in rats by a novel UPLC-MS/MS assay. Pharm. Biol. 60(1), 621–626 (2022).
Li, X. et al. Comparative pharmacokinetics of six components in normal and rheumatoid arthritis rats after intragastrical administration of Qianghuo Shengshi Decoction granules by LC-MS/MS. Chin. Herb. Med. 16(3), 457–465 (2024).
Wei, P. et al. Identification and characterization of chemical constituents in Mahuang Guizhi Decoction and their metabolites in rat plasma and brain by UPLC-Q-TOF/MS. Chin. Herb. Med. 16(3), 466–480 (2024).
Xiong, S. et al. Comparative pharmacokinetics of four major compounds after oral administration of Mori Cortex total flavonoid extract in normal and diabetic rats. Front. Pharmacol. 14, 1148332 (2023).
Cheng, W. et al. Simultaneous determination of five compounds of fried Radix Paeoniae Alba extract in beagle dogs plasma by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry and its application in a pharmacokinetic study. Biomed. Chromatogr. 38(3), e5803 (2024).
Yu, X. et al. Simultaneous determination and pharmacokinetic study of six components in beagle dog plasma by UPLC-MS/MS after oral administration of Astragalus Membranaceus aqueous extract. Biomed. Chromatogr. 36(12), e5488 (2022).
Barsouk, A. et al. Epidemiology, staging and management of prostate cancer. Med. Sci. (Basel). 8(3), 28–41 (2020).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70(1), 7–30 (2020).
Trachtenberg, J. et al. A phase 3, multicenter, open label, randomized study of abarelix versus leuprolide plus daily antiandrogen in men with prostate cancer. J. Urol. 167(4), 1670–1674 (2002).
Klotz, L. et al. The efficacy and safety of degarelix: A 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 102(11), 1531–1538 (2008).
Gopinath, C., Nathar, T. J., Ghosh, A., Hickstein, D. D. & Nelson, E. J. R. Contemporary animal models for human gene therapy applications. Curr. Gene Ther. 15(6), 531–540 (2015).
Author information
Authors and Affiliations
Contributions
Jinglai Li conceived and designed the experiments; Lan Yin wrote the paper; Yuexin Li performed the experiments; Yutao Xue analyzed the data; Xiaoying Wang conceived the experiments; Wenxia Xu revised the paper; Zhenqing Zhang designed the experiments; Shan Xiong designed the experiments and revised the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
Animal experiments were demonstrated to be ethically acceptable and were carried out according to the Guidelines of the Experimental Animal Care and Use of Laboratory Animals of the Beijing Institute of Pharmacology and Toxicology. All the animal protocols for experiments were approved by the Institutional Animal Care and Use Committee of the Beijing Institute of Pharmacology and Toxicology (nos 11400700031606). This study was reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Yin, L., Li, Y. et al. Development and validation of an LC–MS/MS method for quantitative determination of LXT-101 sustained-release suspension, a novel drug in treating prostate cancer, in beagle plasma. Sci Rep 15, 11868 (2025). https://doi.org/10.1038/s41598-025-96764-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96764-3