Abstract
This study investigated the impact of rhizosphere fungi on the quality of Atractylodes macrocephala in China by analyzing the physical and chemical properties, enzyme activities, and community structures of soil samples from four distinct regions: Pan’an (PA), Bozhou (BZ), Zhoukou (ZK), and Anguo (AG). The results indicated that both biomass and active components of A. macrocephala were significantly higher in authentic production areas compared to emerging ones. The rhizosphere soil network in PA, identified as an authentic production area, exhibited the most complex structure, with pH levels significantly negatively correlated with 12 major fungal genera. Notably, fungi such as Rozellomycota, Mortierella, and Basidiomycota were linked to the quality of A. macrocephala through their roles in organic matter decomposition. Additionally, Saitozyma was found to be a central component of the rhizosphere fungal community, with a relative abundance of 2.19%, markedly higher than in emerging production areas (< 0.1%). These findings provide critical insights into the factors affecting A. macrocephala quality across different regions, offering valuable guidance for the sustainable cultivation of this essential medicinal plant in China.
Similar content being viewed by others
Introduction
Atractylodes macrocephala, an important medicinal herb in traditional Chinese medicine (TCM), has garnered significant attention due to its diverse pharmacological properties. It contains various secondary metabolites, including lactones and ketones, which exhibit notable anti-inflammatory, antioxidant, and digestive-promoting effects1. A. macrocephala is primarily cultivated in several regions of China, including Pan’an (PA), Bozhou (BZ), Zhoukou (ZK), and Anguo (AG). The primary medicinal components of A. macrocephala are volatile oils, particularly sesquiterpenoids, which significantly enhance its therapeutic efficacy2. Among these, atractylenolides and atractylone are key quality markers widely employed to evaluate its pharmacological potential and ensure quality consistency3.
The increasing demand for A. macrocephala in TCM highlights the urgent need to ensure its consistent quality and stability. However, limited cultivation areas and environmental variability contribute to significant quality differences among production regions4. Traditional medicinal herbs are often valued for their “authentic” varieties from specific regions, recognized for their superior efficacy and stable quality. Understanding the causes of quality variations, particularly the role of ecological factors, is therefore critical. The quality of A. macrocephala is closely tied to ecological conditions, such as geographic location, climate, and soil composition, all of which significantly influence the content of active ingredients. For instance, sesquiterpenoid lactones, including Butenolide II and III, are key pharmacological components, and their concentrations vary substantially across production regions5. Soil composition, climate, and ecological environment directly affect the synthesis and accumulation of these compounds.
Rhizosphere microorganisms, particularly rhizosphere fungi, are vital to plant growth, development, and the production of bioactive components in medicinal plants. By degrading soil organic matter (SOM), these fungi enhance nutrient absorption by plants6. Additionally, the diversity and abundance of microbial communities influence not only plant health and yield but also the pharmacological composition of medicinal plants like A. macrocephala by regulating secondary metabolite synthesis7. A deeper understanding of the role of microbial ecosystems in A. macrocephala cultivation is essential for improving its quality and stability.
Recent studies have shown that interactions between soil properties and rhizosphere microbial communities significantly impact the quality and bioactivity of medicinal herbs. Soil physicochemical properties, such as potassium content and ammonium nitrogen levels, along with fungal community diversity, influence the synthesis of active components and disease resistance, ultimately affecting medicinal material quality8. For example, research on Astragalus has revealed that soil properties alter fungal community composition, with certain microbial abundances correlating positively or negatively with active compound levels9. Similarly, studies on Glycyrrhiza have demonstrated that soil characteristics and root metabolites regulate the diversity and structure of endophytic fungal communities, thereby influencing licorice quality10. These findings underscore the importance of soil ecology and rhizosphere microbial communities in improving the quality of medicinal plants.
This study aims to investigate the biomass and active compound content of A. macrocephala cultivated in 20 fields across four major production regions in China. It focuses on analyzing the influence of rhizosphere fungal communities, soil enzyme activity, and other physicochemical factors, including organic matter (OM), electrical conductivity (EC), and pH, we seek to understand how these factors influence the quality of A. macrocephala and elucidate the role of fungi in the formation of its medicinal properties on A. macrocephala’s chemical composition. The findings will provide insights into the environmental factors affecting the key active compounds of A. macrocephala and explore the potential role of microorganisms in their biosynthesis11.
Results
Comparative analysis of active ingredient content and biomass of A.macrocephala in four regions
Atractylodes volatile oil is the main component of A. macrocephala, primarily composed of sesquiterpene compounds, including Atractylone, Butenolide I, Butenolide II, and Butenolide III. These compounds exhibit pharmacological properties such as anti-inflammatory, antioxidant, and anticancer effects1. Therefore, in this study, Butenolide I, II, III, and Atractylone were selected as indicators to measure the content of active ingredients in A. macrocephala. In the analysis of active ingredient content in samples from the four regions of PA, BZ, ZK, and AG (Fig. 1A), the average concentrations of Butenolide I and Butenolide III were significantly higher in the PA region than in the other regions, reaching 0.513 mg/g and 1.381 mg/g, respectively. However, there were no significant differences in the concentrations of Atractylone and Butenolide II among the four regions. In the biomass measurement of A. macrocephala (Fig. 1B), PA showed consistently the highest weight in both aboveground and underground measurements. BZ exhibited high weight but with greater variability, ZK generally displayed the lowest weight, and AG showed moderate weight, with underground measurements being more consistent than aboveground ones.
Differences in the physicochemical properties of rhizosphere soil of A. macrocephala in different production areas
Soil enzymes play a vital role in various biochemical processes, including nutrient cycling, organic matter decomposition, and soil microbial activity. In this study, we selected several key soil enzymes—catalase (S-CAT) , urease (S-UE), nitrate reductase (S-NP), and β-glucosidase (S-β-GC)—based on their relevance to soil health and their involvement in critical soil processes12,13. Additionally, we measured organic matter (OM), electrical conductivity (EC), and pH to assess the general soil quality and its influence on enzyme activity (Fig. 2). The results showed that the PA group had significantly higher S-NP activity and OM content than the other groups, with greater variability in EC and pH values. The BZ group had the highest S-β-GC and S-UE activities, with an alkaline pH. The ZK group had balanced indicators, with relatively high S-CAT activity and an alkaline pH. The AG group excelled in S-CAT and S-UE activities but had the lowest OM content and an alkaline pH.
Diversity of fungi in the rhizosphere soil of A. macrocephala in different production areas
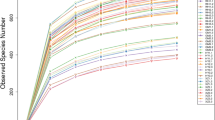
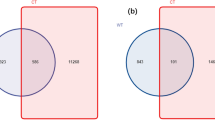
High-throughput sequencing results showed that the constructed library had a coverage rate of over 99%, including 9 fungal phyla, 22 fungal classes, 52 fungal orders, 109 fungal families, and 160 fungal genera, with a total of 5659 fungal OTUs (Supplementary Tables 1 and 2). Venn diagram analysis (Fig. 3A) indicated that, besides the shared core OTUs, the PA region had the highest number of unique OTUs. Comprehensive assessment of fungal diversity (Fig. 3B–G) revealed that Good’s coverage was close to 1 across samples. The PA region showed significant advantages in species richness, Chao1 index, and Ace index, while differences in Simpson and Shannon indices were not significant.
Venn diagram of OTUs in four regions. (A) Fungal diversity assessment. (B) Good’s Coverage, indicating sampling completeness across groups. (C) Species Richness, reflecting the total observed species within each group. (D) Chao1 Richness Estimator, representing the estimated species richness. (E) Abundance-based Coverage Estimator (ACE), showing richness based on species abundance. (F) Simpson’s Diversity Index, highlighting community evenness and dominance. (G) Shannon Diversity Index, reflecting species diversity and evenness in the communities.
Analysis of fungal community structure and composition across different regions
The principal component analysis (PCA) (Fig. 4A) and redundancy analysis (RDA) (Fig. 4B) revealed significant regional variations in fungal community composition and its relationship with soil properties. PCA results showed that the first two principal components explained 74.35% of the total variance, with distinct clustering of samples from different regions, indicating unique fungal community structures influenced by regional environmental factors. The PA region exhibited stable fungal communities under consistent soil conditions, while the AG region displayed distinct environmental characteristics. In contrast, the BZ and ZK regions showed more variability, with overlapping or dispersed patterns in fungal communities. RDA results further highlighted varying degrees of association between soil properties and fungal diversity. While strong correlations were observed in the ZK region, moderate associations were found in the PA region, and weaker relationships were evident in the BZ region. The AG region exhibited distinct environmental drivers influencing both soil and fungal properties. These findings underscore the complex and region-specific interactions between soil health and fungal diversity.
The co-occurrence network of the Atractylodes rhizosphere soil fungi
The top 10 dominant fungi (with relative abundance > 1%) at the genus level in the rhizosphere soil of A. macrocephala across the four production areas were Mortierella, Fusarium, Alternaria, Aureobasidium, Plectosphaerella, Clonostachys, Gibberella, Trechispora, Paraphoma, and unclassified fungal genera (Fig. 5A). At the phylum level, the dominant group was Ascomycota (relative abundance 72.4%) (Fig. 5B).
Rhizosphere fungal network of A. macrocephala from different habitats. (A) Circos plot of dominant fungi at different sampling sites, with internal lines representing corresponding relationships and capital letters indicating the sampling site names, at the genus level. (B) Circos plot at the phylum level. (C) Bar chart showing the contribution values of each species to the intergroup differences at the phylum level. (D) Differential Alternaria fungi at different sampling sites. (E) Differential Gibberella fungi at different sampling sites. (F) Differential Trechispora fungi at different sampling sites.
To explore the fungi that contributed substantially to the group differences, a random forests analysis was conducted, and fungi at the phylum level were screened and contributed > 0.06 to Rozellomycota (relative abundance 0.64%), Mortierellomycota (6.06%), Basidiomycota (13.2%) and unclassified fungi (Fig. 5C). A variance analysis of the relative abundance of dominant fungi at the genus level revealed significant changes in certain fungi in the authentic production area of PA. For example, the relative abundance of Trechispora increased (P < 0.01) (Fig. 5D), the relative abundance of Gibberella also increased (P < 0.01) (Fig. 5E), while the relative abundance of Alternaria significantly decreased (P < 0.01) (Fig. 5F).
The network analysis assessed the complexity of interactions between fungal genera in the rhizosphere soils of Atractylodes in different production areas. Spearman correlation was used to calculate the correlations among the top 80 fungal genera in the soil, and Gephi was used to visualize the co-occurrence networks of the four production areas (Fig. 6A–D), evaluating several topological properties. In the network model, each node represents a microbial genus, and the fungal network is composed of nodes and edges. The authentic production area had the highest number of nodes and links. The average geodesic distance in a network is the shortest path between two nodes, and a smaller average geodesic distance indicates that all the nodes in the network are closer. This indicates a more complex network. Modularity is the degree to which a network can be divided into communities or modules. For ecological networks, the microbial species in a module can be considered to have similar ecological niches11,14. A higher modularity indicates a less complex network. The degree centrality is the number of links on the nodes, and when more differences are observed between connectivity across all the nodes, the value is closer to 1. A higher value indicates a more complex network. The Atractylodes rhizosphere soil network in the authentic production area was found to be the most complex and compact, with the highest degree of centrality (0.074) and network density (0.018) and the lowest mean geodesic distance (4.64) and lower modularity (0.658). The network in AG region was relatively simpler, with a higher modularity (0.801) and a lower degree of centrality (0.03). It also had the highest average geodesic distance (7.052) and the lowest network density (0.011), indicating a less compact and less complex network compared to the authentic production area (Supplementary Table 3).
Correlation analysis of physicochemical properties of rhizosphere soil and fungal community changes in A. macrocephala in the PA region
To analyze the interaction between the rhizosphere soil physicochemical properties of A. macrocephala and the changes of soil fungal communities in the four production areas in more detail, the correlation between the soil physicochemical properties and fungi at the genus level was analyzed by a Pearson correlation. The heat map showed that S-NP and OM significantly positively correlated with the relative abundances of Ilyonectria, Cylindrocarpon, Chaetomium, Lecanicillium, Gibberella, Didymella, Mortierella, Lectera, Saitozyma, Paraphoma and Trechispora. The activities of S-β-G and S-UE significantly negatively correlated with the relative abundance of Gibberella, Didymella, Mortierella, and Trechispora but significantly positively correlated with the relative abundance of Trichoderma. The pH significantly negatively correlated with the relative abundances of Chaetomium, Gibberella, Didymella, Mortierella, Trechispora, Lectera, Saitozyma, Paraphoma and Lecanicillium (Fig. 7). The results showed that the increase in pH significantly inhibited the main fungal genera in the rhizosphere soil of A. macrocephala in the four production areas. The pH of the rhizosphere soil of A. macrocephala in the authentic production areas was significantly lower than that in the emerging production areas, which could promote the complexity of the fungal structure in the rhizosphere soil of A. macrocephala.
Discussion
To explore the influence of soil fungal communities in different production areas on the growth and quality of A. macrocephala, clarify the origin of A. macrocephala from the perspective of soil fungi, and provide a plan for the rational planting of A. macrocephala, this study analyzed both authentic and emerging production areas of A. macrocephala.
Soil enzymes secreted by microorganisms play a critical role in nutrient cycling, soil structure maintenance, and crop production15. This study revealed that the active components and biomass of A. macrocephala were significantly positively correlated with the organic matter content and soil phosphatase activity in the rhizosphere soil. Furthermore, these factors were also significantly positively correlated with the level of alpha diversity in the rhizosphere soil. Variations in soil fertility levels led to changes in the fungal community structure in the rhizosphere. In more fertile soils, higher organic matter content and enhanced soil enzyme activities contributed to the growth of A. macrocephala and the accumulation of its active components, thereby improving its yield and quality16. Notably, differences in organic matter and soil enzyme activities in the rhizosphere soil across different production regions may indirectly influence the growth and development of A. macrocephala. Our findings demonstrated that rhizosphere soils in authentic production areas had higher organic matter content and enzyme activity than those in emerging areas, indicating better soil quality and a more complex and diverse fungal community structure, which promoted the healthy growth of A. macrocephala.
2021Given the high complexity of microbial communities, co-occurrence networks are widely used to analyze microbial community structures17. This study compared the fungal association networks in the rhizosphere soil of A. macrocephala from four production areas, finding significant variability in the topological characteristics, key species, and correlations with environmental variables of fungal community structures across these areas. The presence and distribution of modules in co-occurrence networks reflect the characteristics of the microbial community structure18. Compared to emerging production areas, the rhizosphere soil fungal network of A. macrocephala in authentic production areas had the most nodes, the highest average degree, and the shortest average geodesic distance. The community dissimilarities, including total fungi and fungal functional guilds, increased with the soil fertility index dissimilarity. In more fertile soils, fungal networks exhibited larger size, higher connectivity, and greater potential for inter-module connection19, indirectly enhancing the yield and quality of A. macrocephala.
Soil pH has a complex effect on microbial community structure, with fungi generally preferring acidic soils20. This study showed that the rhizosphere soil of A. macrocephala in authentic production areas is acidic, while soils in emerging areas are alkaline. Correlation analysis revealed that pH negatively correlated with the relative abundance of 12 fungal genera, including Chaetomium, Gibberella, Didymella, Mortierella, Trechispora, Lectera, Saitozyma, and Paraphoma, indicating inhibited fungal community abundance. Chaetomium21, Mortierella22, and Saitozyma23 have been proven to effectively enhance plant disease resistance through various mechanisms. These mechanisms include improving soil fertility, regulating hormone signaling, enhancing the expression of disease resistance genes, and altering the rhizosphere microbial community. Lectera24 and Trechispora25 are also considered potential biocontrol agents. This experiment also provides evidence for the screening of new biocontrol agents. More importantly, due to the increased abundance of fungal communities in acidic soils, A. macrocephala in authentic production areas may have better disease resistance.
Rhizosphere microorganisms are the main factors affecting the formation of high quality medicinal materials and promoting the accumulation of secondary metabolites11.The composition and structure of rhizosphere fungi in authentic production areas of A. macrocephala varied significantly from those in emerging areas. Among the nine detected phyla, Rozellomycota, Mortierella, and Basidiomycetes contributed the most to group differences. Rozellomycota, primarily a parasite in chytrids, algae, or oomycetes, degrades organic matter by secreting enzymes outside the cell and absorbing nutrients via diffusion26. Mortierella may be involved in the mineralization of organic matter in the soil, degrading crop residues into organic matter27. The extracellular enzymes utilized by saprotrophic Basidiomycetes for nutrient acquisition participate in the interspecific interactions with other soil biota but are also involved in the transformation of soil organic matter – lignocellulose and humic compounds28. Therefore, studying the composition and function of rhizosphere microbial communities is of great significance for understanding the quality formation mechanisms of A. macrocephala and other medicinal plants.
Microecological imbalance in rhizosphere caused by variation of the soil microbial community is considered the primary cause of replant disease29. In our study, six out of the top ten most abundant fungi, including the genera Plectosphaerella, Alternaria, Gibberella, Fusarium, Clonostachys, and Paraphoma, were identified as pathogenic. Among them, Fusarium has a significantly negative impact on the health of A. macrocephala during monocropping due to its overabundance in both rhizospheric and endophytic communities associated with root rot disease30. These findings highlight the importance of managing soil microbial communities to mitigate replant disease and ensure the healthy growth of A. macrocephala.
Diverse root-associated fungi can form highly compartmentalized coexistence networks, with networks representing symbiotic fungal coexistence patterns divided into clear modules composed of various functional root-associated fungi. This study points out that although the relative abundance of Saitozyma is low, it serves as a fungal community hub in the rhizosphere soil of A. macrocephala. Other studies have indicated that Saitozyma occupies a central position in the meta-community networks of subtropical and warm temperate forests. In these regions, Saitozyma has a wide geographic and host range and may influence community-level plant–microbe interactions by enhancing host plant growth and pathogen resistance31. Therefore, Saitozyma holds significant ecological importance, warranting further research into its functions and roles in different environments.
In summary, among A. macrocephala production areas in China, Pan’an, the authentic production area, had the most beneficial rhizosphere soil, likely due to the highest abundance of beneficial fungi promoting A. macrocephala growth and effective component accumulation. The alkaline pH of rhizosphere soil in emerging areas negatively correlated with 12 major fungal genera, possibly inhibiting A. macrocephala growth and disease resistance. Rozellomycota, Mortierella, and Basidiomycetes affect A. macrocephala growth by participating in soil organic matter flow, closely related to Atractylodes root formation. Saitozyma’s role is significant and warrants further attention. However, this study’s focus on rhizosphere fungi, excluding bacteria, indicates limitations that future research will address.
Conclusions
There were significant differences in the enzyme activities and microbial community diversity in the rhizosphere soil of A. macrocephala in the different production areas. Compared with the emerging production areas, A. macrocephala in the authentic production areas had the highest concentrations of beneficial active ingredients, such as Butenolide I and Butenolide III, as well as superior soil enzyme activities, including phosphatase, urease, catalase, and β-glucosidase. These factors contributed to the improved soil quality and facilitated better growth and development of A. macrocephala. There were significant differences in the composition of the microbial community in the rhizosphere soil of A. macrocephala between the authentic and emerging production areas. In the authentic production area Pan’an, the dominant phylum in the rhizosphere soil was Ascomycota, with a relative abundance of 72.4%, which was notably higher than in other areas. Additionally, Rozellomycota, Mortierella, and Basidiomycota were more abundant in the rhizosphere soil of the authentic production areas. These fungi play important roles in organic matter decomposition and soil quality improvement, potentially promoting the growth and active ingredient accumulation of A. macrocephala. The Atractylodes rhizosphere soil fungal network was the most complex and compact in the authentic production areas. These soil microbes influence the health of A. macrocephala through a close and complex network of relationships. Simultaneously, this study revealed that the differences in soil microbial communities in the rhizosphere of A. macrocephala were influenced by changes in the soil environmental factors. In future studies, we aim to isolate beneficial microorganisms, such as Saitozyma, Rozellomycota, Mortierella, Basidiomycota, Trichoderma, Chaetomium, and Trechispora, from healthy rhizosphere soil samples to explore how these microorganisms can promote the growth of A. macrocephala and enhance its disease resistance. In addition, further studies are needed at the prokaryotic level in this study to determine the role that bacterial communities play in the growth of A. macrocephala. These types of research will help to clarify the reasons for the differences in quality of A. macrocephala in different production areas, as well as the cultivation and regional distribution of A. macrocephala in China.
Materials & methods
Sample collection and site description
Since A. macrocephala is one of the authentic medicinal materials in Zhejiang Province, Bozhou is the second largest production area, and Henan and Hebei are emerging production areas. Thus, the A. macrocephala and the rhizosphere soil samples were collected from the four sampling areas of PA in Zhejiang, BZ in Anhui, ZK in Henan, and AG in Hebei for this study. A. macrocephala had been planted for approximately 10 years in each sampling area. The crops that had been planted previously were graminaceous, and the A. macrocephala variety was a biennial improved species No. 1. The soil samples were collected during the harvest period in mid-October 2020. Five fields were selected, and the distance between the adjacent sampling points > 1 km. The five-point sampling method was used to collect A. macrocephala and the rhizosphere soil from each field. The samples were mixed together to form one sample. A total of 20 samples were established for this study.
The annual average temperature and atmospheric pressure data were obtained from the China National Meteorological Centre Database (https://data.cma.cn/). PA is located in a mountainous area, and A. macrocephala is mostly planted on hillsides with an altitude > 300 m, while BZ, AG, and ZK are all plains or hills. The annual average high temperature and low temperature are 17 °C and 7 °C in PA, respectively; 10 °C and 0 °C in BZ, respectively; 11 °C and − 2 °C in AG respectively; and 10 °C and 0 °C ZK, respectively. The specific sampling locations are marked in the tables (Table 1). The average annual precipitation in PA is 1,450 mm, in ZK it is 752.4 mm, in AG it is 555.3 mm, and in BZ it is 744.3 mm.
The organic debris and topsoil were removed, and the entire Atractylodes plant was dug up and removed from the soil. The root soil was gently shaken to obtain the rhizosphere soil. The sample was identified as A. macrocephala Koidz by the Zhejiang Institute of Traditional Chinese Medicine (Zhejiang Chinese Medical University, Hangzhou, China), and the plants were brought back to the laboratory for preservation. Part of the soil samples were stored at − 80 °C for high-throughput sequencing, and the other part of the soil samples was air-dried to measure its physicochemical properties.
Soil physical and chemical properties and enzyme assays
A portion of the mixed air-dried soil sample was passed through a 20-mesh sieve, and a pH meter was used to determine the pH value of the soil and aqueous solution at a ratio of 1:2 (w/v). The conductivity of the soil and the ultrapure aqueous solution was measured using a conductivity meter 1:5 (w/v), and there were three replicates.
The phenol-sodium hypochlorite colorimetric method was used to assay the soil urease. The benzene disodium phosphate colorimetric method was used to assay the soil acid phosphatase, and UV spectrophotometry was used to assay the soil catalase. β-glucosidase was assayed using the nitrophenol colorimetric method.
Extraction and identification of the active components of A. macrocephala
The dried A. macrocephala rhizomes were crushed, passed through a 50-mesh sieve, weighed to 1.0 g, mixed with 50 mL of methanol and sonicated for 30 min. High-performance liquid chromatography (HPLC) was used to determine the concentrations of Butenolide I, Butenolide II, Butenolide III and Atractylone using wavelengths of 220 nm and 270 nm20. A volume of 10 μL was injected, and the temperature was 25 °C. Its content was calculated by the linear equation method and repeated three times.
DNA extraction and high-throughput sequencing
The total DNA was extracted from 200 mg of each rhizosphere sample using a commercial DNA extraction kit (Q10212; Omega Bio-Tek, Norcross, GA, USA), according to the manufacturer’s directions. The DNA quality was assessed using a NanoDrop® ND-1000 Spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 0.8% agarose gel electrophoresis. The extracted DNA was stored at − 20 °C for further processing. The ITS high-throughput sequencing was performed by OE Biotech. Co., Ltd. (Shanghai, China) using an Illumina MiSeq platform (Illumina, San Diego, CA, USA). The ITS regions of the fungi were amplified using the primers ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2 R (5′-GCTGCGTTCTTCATCGATGC-3′).
High-throughput sequencing obtains double-ended sequence data, and the sequences contain barcode sequences, as well as primers and adapter sequences, that were added during sequencing. The primer linker sequences were removed, and the paired reads were merged into one sequence based on the overlap relationship between the PE reads. The samples were identified and distinguished according to the barcode tag sequence to obtain the sample data, and finally the quality of each sample data was checked. Quality control filtering was used to obtain valid data for each sample. The ITS sequences were then clustered into operational taxonomic units (OTUs) with 97% identity by USEARCH, and BLAST was used to align the sequences with the corresponding databases to identify the fungi.
Network analysis
The online tool Molecular Ecological Network Analysis pipeline (MENAP) was used to construct a phylogenetic Molecular Ecological Network (pMEN) (http://ieg2.ou.edu/MENA). Of these, only the OTUs detected in > half of the samples were used for each network construction. Briefly, the pairwise similarity of the relative abundance data for the different OTUs were calculated based on the Spearman correlation coefficient. The correlation matrix was then converted into a similarity matrix, and the appropriate similarity threshold (St) was automatically defined using the random matrix theory (RMT) before the network was constructed. Once the St had been determined, the adjacency matrix was obtained by retaining an OTU with all similarity values > St determined. It could then be used to construct the molecular ecological networks using MENA. After the molecular ecological network had been constructed, the MENA pipeline was used to calculate various network topological features based on the adjacency matrix and obtain the node and edge files of the corresponding network. All the co-occurrence networks were visualized using Gephi (v. 0.9.3).
Statistical analysis
A Pearson’s correlation test and the relationship between environmental factors and Atractylodes Lactone and Atractylon contents were analyzed by SPSS 20.0 (IBM, Inc., Armonk, NY, USA). A Principal Coordinate Analysis (PCA) was utilized based on the Bray–Curtis Distance using Origin. A Spearman correlation analysis between the soil physiochemical traits and fungal communities was performed using the R (v. 4.1.2) psych package, and the correlation was visualized using the R (Version 4.1.2) ggplot2 package. GraphPad 8.0 (San Jose, CA, USA) was used for the solid drawings.
Data availability
Sequence data that support the findings of this study have been deposited in the National Center for Biotechnology Information with the primary accession code PRJNA1129807: https://www.ncbi.nlm.nih.gov/bioproject PRJNA1129807. All other data generated or analyzed during this study are included in this published article [and its supplementary material files].
References
Zhu, B., Zhang, Q., Hua, J., Cheng, W. & Qin, L. The traditional uses, phytochemistry, and pharmacology of Atractylodes Macrocephala Koidz: A review. J. Ethnopharmacol. 226, 143–167 (2018).
Hai, C. T. et al. Atractylodes Macrocephala rhizomes contain anti-inflammatory sesquiterpenes. Chem. Pharm. Bull. (Tokyo) 71, 451–453 (2023).
Ma, Z. et al. Species Differentiation and quality evaluation for Atractylodes medicinal plants by GC/MS coupled with chemometric analysis. Chem. Biodivers. 20, e202300793 (2023).
Yang, R. et al. Geographical origin traceability of Atractylodis Macrocephalae rhizoma based on chemical composition, chromaticity, and electronic nose. Molecules https://doi.org/10.3390/molecules29214991 (2024).
Zhou, J., Qu, F. & Yu, Y. Chemical and ecological evaluation of a genuine Chinese Medicine: Atractylodes Macrocephala Koidz. Afr. J. Tradit Compl. Altern Med. 8, 405–411 (2011).
Shah, K. K. et al. Role of soil microbes in sustainable crop production and soil health: A review. Agricult. Sci. Technol. https://doi.org/10.15547/ast.2021.02.019 (2021).
Cai, H. et al. Study on chemical fingerprinting of crude and processed Atractylodes Macrocephala from different locations in Zhejiang Province by reversed-phase high-performance liquid chromatography coupled with hierarchical cluster analysis. Pharmacogn. Mag. 8, 300–307 (2012).
Zhang, M., Wang, N., Hu, Y. & Sun, G. Changes in soil physicochemical properties and soil bacterial community in mulberry (Morus Alba L.)/Alfalfa (Medicago Sativa L.) intercropping system. Microbiologyopen 7, 100555 (2018).
Li, Y. et al. The composition of root-associated bacteria and fungi of Astragalus Mongholicus and their relationship with the bioactive ingredients. Front. Microbiol. 12, 642730 (2021).
Dang, H. et al. Differences in the endophytic fungal community and effective ingredients in root of three glycyrrhiza species in Xinjiang, China. PeerJ 9, e11047 (2021).
Song, P. et al. Diversity and structural analysis of rhizosphere soil microbial communities in wild and cultivated rhizoma Atractylodis Macrocephalae and their effects on the accumulation of active components. PeerJ 11, e14841 (2023).
Li, X. et al. Impacts of partial substitution of chemical fertilizer with organic fertilizer on soil organic carbon composition, enzyme activity, and grain yield in wheat-maize rotation. Life-Basel https://doi.org/10.3390/life13091929 (2023).
Borowik, A., Wyszkowska, J., Zaborowska, M. & Kucharski, J. Microbial diversity and enzyme activity as indicators of permethrin-exposed soil health. Molecules 28(12), 4756 (2023).
Shah, K. et al. Role of soil microbes in sustainable crop production and soil health: A review. Agricult. Sci. Technol. 13, 109–118 (2021).
Tan, X. et al. Soil chemical properties rather than the abundance of active and potentially active microorganisms control soil enzyme kinetics. Sci. Total Environ. 770, 144500 (2021).
Hao, L. et al. Arbuscular mycorrhizal fungi alter microbiome structure of rhizosphere soil to enhance maize tolerance to la. Ecotoxicol. Environ. Saf. 212, 111996 (2021).
Hirano, H. & Takemoto, K. Difficulty in inferring microbial community structure based on co-occurrence network approaches. BMC Bioinform. 20, 329 (2019).
Centler, F., Günnigmann, S., Fetzer, I. & Wendeberg, A. Keystone species and modularity in microbial hydrocarbon degradation uncovered by network analysis and association rule mining. Microorganisms https://doi.org/10.3390/microorganisms8020190 (2020).
Guo, J. et al. Soil fungal assemblage complexity is dependent on soil fertility and dominated by deterministic processes. New Phytol. 226, 232–243 (2020).
Sridhar, B. et al. Microbial community shifts correspond with suppression of decomposition 25 years after liming of acidic forest soils. Glob. Change Biol. 28, 5399–5415 (2022).
Feng, C. et al. Biological control of fusarium crown rot of wheat with Chaetomium Globosum 12XP1-2-3 and its effects on rhizosphere microorganisms. Front. Microbiol. 14, 1133025 (2023).
Wang, Y. et al. Regulating root fungal community using Mortierella Alpina for Fusarium Oxysporum resistance in Panax Ginseng. Front. Microbiol. 13, 850917 (2022).
Das, S., Rabha, J. & Narzary, D. Assessment of soil yeasts Papiliotrema Laurentii S-08 and Saitozyma Podzolica S-77 for plant growth promotion and biocontrol of fusarium Wilt of Brinjal. J. Appl. Microbiol. https://doi.org/10.1093/jambio/lxad252 (2023).
van Loon, L. C. Plant responses to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 119, 243–254 (2007).
Vanegas-León, M. L. et al. Are trechisporales ectomycorrhizal or non-mycorrhizal root endophytes?. Mycol. Prog. 18, 1231–1240 (2019).
Gleason, F. H., Carney, L. T., Lilje, O. & Glockling, S. L. Ecological potentials of species of rozella (Cryptomycota). Fungal Ecol. 5, 651–656 (2012).
Grzyb, A., Wolna-Maruwka, A. & Niewiadomska, A. Environmental factors affecting the mineralization of crop residues. Agronomy https://doi.org/10.3390/agronomy10121951 (2020).
Baldrian, P. Wood-inhabiting ligninolytic basidiomycetes in soils: Ecology and constraints for applicability in bioremediation. Fungal Ecol. 1, 4–12 (2008).
Yuan, X. F., Song, T. J., Yang, J. S., Huang, X. G. & Shi, J. Y. Changes of microbial community in the rhizosphere soil of Atractylodes Macrocephala when encountering replant disease. S. Afr. J. Bot. 127, 129–135 (2019).
Zhu, B. et al. Diversity of rhizosphere and endophytic fungi in Atractylodes Macrocephala during continuous cropping. PeerJ 8, e8905 (2020).
Toju, H., Tanabe, A. S. & Sato, H. Network hubs in root-associated fungal metacommunities. Biorxiv. 270371 (2018).
Feng, K. et al. INAP: An integrated network analysis pipeline for microbiome studies. Imeta 1, e13 (2022).
Acknowledgements
The authors appreciate the assistance from the Public Platform of the Medical Research Centre, Academy of Chinese Medical Science, and Zhejiang Chinese Medical University.
Funding
This work was funded by National Natural Science Foundation of China, 82173920.
Author information
Authors and Affiliations
Contributions
YaZ and CG wrote the main manuscript text, JX summarized and organized the raw data and created Figs. 1, 2, and 4. YuZ, SF, NZ, and QM contributed to the creation of the remaining figures. SD and BZ participated in the preliminary investigation of the article. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
In this study, the samples of Atractylodes macrocephala used were collected in accordance with relevant laws, regulations, and institutional requirements. We have obtained the official permit for collecting Atractylodes macrocephala from School of Life Sciences, Zhejiang Chinese Medical University. Sequence data that support the findings of this study have been deposited in the National Center for Biotechnology Information with the primary accession code PRJNA1129807.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, Y., Gu, X., Xu, J. et al. Fungal diversity and network analysis in rhizosphere soil of Atractylodes macrocephala across different cultivation regions. Sci Rep 15, 19889 (2025). https://doi.org/10.1038/s41598-025-96810-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96810-0