Abstract
Obesity, particularly visceral fat, is a well-established risk factor for various gastrointestinal disorders, including constipation, possibly due to its metabolic and hormonal effects on bowel motility, though the mechanisms remain unclear. The Body Roundness Index (BRI), a novel and reliable anthropometric measure, has been validated as a precise indicator of visceral fat. However, its relationship with constipation remains uncertain. This population-based, cross-sectional study analyzed data from 6,898 participants in the National Health and Nutrition Examination Survey (NHANES, 2005–2010) to assess this association. Binary logistic regression analyses, adjusted for multiple covariates, revealed that higher BRI levels were significantly associated with a lower likelihood of constipation. Participants in the highest quartile had 32% lower odds of constipation than those in the lowest quartile (adjusted OR: 0.68; 95% CI: 0.54–0.86; P: 0.0013). As this was a cross-sectional study, the findings indicate an association between BRI levels and constipation rather than causality. These results suggest that BRI could be a practical and accessible marker for identifying individuals with different susceptibility to constipation, potentially aiding clinical assessment and guiding preventive strategies.
Similar content being viewed by others
Introduction
Constipation is a prevalent gastrointestinal disorder affecting a substantial proportion of the global population. Epidemiological studies estimate that approximately 9–20% of individuals in the United States experience constipation1. Chronic constipation not only diminishes quality of life but also contributes to anxiety, depression, and a higher healthcare burden2,3,4. The etiology of constipation is multifactorial and is generally classified as organic, functional, or medication-induced5,6,7.
Recent studies have explored the potential role of visceral fat accumulation in constipation development, as obesity is increasingly recognized as a risk factor for gastrointestinal dysfunction. Several studies have reported a higher prevalence of constipation in obese individuals, particularly those with excessive visceral fat. For example, one study found that adults with a BMI of 25 to 39.9 kg/m² had a significantly higher incidence of constipation8. Another study in Iran reported that approximately 60% of patients with functional constipation had elevated BMI, which may be associated with alterations in biologically active substances and gut microbiota. Biologically active substances (such as pro-inflammatory cytokines like TNF-α, IL- 6, and IL- 1β) may contribute to the risk of gastrointestinal disorders and the imbalance in gut microbiota, which is related to obesity, may reduce gut motility and contribute to constipation9. A large-scale study in China further confirmed that obese individuals (BMI ≥ 30 kg/m²) had a higher prevalence of chronic constipation than those with normal weight10.
Conversely, some studies have failed to establish a significant association between obesity and constipation11, and others have paradoxically reported a lower prevalence of constipation among obese individuals compared to the general population12. These discrepancies may arise from differences in study populations, constipation definitions, and lifestyle factors, highlighting the need for further investigation.
While BMI is commonly used to assess obesity, it does not differentiate between visceral and subcutaneous fat, limiting its utility in predicting metabolic health risks. Alternative anthropometric indices, such as waist circumference (WC), hip circumference (HC), and body fat percentage (BFP), are also frequently used to assess body fat distribution13,14,15,16,17. Among these, WC is commonly used as a surrogate marker for central obesity and has been shown to correlate with visceral adipose tissue (VAT). However, WC alone cannot accurately reflect visceral fat18,19. HC primarily reflects subcutaneous fat in the gluteofemoral region, which may have different metabolic implications20. FP, measured using bioelectrical impedance analysis (BIA) or dual-energy X-ray absorptiometry (DXA), provides an estimate of total adiposity but does not distinguish fat distribution. These limitations underscore the need for improved anthropometric measures to more accurately assess visceral adiposity.
Given the limitations of BMI and other conventional indices, the Body Roundness Index (BRI) has emerged as a promising alternative for assessing visceral adiposity. By incorporating waist circumference and height, BRI provides a more precise estimation of abdominal obesity21. Unlike BMI, which does not account for fat distribution, the BRI specifically quantifies central adiposity, a key determinant of metabolic and gastrointestinal disorders. Emerging evidence suggests that BRI outperforms BMI in predicting visceral adipose tissue (VAT) accumulation and obesity-related health risks22,23. And BRI has demonstrated superior predictive ability for various clinical conditions, including cardiometabolic diseases24,25, kidney disease26, and cancer27. Moreover, while CT and MRI remain the gold standard for visceral fat measurement, their high costs and technical constraints limit large-scale application. In contrast, BRI offers a non-invasive, cost-effective, and accessible alternative for visceral adiposity assessment in epidemiological and clinical research28,29.
Despite its advantages, research on the association between the BRI and constipation remains limited. As BRI provides a more precise assessment of visceral adiposity, it offers a novel perspective on its potential role in constipation among overweight and obese individuals. Given the strong association between visceral fat accumulation and gastrointestinal motility disorders, investigating BRI in relation to constipation may enhance risk stratification and deepen our understanding of obesity-related gastrointestinal dysfunction.
This study, utilizing data from 6,898 individuals obtained through the NHANES, aimed to achieve two primary objectives: (1) to investigate the association between the BRI and constipation risk, and (2) to propose methods for further elucidating the relationship between visceral fat and constipation.
Materials and methods
Survey description and study population
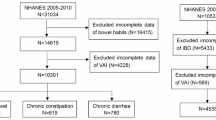
The complete dataset is publicly accessible on the NHANES website. All NHANES participants provided written informed consent through an opt-in process, ensuring voluntary participation in accordance with established ethical guidelines. This study included individuals from NHANES survey cycles conducted between 2005 and 2010, excluding participants younger than 20 years, those with incomplete clinical data, pregnant individuals, those diagnosed with malignant disease or inflammatory bowel disease, and individuals classified as type 6 or 7 on the Bristol Stool Scale. After excluding participants with missing data in the independent variable, dependent variable, or covariates, a total of 6,898 participants were included in the final analysis (Fig. 1).
Data collection and measurements
Data collection and measurements were obtained from the NHANES database. The database encompasses a wide range of information, including demographic data (e.g., race, gender, and age); nutritional information; anthropometric measurements such as waist circumference (WC) and height; laboratory data (e.g., fasting blood glucose, glycosylated hemoglobin, and arterial pressure); and questionnaire responses covering alcohol consumption, tobacco use, and pre-existing health conditions. And dietary intake data were collected using two 24-hour dietary recall interviews, with a follow-up conducted via telephone 3 to 10 days later. Nutrient and energy intakes were calculated using the Food and Nutrient Database for Dietary Studies (FNDDS). Interviews were conducted by trained personnel in a Mobile Examination Center (MEC) using the USDA Automated Multiple-Pass Method, a validated approach designed to ensure accurate and comprehensive dietary data collection.
Assessment of body roundness index
BRI was utilized as the independent variable in this study. The BRI was calculated using the formula established in previous studies. The BRI was calculated according to the following formula: BRI = 364.2–365.5 × √(1 - ([WC (m)/2π]²/[0.5 × height (m)]²))21. To facilitate its calculation, we have developed a web-based calculator, which we have included as supplementary material for this study.
Definition of constipation
Between 2005 and 2010, NHANES introduced the Bowel Health Questionnaire to document bowel movement frequency and stool characteristics. Stool characteristics were assessed using vividly colored cards depicting the seven-point Bristol Stool Form Scale (BSFS), from which participants selected the number corresponding to their typical stool form. Bowel movement frequency was determined based on self-reported weekly counts. These data were essential for identifying individuals classified as constipated30. Our research employed two definitions of constipation31. In the first definition, participants were classified as constipated if they reported stool characteristics corresponding to type 1 (separate hard lumps, like nuts) or type 2 (sausage-shaped but lumpy) on the Bristol Stool Form Scale (BSFS). Stool types 3 to 5 were categorized as normal: type 3 (sausage-like with cracks on the surface), type 4 (smooth and soft, sausage- or snake-like), and type 5 (soft blobs with clear edges). Stool types 6 (fluffy pieces with ragged edges, mushy stool) and 7 (watery with no solid pieces) were not considered part of the normal population and were excluded from the study32. Additionally, constipation was defined based on self-reported bowel movement frequency. Participants were classified as constipated if they reported having two or fewer bowel movements per week. To minimize bias arising from different constipation definitions, we conducted separate analyses using stool form and bowel movement frequency as independent criteria. The Rome IV criteria were not applied, as NHANES lacks detailed symptom-based assessments, such as straining or incomplete evacuation, which are essential for a full Rome IV diagnosis. Furthermore, applying these criteria ensures consistency with previous NHANES-based studies and facilitates epidemiological analyses in large populations.
Covariates
To address potential confounding effects in the relationship between BRI and constipation, we utilized multivariable adjustment models to account for various confounding factors. In this study, we adjusted for several factors, including sex (male or female), age (< 40 years, 40–64 years, and ≥ 65 years), race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, and Other Race), education level (below high school, high school graduate, and above high school), poverty-to-income ratio (PIR; < 2 or ≥ 2), metabolic equivalent of task (MET; < 500 or ≥ 500), depressive symptoms (non-depressed or depressed), sleep duration (< 5 h, 5–6 h, and ≥ 9 h), smoking and alcohol use habits, and medical conditions such as diabetes and hypertension. Additionally, we assessed the influence of dietary components, including caloric intake, protein, carbohydrates, dietary fiber, fat, magnesium, selenium, and caffeine consumption. Moreover, serum vitamin D concentrations were assessed in the study.
Depression was assessed using the NHANES Mental Health Questionnaire, with a PHQ- 9 score ≥ 10 indicating depression. Diabetes was defined as: (1) physician-diagnosed diabetes; (2) 2-hour post-OGTT or random blood glucose ≥ 11.1 mmol/L; (3) fasting blood glucose ≥ 7.0 mmol/L; or (4) HbA1c ≥ 6.5%. Hypertension was defined as blood pressure ≥ 140/90 mmHg or current antihypertensive use. Physical activity was quantified using metabolic equivalents (METs), derived from a physical activity questionnaire. Following U.S. Department of Health and Human Services guidelines, participants with < 500 MET-min/week were classified as non-active, while those with ≥ 500 MET-min/week were considered active. Alcohol consumption was categorized based on self-reported intake over the past 12 months as “nondrinkers” or “drinkers.” Smoking status was classified as “smokers” (≥ 100 lifetime cigarettes) or “nonsmokers.” Dietary intake was averaged from two 24-hour dietary recalls, providing detailed nutrient data. Measurement and quantification details are available on the NHANES website.
Statistical analyses
The statistical analyses were conducted in accordance with the recommendations of the Centers for Disease Control and Prevention (CDC). Advanced statistical methods were employed in this investigation. The methodology involved dividing the BRI into quartiles, with the lowest quartile (Q1) serving as the reference group. Categorical data were presented as frequencies and percentages, while continuous variables were reported as means and standard deviations.
Three multivariable logistic regression models were employed to investigate the association between BRI and constipation, with constipation explicitly defined as the outcome variable. Model 1 was unadjusted. Model 2 was adjusted for sex, age, and race. Model 3 included further adjustments for education level, income-to-poverty ratio, MET score, depressive symptoms, sleep duration, smoking status, drinking status, diabetes, hypertension, serum vitaminD, and nutritional factors—specifically energy, protein, carbohydrates, dietary fiber, fat intake, magnesium, selenium, and caffeine—while retaining adjustments for sex, age, and race. Given the significance of dietary factors, we examined their impact on the BRI-constipation relationship. As no significant changes were observed, the fully adjusted model was adopted.
This study examined the nonlinear relationship using smoothed curve fitting and applied a segmented regression model to fit each interval and assess the threshold effect. As a single linear model might not fully capture the relationship, a log-likelihood ratio test was performed to compare it with a two-segment model, confirming the presence of a threshold. The inflection point (K) was further identified using a two-step recursive method. Subgroup analyses and interaction tests were conducted to comprehensively explore potential variations within the population. Logistic regression was applied to analyze binary dependent variables and to test linear trends between ordinal categorical variables and the dependent variable. The chi-square test was employed to compare percentages (%) of categorical variables. The Kruskal-Wallis H test was employed to evaluate continuous variables, with results reported as means ± standard deviations (SD).
All analyses were performed using R software (version 4.4.2; http://www.R-project.org) and EmpowerStats (version 4.2; http://www.empowerstats.com). And the P-value < 0.05 was considered statistically significant.
Ethics approval and consent to participate
This study is based on publicly available data from the National Health and Nutrition Examination Survey, which is conducted by the National Center for Health Statistics (NCHS). The NHANES protocol was approved by the NCHS Ethics Review Board, and all participants provided written informed consent.
Results
Baseline characteristics of participants
Table1 summarizes the baseline characteristics of participants selected from the NHANES 2005–2010 cycles. A total of 6,898 participants were included in the study. Significant differences were observed between the constipation and non-constipation groups in terms of gender, age, ethnicity, education level, PIR, smoking status, alcohol consumption, sleep duration, depressive symptoms, MET, and dietary factors, including total energy intake, protein, carbohydrates, dietary fiber, total fat, magnesium, selenium, and caffeine intake. However, no significant differences were found in diabetes prevalence, or vitamin D levels between the two groups
Relationship between BRI and constipation
The present study identified an inverse relationship between BRI and the likelihood of experiencing constipation. BRI was analyzed as a categorical variable stratified into quartiles. As presented in Table 2, individuals in the highest quartile (Q4) exhibited a 32% lower likelihood of experiencing constipation compared to those in the lowest quartile (Q1) in a fully adjusted model accounting for all covariates (OR: 0.68; 95% CI: 0.54–0.86; P: 0.0013). We also found no significant results when using stool shape or bowel movement frequency alone. Therefore, we retained the combined definition and reported alternative results in the supplementary materials. These findings suggest a potential inverse association between higher BRI and constipation risk. Given the high prevalence of constipation and its impact on quality of life, BRI may serve as a modifiable factor in public health interventions. Maintaining an appropriate level of visceral fat could help reduce constipation risk, supporting the development of preventive strategies.
Non-linear relationship between BRI and constipation
This study examined the nonlinear association between BRI and the risk of constipation, as illustrated in Fig. 2. A detailed analysis of the smoothed curves was conducted to elucidate this relationship. The likelihood ratio test showed a p-value of 0.004 for the association between BRI and constipation occurrence. A two-segment linear regression model with a recursive algorithm was applied to determine the inflection point, which was identified at a BRI of 3.19. Below this threshold, each unit increase in BRI was associated with a 45% higher risk of constipation (OR: 1.45; 95%CI: 1.07–1.96; P: 0.017). Conversely, when BRI exceeded 3.19, each unit increase was associated with a 8% reduction in constipation risk (OR: 0.92; 95%CI: 0.88–0.95; P < 0.001). To evaluate the potential impact of outlier-truncated data, we excluded the top and bottom 1% of participants and conducted a new threshold effect analysis. The results remained consistent with the original findings, showing no significant differences, and the results are placed in the supplementary materials. Therefore, we retained the original results (Table 3).
Subgroup analyses
In order to test the reliability of the association between BRI and constipation, further subgroup analyses were conducted. The findings revealed that the negative relationship between BRI and constipation was observed in most subgroups (P < 0.05) (Fig. 3). Interaction terms were not significant in any subgroup (all P-values for interaction > 0.05). Additionally, subgroup analyses based on energy intake and dietary fiber yielded similar results, which are provided in the supplementary materials.
Disscussion
This study provides novel insights into the relationship between the BRI and constipation, highlighting a distinct nonlinear association. Using data from a large and representative NHANES cohort of 6,898 participants, we identified an inflection point (BRI = 3.19) that marks a shift in the relationship. Below this threshold, each unit increase in BRI was associated with a 45% increase in the odds of constipation. However, for BRI values exceeding 3.19, the possibility of constipation decreased by 8% with each unit increase. These findings suggest that the influence of visceral adiposity on bowel function varies depending on the degree of adiposity, with opposing effects observed at different BRI levels.
These findings align with and expand upon previous research on obesity indices and bowel symptoms. Xiang et al.13reported that a higher Waist-to-Waist Index (WWI) was associated with an increased risk of constipation, underscoring the role of central obesity in gastrointestinal motility disorders. Similarly, previous studies have shown that abdominal obesity, commonly assessed using the visceral adiposity index (VAI) and waist-to-height ratio (WHtR), is associated with altered bowel habits and functional constipation14,15. Compared to traditional anthropometric measures such as BMI and waist circumference, the BRI accounts for both body shape and fat distribution, potentially offering a more accurate assessment of adiposity-related health risks. Previous studies have demonstrated that BRI is a strong predictor of metabolic syndrome and cardiovascular diseases25, and our findings suggest that it may also be useful in assessing gastrointestinal dysfunction.
The relationship between body composition and gut microbiota has garnered increasing attention in recent years. Visceral fat accumulation has been linked to microbiota dysbiosis, characterized by reduced microbial diversity and an increase in pro-inflammatory bacterial species33,34. This altered microbiota profile may contribute to slowed colonic transit and an increased probability of constipation. Other potential mechanisms include obesity-related gut dysbiosis, such as small intestinal bacterial overgrowth (SIBO), as well as hypothalamic-pituitary-adrenal (HPA) axis overactivity, which may reduce the likelihood of constipation by stimulating intestinal motility, enhancing secretion, and modulating gut inflammation35.
Dietary intake plays a crucial role in regulating bowel habits. Insufficient fiber and fluid intake have been identified as major contributors to constipation30. Physical activity has also been shown to modulate the effects of dietary fiber on constipation risk32. Given that individuals with higher BRI often exhibit poorer dietary habits, including lower fiber intake and higher consumption of processed foods, it is possible that dietary factors may mediate the observed nonlinear relationship between BRI and constipation. Future studies should further investigate these interactions.
Interestingly, our study found that individuals with lower BRI exhibited a paradoxical increase in susceptibility to constipation. One possible explanation is that lower visceral fat levels may contribute to pelvic floor instability, impairing normal defecation mechanics36,37. Additionally, lean individuals with constipation may have underlying functional bowel disorders, such as irritable bowel syndrome with constipation (IBS-C), which is influenced by factors beyond adiposity, including gut-brain axis dysfunction and psychological stress. Further research is needed to explore these mechanisms in greater depth.
This study has several strengths, including its large and representative sample, rigorous quality control, and comprehensive adjustment for potential confounders. However, certain limitations should be acknowledged. First, the cross-sectional design prevents the establishment of causal relationships between BRI and constipation risk. Second, reliance on self-reported data for constipation diagnosis may introduce recall bias and subjectivity. Third, while we adjusted for multiple covariates, the possibility of unmeasured confounders influencing the association cannot be entirely ruled out.
Conclusion
The findings of this study demonstrate a negative association between a higher BRI and the likelihood of constipation, suggesting that an appropriate increase in visceral fat levels may help prevent constipation. However, this observational study cannot establish causality, and the underlying mechanisms remain unclear. It is possible that visceral adiposity influences bowel function through metabolic or hormonal pathways, but further research is needed to clarify this relationship. Prospective and clinical studies are necessary to confirm the potential role of visceral fat in constipation development and to explore the biological mechanisms underlying this association.
Data availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References. The dataset supporting the conclusions of this article is available in the NHANES repository, https://www.cdc.gov/nchs/nhanes/index.htm.
References
Oh, S. J. et al. Chronic constipation in the united States: results from a Population-Based survey assessing healthcare seeking and use of pharmacotherapy. Am. J. Gastroenterol. 115 (6), 895–905 (2020).
Rao, S. S. et al. Psychological profiles and quality of life differ between patients with dyssynergia and those with slow transit constipation. J. Psychosom. Res. 63 (4), 441–449 (2007).
Benninga, M. A. & Scott, S. M. Chronic constipation. Preface. Best Pract. Res. Clin. Gastroenterol. 25 (1), 1–2 (2011).
Rao, S. S., Rattanakovit, K. & Patcharatrakul, T. Diagnosis and management of chronic constipation in adults. Nat. Reviews Gastroenterol. Hepatol. 13 (5), 295–305 (2016).
Milosavljevic, T. et al. Chronic constipation: gastroenterohepatologist’s approach. Dig. Dis. (Basel Switzerland). 40 (2), 175–180 (2022).
Morshedzadeh, N. et al. The effects of flaxseed supplementation on metabolic syndrome parameters, insulin resistance and inflammation in ulcerative colitis patients: an open-labeled randomized controlled trial. Phytother. Res. 35 (7), 3781–3791 (2021).
Keshavarz, Z. et al. Non-alcoholic fatty liver disease and dairy products consumption: results from FASA Persian cohort study. Front. Nutr. 9, 962834 (2022).
Silveira, E. A. et al. Prevalence of constipation in adults with obesity class II and III and associated factors. BMC Gastroenterol. 21 (1), 217 (2021).
Pourhoseingholi, M. A. et al. Obesity and functional constipation; a community-based study in Iran. J. Gastrointest. Liver Diseases: JGLD. 18 (2), 151–155 (2009).
Chen, K. et al. Prevalence of obesity and associated complications in China: A cross-sectional, real-world study in 15.8 million adults. Diabetes Obes. Metab. 25 (11), 3390–3399 (2023).
Delgado-Aros, S. et al. 3rd: obesity is associated with increased risk of Gastrointestinal symptoms: a population-based study. Am. J. Gastroenterol. 99 (9), 1801–1806 (2004).
Talley, N. J., Howell, S. & Poulton, R. Obesity and chronic Gastrointestinal tract symptoms in young adults: a birth cohort study. Am. J. Gastroenterol. 99 (9), 1807–1814 (2004).
Xiang, N., Xu, L., Qian, H. & Zhang, D. Multiple obesity indices suggest a close relationship between obesity and constipation: evidence from NHANES. BMC Public. Health. 24 (1), 1273 (2024).
Yang, X. et al. Association between visceral adiposity index and bowel habits and inflammatory bowel disease: a cross-sectional study. Sci. Rep. 14 (1), 23923 (2024).
Wan, Y. et al. The impact of visceral obesity on chronic constipation, inflammation, immune function and cognitive function in patients with inflammatory bowel disease. Aging 13 (5), 6702–6711 (2021).
Zazai, R., Wilms, B., Ernst, B., Thurnheer, M. & Schultes, B. Waist circumference and related anthropometric indices are associated with metabolic traits in severely obese subjects. Obes. Surg. 24 (5), 777–782 (2014).
Jabłonowska-Lietz, B., Wrzosek, M., Włodarczyk, M. & Nowicka, G. New indexes of body fat distribution, visceral adiposity index, body adiposity index, waist-to-height ratio, and metabolic disturbances in the obese. Kardiologia Polska. 75 (11), 1185–1191 (2017).
Yumuk, V. et al. European guidelines for obesity management in adults. Obes. Facts. 8 (6), 402–424 (2015).
Salmón-Gómez, L., Catalán, V., Frühbeck, G. & Gómez-Ambrosi, J. Relevance of body composition in phenotyping the obesities. Reviews Endocr. Metabolic Disorders. 24 (5), 809–823 (2023).
Christiansen, M. R. et al. Abdominal and gluteofemoral fat depots show opposing associations with postprandial lipemia. Am. J. Clin. Nutr. 114 (4), 1467–1475 (2021).
Thomas, D. M. et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obes. (Silver Spring Md). 21 (11), 2264–2271 (2013).
Liu, X. et al. Association between a body shape index and prostate cancer: a cross-sectional study of NHANES 2001–2018. Int. Urol. Nephrol. 56 (6), 1869–1877 (2024).
Zhang, Y. et al. Association between body roundness index and overactive bladder: results from the NHANES 2005–2018. Lipids Health Dis. 23 (1), 184 (2024).
Cai, X. et al. Body roundness index improves the predictive value of cardiovascular disease risk in hypertensive patients with obstructive sleep apnea: a cohort study. Clinical and experimental hypertension (New York, NY: 2023, 45(1):2259132. (1993).
Rico-Martín, S. et al. Effectiveness of body roundness index in predicting metabolic syndrome: A systematic review and meta-analysis. Obes. Reviews: Official J. Int. Association Study Obes. 21 (7), e13023 (2020).
Zhang, Y. et al. Body roundness index is related to the low estimated glomerular filtration rate in Chinese population: A cross-sectional study. Front. Endocrinol. 14, 1148662 (2023).
Gao, W. et al. The association between the body roundness index and the risk of colorectal cancer: a cross-sectional study. Lipids Health Dis. 22 (1), 53 (2023).
Xu, Z. et al. Measurement of visceral fat and abdominal obesity by single-frequency bioelectrical impedance and CT: a cross-sectional study. BMJ Open. 11 (10), e048221 (2021).
Maskarinec, G. et al. Subcutaneous and visceral fat assessment by DXA and MRI in older adults and children. Obes. (Silver Spring Md). 30 (4), 920–930 (2022).
Markland, A. D. et al. Association of low dietary intake of fiber and liquids with constipation: evidence from the National health and nutrition examination survey. Am. J. Gastroenterol. 108 (5), 796–803 (2013).
Wilson, P. B. Associations between physical activity and constipation in adult Americans: results from the National health and nutrition examination survey. Neurogastroenterol. Motil. 32 (5), e13789 (2020).
Li, Y., Tong, W. D. & Qian, Y. Effect of physical activity on the association between dietary fiber and constipation: evidence from the National health and nutrition examination survey 2005–2010. J. Neurogastroenterol. Motil. 27 (1), 97–107 (2021).
Yan, H. et al. Gut Microbiome alterations in patients with visceral obesity based on quantitative computed tomography. Front. Cell. Infect. Microbiol. 11, 823262 (2021).
Nie, X. et al. A metagenome-wide association study of gut Microbiome and visceral fat accumulation. Comput. Struct. Biotechnol. J. 18, 2596–2609 (2020).
Jung, S. E., Joo, N. S., Han, K. S. & Kim, K. N. Obesity is inversely related to hydrogen-producing small intestinal bacterial overgrowth in non-constipation irritable bowel syndrome. J. Korean Med. Sci. 32(6), 948–953 (2017).
Joubert, K. & Laryea, J. A. Abdominal approaches to rectal prolapse. Clin. Colon Rectal Surg. 30 (1), 57–62 (2017).
Jeong, H. Y., Park, D. H. & Lee, J. K. Levator plate descent angle in pelvic floor disorders. Tech. Coloproctol. 25 (9), 1011–1018 (2021).
Acknowledgements
This work was supported by grants from the Major project of Natural Science Foundation of China (92148201).
Author information
Authors and Affiliations
Contributions
Conceptualization: CG, XY. Methodology: CG, QP Software: CG, QP, XY. Validation: CG, QP, XY. Formal analysis: CG, QA. Investigation: CG, ZZ. Resources: QP. Data curation: CG. Writing-original draft: CG, QP. Visualization: CG, QA. Supervision: XY. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, C., Peng, Q., Ao, Q. et al. Association between body roundness index and constipation using data from NHANES 2005–2010. Sci Rep 15, 13271 (2025). https://doi.org/10.1038/s41598-025-96876-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96876-w