Abstract
Spaceflight exposes astronauts to unique conditions like microgravity, which may affect brain function, though it remains underexplored compared to other physiological systems. Astronauts often report temporary neurological symptoms, such as disorientation, visual disturbances, and motor issues, potentially linked to structural and electrophysiological brain changes. To investigate this, electroencephalography (EEG) is a reliable tool to study brain activity in space, measuring oscillatory activity and functional connectivity (FC). This study analyzed EEG data from five male astronauts during three stages: pre-flight, during low Earth orbit (LEO), and post-flight in a 2-min task-free eyes-closed (EC) condition followed by another 2-min of eyes-open (EO) condition. The focus was on beta band (12–30 Hz) activity, which is associated with motor control and proprioception. Results showed increased beta power during spaceflight when compared to pre-flight (EC: p < 0.01) and post-flight (EC: p < 0.01; EO: p < 0.05) conditions. FC strength also increased during spaceflight when compared to pre-flight (EO: p < 0.05) and post-flight (EC: p < 0.01; EO: p < 0.01) conditions. These differences were found primarily in the sensorimotor cortex (SMC) and frontotemporal regions, suggesting the brain’s adaptation to altered vestibular and proprioceptive inputs during microgravity. As these results reflect astronaut’s movement adaptation to microgravity, this study highlights the importance of understanding central nervous system (CNS) changes during spaceflights to ensure optimal performance and protect astronaut’s health during long-duration missions.
Similar content being viewed by others
Introduction
During spaceflights, human’s health and performance is affected by environmental conditions such as isolation, radiation, and microgravity1,2. Understanding how such an extreme environment could affect human physiology is crucial to safeguard the well-being of the astronauts and mission success.
Although research in different systems such as cardiovascular, ocular, and others have been frequently investigated, research on the impact of spaceflight on the central nervous system has been scarce. Research on changes in brain functions and structure3,4,5 is of great importance to develop strategies that could mitigate some of the negative effects during exploration class missions6,7,8. While severe impairments have not been documented in astronauts, they have typically reported temporary disorientation, spatial illusions, visual disturbances, sleep alterations, motor deficiencies, and reduced performance9,10,11. These subjective reports seem to be associated with morphological/structural brain changes12,13 as well as with electrophysiological changes14,15,16. Surveying the brain’s function during all aspects of missions will help address their relevance and potential clinical significance.
Although the assessment of crewmember’s cognitive function and performance has been primarily dominated by neuropsychological tests5,14,17,18, these batteries are subjected to limitations19, especially in differentiating subjects in preclinical stages from healthy participants20. That is why techniques such as electroencephalography (EEG) and magnetoencephalography (MEG) are effective in identifying preclinical biomarkers and monitoring the progression of brain changes21.
Structural changes have been observed in astronaut’s brain MRIs (Magnetic Resonance Imaging) after returning from a 6-months mission aboard the International Space Station (ISS), and it has been estimated that more than 50% of the crewmembers may be affected by such structural changes12,22. However, due to the limitations in measuring these changes in space, alternative methods are required. In this context, EEG has proven to be a feasible and reliable neurophysiological technique better suited for studying brain changes before, after, and most importantly during spaceflight14,15,16,23,24,25. EEG is non-invasive, the recordings can be obtained as many times as needed, the equipment is light and small, and the feasibility for high-quality recordings with dry-EEG electrodes has been already demonstrated24.
EEG directly measures global neuronal electrical activity, and it has been used on more than 50 previous flight missions as a part of polysomnography and cognitive studies26,27,28. The dynamics of EEG rhythms can be characterized by power spectrum and synchrony measurements29, which can be interpreted as the responsiveness of brain areas and their communication, respectively. Spectral power represents the amount of activity in a specific frequency band [delta (2–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and gamma (30–45 Hz)]. Synchrony can be measured by functional connectivity (FC) analysis, which refers to the synchronization between the measured activity of two or more brain regions in terms of phase or amplitude, indicating statistical dependencies between them29,30.
EEG high temporal resolution31 allows the detection of both rapid and long shifts of brain activity / responsiveness. Considering this evidence, it becomes vital to broaden our insight into how cognitive processes are influenced by the conditions during short (from a few minutes to a few weeks), and long (from one month to a year or longer) spaceflights. The relevance of utilizing EEG for functional assessment is emphasized by the fact that modifications in brain function could manifest before structural brain abnormalities32. The reliability of this method has been proven in clinical research33.
When in low Earth orbit (LEO), astronauts are in a weightlessness condition. During this time, astronauts need to adapt to the new environment, where the microgravity causes altered vestibular and proprioceptive inputs that lead to a sensorimotor adaptation6,12,34. Because of this, upon return to Earth, the astronauts exposed to long-duration missions need to readapt to the gravitational environment. Several astronauts have reported disturbances in perception, spatial orientation, posture, walking patterns, and eye-head coordination35,36.
One of the EEG frequency bands typically associated with sensorimotor activity is the beta band. The beta band activity is prevalent in the sensorimotor cortex (SMC), both in the precentral gyrus and postcentral gyrus37, and it is related to proprioceptive processing, motor activity, and motor learning37,38,39. These oscillations increase during stable postures and movement cancelation, and decrease during movement planning and execution40. This cortical beta activity has been associated with an increased interneuron-mediated GABA activity41,42 and acts as a top-down inhibitory rhythm40.
The analyzed dataset used in this study is a part of the NEUROSPAT experiment15,16. We performed a retrospective analysis of the EEG data obtained during long-duration ISS missions, to evaluate functional brain characteristics in three different conditions: pre-flight (ground level), in LEO, and post-flight (ground level). Our objective is to assess the changes of power and FC in the beta band, and the persistence of these changes after returning to Earth. Due to the relationship between beta frequency band and motor control, we hypothesize that microgravity conditions could modify the brain activity in this frequency band.
Methods
The dataset was collected as part of the NEUROSPAT study (AO-2004, 118)10,15,16,43, which involved five male astronauts with an average age of 54.2 ± 2.6 years. These participants spent approximately six months (174.6 ± 19.9 days) in low Earth orbit. To standardize sleep duration before the recordings, astronauts completed a sleep survey and were given 8.5 h of rest the preceding night. The recordings were not carried out within 48 h after air travel that crossed more than 4 time zones, nor after work shifts leading to a time shift greater than 4 h, nor the day following imposed sleep deprivation, nor following demanding mental or physical activities, such as centrifuge training, vestibular countermeasures tests, and extravehicular activities. Astronauts were instructed to maintain their usual caffeine intake and to abstain from alcohol or medication for 16 h before the recordings. Recordings were conducted at approximately the same time each day, with a margin of ± 2 h, preferably in the morning. Participants were assessed in three conditions (Table 1).
Ethics statement
All experimental procedures were approved by the European Space Agency Medical Care Committee and the NASA Johnson Space Center Institutional Review Board for Human Testing, in compliance with the Helsinki Declaration of 1964. Each participant provided written informed consent before the experiment began.
Data acquisition
EEG was used to measure brain activity in all participants during two-minute, task-free eyes-closed (EC) and eyes-open (EO) conditions15. On Earth, participants were comfortably seated in a chair, while in space, they were free-floating with their movement restricted by a waist belt attached to straps and secured to metal rings on the racks of the Columbus module in the ISS. To avoid visual distractions, astronauts wore a face mask within a cylindrical tube attached to the laptop screen. This setup was identical for recordings on Earth and in the ISS.
EEG data were collected at a sampling rate of 1116 Hz using the 59-channel electroencephalogram mapping module (MEEMM) of the European physiology module. This system was installed in the Columbus module of the ISS, and recordings were made at the European Astronaut Center (Köln, Germany) or in Star City (Moscow). The MEEMM employed a dedicated physical reference electrode located on the right earlobe. Some post-flight sessions at the Johnson Space Center (Houston) used an asalab 64-channel amplifier (ANT Neuro BV, Hengelo, Netherlands) in a standard lab environment with a 1024 Hz sampling rate. This amplifier is a stationary DC-EEG system with a common average reference. Electrode impedance on the scalp was maintained below 5 kΩ throughout all sessions.
Data preprocessing
A set of identical 55 channels, standardized according to the 10-10 system, was selected from both the MEEMM and asalab systems to ensure a homogeneous layout and spatial coverage of the head. Bad channels were automatically detected by assessing the mean power spectral density (PSD) in the 70–100 Hz frequency range. A channel was marked as bad if its PSD exceeded the mean PSD + three standard deviations across all 55 channels in the dataset24. These identified bad channels were then interpolated using spherical splines44, and the DC offset of each channel was removed. After bad channel correction, data were re-referenced to a common average reference. Ocular artifacts were identified and removed using principal component analysis (PCA) in ASA software (ANT Neuro BV, Hengelo, Netherlands), with components removed if they accounted for 95% of the variance within the noise subspace. Muscle artifacts were automatically detected and excluded using the FieldTrip package45. Any remaining artifacts were removed following expert visual inspection. The resulting clean data was divided into four-second epochs with a two-second overlap, yielding an average of 23 ± 3 epochs for eyes-closed (EC) and 18 ± 4 epochs for eyes-open (EO) across subjects and conditions. Finally, for the power and functional connectivity analysis, these clean EEG time series were band-pass filtered between 2 and 45 Hz. This filtering used a high-order (1500) finite impulse response (FIR) filter with a Hamming window and was executed using a two-pass filtering process with 2 s padding. We therefore applied it to avoid any signal characteristics changes due to filter-related phase shifts.
Source reconstruction
Source activity was estimated using a template MRI based on the New York Head (ICBM-NY)46, with a 3-layer BEM head model, a regular volumetric grid with a 10 mm spacing source model, and default electrode positions. The forward model was solved using OpenMEEG47. For each subject, sources were independently reconstructed using the exact low-resolution brain electromagnetic tomography (eLORETA) method48 with a regularization factor of 10⁻⁸. Each source location was assigned a label based on the 90 regions defined in the Automated Anatomical Labeling (AAL) atlas49.The whole brain anatomical model here used comprised 78 cortical and subcortical areas mapped by the AAL atlas (excluding the cerebellum, basal ganglia, thalamus, and olfactory cortices), resulting in a total of 1202 source positions for subsequent analysis.
The power spectrum for each source was computed for each trial using the averaged perdiodogram approach with multi-taper Discrete Prolate Spheroidal Sequences (DPSS) with 1 Hz smoothing. Relative power was calculated by normalizing the power spectrum at each source position to the total power across the 2–45 Hz range. The average power for the anatomical model within the beta frequency band was determined by averaging across all epochs and relevant sources, and summing the corresponding frequency steps. This resulted in a reconstructed power matrix for each condition, organized by stages x participants.
To quantify FC, we employed the phase locking value (PLV) metric50. PLV is a robust measure of phase synchronization that assesses the consistency of phase differences between the time series of two brain source positions. The reliability of this metric has been substantiated by its high inter-session stability51. For the anatomical model, symmetrical matrices of 1202 × 1202 sources were obtained by averaging PLV values across trials for each condition and participant. Then, these matrices were averaged to obtain one single nodal strength value (also known as weighted global connectivity), that represented a marker of whole brain FC, for each participant and condition.
Statistical analysis
Statistical analysis was conducted using Prism 10 Software (GraphPad version 10.0.2 https://www.graphpad.com/, San Diego, CA, USA). The power spectrum and functional connectivity (FC) values obtained under the three conditions were statistically compared. Differences in beta band relative power and functional connectivity (FC) strength were also assessed between the eyes-closed and eyes-open conditions. Depending on the number of independent variables, data normality (Shapiro–Wilk test), and equality of variances (sphericity test) of the groups, either a one-way ANOVA or a two-way repeated measures ANOVA with Tukey’s multiple comparison test was applied. For repeated measures with reduced sphericity, Geisser and Greenhouse’s correction method was used. Results are presented as mean ± standard deviation (SD), with p values noted as follows: *p < 0.05, **p < 0.01, ***p < 0.001. Additionally, q values with a significance level > 5 corresponds with a minimum p value of 0.05.
Results
Changes in beta band relative power
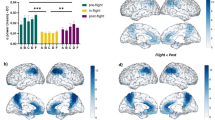
All individual subjects showed an increase of beta band relative power during eyes-closed (EC) under the in-flight condition (aboard ISS station) (Fig. 1). As a cohort, the beta band power was found to be significantly increased (F = 11.92, p < 0.001) during the in-flight condition when compared to the pre-flight (p < 0.01) and post-flight conditions (p < 0.01) (Fig. 1a). These changes were observed across different areas (Fig. 1b–d). Right hippocampus, right Rolandic operculum, and right inferior frontal gyrus (opercular), showed the most considerable differences in beta band power (higher q value) during the in-flight condition compared to the pre-flight condition (Fig. 1b). Left precentral gyrus and left postcentral gyrus showed the most significant differences in beta band power during the in-flight condition compared to the post-flight condition (Fig. 1c). Although no statistically significant overall differences were observed between the pre-flight and post-flight conditions, the medial right superior frontal gyrus showed the most considerable differences (Fig. 1d).
Changes in beta band relative power (eyes-closed) between flight conditions. (a) Statistical comparison between conditions. The bar graph depicts the mean ± SD of the beta band power for each flight condition (*p < 0.05, **p < 0.01, ***p < 0.001). (b–d) Brain figures in the dashed boxes represent the areas with higher statistical power changes in the beta band comparing areas between (b) pre-flight versus in-flight conditions, (c) post-flight versus in-flight conditions, (d) pre-flight versus post-flight conditions. The colorbar is displayed as a family-wise corrected significance level of q value > 5, corresponding with a minimum p value of 0.05. The q statistic value was obtained from the results of the post-hoc Tuckey test of the multiple comparison corrections. Thus, the darker the blue color represents brain regions with higher statistical power. The five subjects are mentioned by the respective code letter under each bar. Brain figures were generated using ‘Image Processing Toolbox’ and ‘Medical Imaging Toolbox’ from Matlab R2023b (version 23.2, https://www.mathworks.com/products/matlab.html).
Additionally, we evaluated the beta band relative power during eyes-open (EO) and found that not all subjects showed an increase of beta band power under the in-flight conditions (Fig. 2) when compared to the pre-flight or post-flight conditions. As a cohort, the beta band power was found to be significantly increased (F = 11.15, p < 0.001) during the in-flight condition when compared to the post-flight condition (p < 0.05) (Fig. 2a). These slightly significant changes were observed in the left cuneus and the left superior occipital lobe (Fig. 2c). No statistically significant overall differences were observed during the in-flight condition when compared to the pre-flight condition, or in the pre-flight versus post-flight comparison (Fig. 2a). However, at the area level, the pre-flight condition showed lower beta band power when compared to the in-flight condition principally in the occipital lobe (Fig. 2b). For the pre-flight versus post-flight comparison, the pre-flight condition showed a slightly higher beta band power in the frontal gyrus (Fig. 2d).
Changes in beta band relative power (eyes-open) between flight conditions. (a) Statistical comparison between conditions. The bar graph depicts the mean ± SD of the beta band power for each flight condition (*p < 0.05, **p < 0.01, ***p < 0.001). (b–d) Brain figures in the dashed boxes represent the areas with higher statistical power changes in the beta band comparing areas between (b) pre-flight versus in-flight conditions, (c) post-flight versus in-flight conditions, (d) pre-flight versus post-flight conditions. The colorbar is displayed as a family-wise corrected significance level of q value > 5, corresponding with a minimum p value of 0.05. The q statistic value was obtained from the results of the post-hoc Tuckey test of the multiple comparison corrections. Thus, the darker the blue color represents brain regions with higher statistical power. The five subjects are mentioned by the respective code letter under each bar. Brain figures were generated using ‘Image Processing Toolbox’ and ‘Medical Imaging Toolbox’ from Matlab R2023b (version 23.2, https://www.mathworks.com/products/matlab.html).
There were beta band power differences between eyes-closed and eyes-open conditions (Fig. 3). Higher relative power was found during eyes-open in the post-flight condition (p < 0.0001) (Fig. 3c).
Differences between eyes-closed and eyes-open in beta band relative power between flight conditions. (a) Comparison between eyes-closed and eyes-open in the pre-flight condition (p = 0.1184). (b) Comparison between eyes-closed and eyes-open in the in-flight condition (p = 0.3495). (c) Comparison between eyes-closed and eyes-open in the post-flight condition (p < 0.0001). Bar graphs depict the mean ± SD of the beta band power for each flight condition per subject. The dark blue in a bar indicates the eyes-closed condition, whereas the light blue bar indicates eyes-open condition. The five subjects are mentioned by their respective code letter under each bar. (*p < 0.05, **p < 0.01, ***p < 0.001).
Changes in beta band FC strength
As a cohort, beta band eyes-closed (EC) FC strength significantly increased (F = 9.908, p < 0.001) during the in-flight condition when compared to the post-flight (p < 0.01) condition (Fig. 4a). These changes were observed across different areas. Significant changes in beta band FC strength were mainly observed in the prefrontal cortex, showing the most significant differences (highest q values), and in the superior temporal gyrus (Fig. 4c). No significant differences were observed between pre-flight and either in-flight (Fig. 4b) or post-flight (Fig. 4d).
Changes in beta FC strength (eyes-closed) between flight conditions. (a) Statistical comparison between conditions. The bar graph depicts the mean ± SD of the beta band FC strength for each flight condition (*p < 0.05, **p < 0.01, ***p < 0.001). (b–d) Brain figures in the dashed boxes represent the areas with higher statistical FC changes in the beta band comparing areas between (b) pre-flight versus in-flight conditions, (c) post-flight versus in-flight conditions, (d) pre-flight versus post-flight conditions. The colorbar is displayed as a family-wise corrected significance level of q value > 5, corresponding with a minimum p value of 0.05. The q statistic value was obtained from the results of the post-hoc Tuckey test of the multiple comparison corrections. Thus, the darker the red color represents brain regions with higher statistical power. The five subjects are mentioned by the respective code letter under each bar. Brain figures were generated using ‘Image Processing Toolbox’ and ‘Medical Imaging Toolbox’ from Matlab R2023b (version 23.2, https://www.mathworks.com/products/matlab.html).
Additionally, we evaluated the beta band FC strength during eyes-open (EO) and found an increase of FC strength (F = 10.62, p < 0.001) in all subjects under in-flight conditions compared to pre-flight (p < 0.05) and post-flight (p < 0.01) conditions (Fig. 5a). These changes were observed across different areas (Fig. 5b–d). Left Rolandic operculum, right angular gyrus, and bilateral precentral gyrus showed the most considerable differences in beta band FC (higher q value) during the in-flight condition compared to the pre-flight condition (Fig. 5b). Left superior frontal gyrus showed the most significant differences in beta band FC during the in-flight condition compared to the post-flight condition (Fig. 5c). Although no statistically significant overall differences were observed between the pre-flight and post-flight conditions, the superior temporal gyrus and the posterior part of the cingulate gyrus showed the most considerable differences (Fig. 5d).
Changes in beta FC strength (eyes-open) between flight conditions. (a) Statistical comparison between conditions. The bar graph depicts the mean ± SD of the beta band FC strength for each flight condition (*p < 0.05, **p < 0.01, ***p < 0.001). (b–d) Brain figures in the dashed boxes represent the areas with higher statistical FC changes in the beta band comparing areas between (b) pre-flight versus in-flight conditions, (c) post-flight versus in-flight conditions, (d) pre-flight versus post-flight conditions. The colorbar is displayed as a family-wise corrected significance level of q value > 5, corresponding with a minimum p value of 0.05. The q statistic value was obtained from the results of the post-hoc Tuckey test of the multiple comparison corrections. Thus, the darker the red color represents brain regions with higher statistical power. The five subjects are mentioned by the respective code letter under each bar. Brain figures were generated using ‘Image Processing Toolbox’ and ‘Medical Imaging Toolbox’ from Matlab R2023b (version 23.2, https://www.mathworks.com/products/matlab.html).
There were beta band FC strength differences between eyes-closed and eyes-open conditions (Fig. 6). Higher FC strength was found during eyes-closed in all flight conditions (Fig. 6a–c) (pre-flight: p < 0.05; in-flight: p < 0.05, post-flight: p < 0.001).
Differences between eyes-closed and eyes-open in beta band FC strength between flight conditions. (a) Comparison between eyes-closed and eyes-open in the pre-flight condition (p < 0.05). (b) Comparison between eyes-closed and eyes-open in the in-flight condition (p < 0.05). (c) Comparison between eyes-closed and eyes-open in the post-flight condition (p < 0.001). Bar graphs depict the mean ± SD of the beta band power for each flight condition per subject. The dark blue in a bar indicates the eyes-closed condition, whereas the light blue bar indicates eyes-open condition. The five subjects are mentioned by their respective code letter under each bar (*p < 0.05, **p < 0.01, ***p < 0.001).
Discussion
In our research we have assessed electrophysiological changes, measured with whole brain EEG before, during, and after a spaceflight. We have shown that power and FC signatures are altered by the different environments that astronauts endure during a spaceflight. In particular, our results point to an increase in beta power and FC during the time in space that appears to recover when astronauts return home. Monitoring these alterations emerges as an important milestone in understanding how spaceflight can affect brain function.
Spaceflight plays a crucial role in advancing our understanding of the universe and pushing the boundaries of human capability. In recent years, there have been significant advancements in this field, notably with private companies joining traditional space agencies like NASA and ESA. The unique environment of space, including microgravity, radiation exposure, and isolation, poses significant challenges to human health1,2. Understanding these health risks helps scientists develop countermeasures, such as exercise routines, nutrition plans, and medical interventions, to maintain astronauts’ well-being. Investigating the health of astronauts in space is also vital for ensuring the safety and success of long-duration missions.
Previous studies have already identified anatomical22,34, electrophysiological15,23, and physical35,52 modifications after long-duration spaceflight missions. However, the implications of these changes are still unclear. Proprioception, widely known to be partially localized in the SMC53, plays a key role in the fine coordination of gross and fine motor actions by integrating multiple sensory inputs, including vision, touch, and vestibular functions54. In microgravity, all these inputs experience significant modifications, leading to alterations in proprioception-related mechanisms such as: lower limb strength, gaze stabilization, balance, or eye-head coordination, among others6,36,52. Due to these alterations, astronauts need to adjust to a new model of movement by depending more on visual cues55.
These changes in the sensory inputs would be reflected as electrophysiological and/or anatomical modifications in the brain. The beta frequency activity is commonly linked with motor control39 and proprioceptive processing56, and has been localized in the SMC37. Our study findings indicate that long-term missions can produce related neurophysiological changes, as evidenced through EEG-derived whole brain analyzes.
Compared to pre- and post-flight conditions, the SMC showed a significant increase in beta band power during the flight, especially on the left side, in the five subjects (Fig. 1). These variations across different flight stages may be attributed to adaptations to the microgravity environment. However, as beta activity is associated with posture stabilization and movement cancellation40, another potential explanation for these results is the difference in participants’ postural tone during EEG recordings; they were seated during the pre- and post-conditions, whereas in space they were floating, secured only by a belt around their waist. Additional research is necessary to clarify the cause of these results.
Regarding the FC strength results (Fig. 4), the largest increases in beta band were localized in areas linked with highly important cognitive functions during weightlessness in space: the superior frontal gyrus is involved in spatial working memory57, the medial frontal gyrus in performance monitoring58, the superior temporal gyrus in visual search and spatial perception59, and the angular gyrus in spatial processing, specifically in the verticality assessment60. The altered proprioceptive integration would trigger the reorganization of motor control strategies, requiring new plans and the development of new motor schemes. This would induce a reorganization of local primary motor regions as well as brain areas involved in motor control and motor schemes evidenced in the increased FC over the prefrontal regions.
The available data from this study alone leaves some unanswered questions. We have to consider that in these extreme conditions during spaceflight, other factors than microgravity could be influencing the beta band activity (isolation, radiation, mood disorders). Although our results could be partially influenced by these factors, the specificity of the brain changes over the primary motor cortex as well as over the motor control regions lead us to link these EEG results to the effects of microgravity. In addition, we excluded the possibility that these changes were driven by arousal or excitation levels, as previous studies have reported opposite results to our findings7,61. Another important question is the timing and progression of changes during spaceflight. This cannot be reliably inferred from just two in-flight recordings used in our analysis. Moreover, the dataset had a very limited number of participants, and gender balance was lacking in both datasets, with no female participants included despite known gender differences in neurophysiological data62,63. Furthermore, the lack of cognitive, structural, cardiovascular, or physiological markers that would be useful to establish correlations, make it difficult to interpret some of these results. One limitation is the lack of both electrode positions and anatomical information (MRIs) of the astronauts at any point during spaceflight, making it necessary the use of a template to estimate the source activity. As a result, the errors introduced by brain shifts occurring in microgravity will be smaller than those arising from variations in electrode placement between sessions. Subsequent studies with a larger cohort and these additional markers to measure are crucial to elucidate the clinical and operational significance of these changes. Definitively, new studies simulating microgravity on earth should confirm these findings. However, it is crucial to continue acquiring more data during spaceflights to extend the interpretability of these results.
Data availability
All relevant data will be available from the Laboratory of Neurophysiology and Movement Biomechanics at Université Libre de Bruxelles (acebolla@ulb.ac.be) upon request and after approval from the European Space Agency Medical Board (ESA-MB) and the NASA Johnson Space Centre Institutional Review Board (NASA-IRB). Authors do not own the data, but others can request data from ESA and NASA. The authors confirm that they did not have any special access or request privileges that others would not have.
References
Clément, G. R. et al. Challenges to the central nervous system during human spaceflight missions to Mars. J. Neurophysiol. 123, 2037–2063 (2020).
Afshinnekoo, E. et al. Fundamental biological features of spaceflight: Advancing the field to enable deep-space exploration. Cell 183, 1162–1184 (2020).
Seidler, R. D., Mao, X. W., Tays, G. D., Wang, T. & Zu Eulenburg, P. Effects of spaceflight on the brain. Lancet Neurol. 23, 826–835 (2024).
McGregor, H. R. et al. Impacts of spaceflight experience on human brain structure. Sci. Rep. 13, 7878 (2023).
Basner, M. et al. Development and validation of the cognition test battery for spaceflight. Aerosp. Med. Hum. Perform. 86, 942–952 (2015).
Tays, G. D. et al. The effects of long duration spaceflight on sensorimotor control and cognition. Front. Neural Circuits 15, 723504 (2021).
Van Ombergen, A. et al. The effect of spaceflight and microgravity on the human brain. J. Neurol. 264, 18–22 (2017).
Wu, B. et al. On-orbit sleep problems of astronauts and countermeasures. Mil. Med. Res. 5, 17 (2018).
Christensen, J. M. & Talbot, J. M. A review of the psychological aspects of space flight. Aviat. Space Environ. Med. 57, 203–212 (1986).
Petit, G. et al. Local sleep-like events during wakefulness and their relationship to decreased alertness in astronauts on ISS. Npj Microgravity 5, 10 (2019).
Roy-O’Reilly, M., Mulavara, A. & Williams, T. A review of alterations to the brain during spaceflight and the potential relevance to crew in long-duration space exploration. Npj Microgravity 7, 5 (2021).
Koppelmans, V., Bloomberg, J. J., Mulavara, A. P. & Seidler, R. D. Brain structural plasticity with spaceflight. Npj Microgravity 2, 2 (2016).
Marshall-Goebel, K. et al. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw. Open 2, e1915011 (2019).
Cheron, G. et al. Gravity influences top-down signals in visual processing. PLoS ONE 9, e82371 (2014).
Cebolla, A. M. et al. Cerebellar contribution to visuo-attentional alpha rhythm: insights from weightlessness. Sci. Rep. 6, 37824 (2016).
Cebolla, A. M., Petieau, M., Palmero-Soler, E. & Cheron, G. Brain potential responses involved in decision-making in weightlessness. Sci. Rep. 12, 12992 (2022).
De Saedeleer, C. et al. Weightlessness alters up/down asymmetries in the perception of self-motion. Exp. Brain Res. 226, 95–106 (2013).
Casario, K. et al. Acceptability of the Cognition Test Battery in astronaut and astronaut-surrogate populations. Acta Astronaut. 190, 14–23 (2022).
Howieson, D. Current limitations of neuropsychological tests and assessment procedures. Clin. Neuropsychol. 33, 200–208 (2019).
Jessen, F. et al. The characterisation of subjective cognitive decline. Lancet Neurol. 19, 271–278 (2020).
López-Sanz, D. et al. Network disruption in the preclinical stages of Alzheimer’s disease: From subjective cognitive decline to mild cognitive impairment. Int. J. Neural Syst. 27, 1750041 (2017).
Roberts, D. R. et al. Effects of spaceflight on astronaut brain structure as indicated on MRI. N. Engl. J. Med. 377, 1746–1753 (2017).
Cheron, G. et al. Effect of gravity on human spontaneous 10-Hz electroencephalographic oscillations during the arrest reaction. Brain Res. 1121, 104–116 (2006).
Fiedler, P. et al. Noise characteristics in spaceflight multichannel EEG. PLoS ONE 18, e0280822 (2023).
De La Torre, G. G. et al. Future perspectives on space psychology: Recommendations on psychosocial and neurobehavioural aspects of human spaceflight. Acta Astronaut. 81, 587–599 (2012).
Gundel, A., Nalishiti, V., Reucher, E., Vejvoda, M. & Zulley, J. Sleep and circadian rhythm during a short space mission. Clin. Investig. https://doi.org/10.1007/BF00209726 (1993).
Monk, T. H., Buysse, D. J., Billy, B. D., Kennedy, K. S. & Willrich, L. M. Sleep and circadian rhythms in four orbiting astronauts. J. Biol. Rhythms 13, 188–201 (1998).
Dijk, D.-J. et al. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 281, R1647–R1664 (2001).
Lejko, N., Larabi, D. I., Herrmann, C. S., Aleman, A. & Ćurčić-Blake, B. Alpha power and functional connectivity in cognitive decline: A systematic review and meta-analysis. J. Alzheimers Dis. 78, 1047–1088 (2020).
Friston, K. J. et al. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 2, 189–210 (1994).
Gevins, A., Smith, M. E., McEvoy, L. K., Leong, H. & Le, J. Electroencephalographic imaging of higher brain function. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 354, 1125–1134 (1999).
Dunlop, K., Talishinsky, A. & Liston, C. Intrinsic brain network biomarkers of antidepressant response: A review. Curr. Psychiatry Rep. 21, 87 (2019).
Martín-Buro, M. C., Garcés, P. & Maestú, F. Test-retest reliability of resting-state magnetoencephalography power in sensor and source space: Reliability of resting-state MEG power. Hum. Brain Mapp. 37, 179–190 (2016).
Demertzi, A. et al. Cortical reorganization in an astronaut’s brain after long-duration spaceflight. Brain Struct. Funct. 221, 2873–2876 (2016).
Reschke, M. F. et al. Posture, locomotion, spatial orientation, and motion sickness as a function of space flight. Brain Res. Rev. 28, 102–117 (1998).
Layne, C. S. et al. Effect of long-duration spaceflight on postural control during self-generated perturbations. J. Appl. Physiol. 90, 997–1006 (2001).
Witham, C. L. & Baker, S. N. Network oscillations and intrinsic spiking rhythmicity do not covary in monkey sensorimotor areas. J. Physiol. 580, 801–814 (2007).
Espenhahn, S., De Berker, A. O., Van Wijk, B. C. M., Rossiter, H. E. & Ward, N. S. Movement-related beta oscillations show high intra-individual reliability. Neuroimage 147, 175–185 (2017).
Sugata, H. et al. Role of beta-band resting-state functional connectivity as a predictor of motor learning ability. Neuroimage 210, 116562 (2020).
Barone, J. & Rossiter, H. E. Understanding the role of sensorimotor beta oscillations. Front. Syst. Neurosci. 15, 655886 (2021).
Baumgarten, T. J. et al. Beta peak frequencies at rest correlate with endogenous GABA+/Cr concentrations in sensorimotor cortex areas. PLoS ONE 11, e0156829 (2016).
Rempe, M. P. et al. Spontaneous sensorimotor beta power and cortical thickness uniquely predict motor function in healthy aging. Neuroimage 263, 119651 (2022).
Takács, E. et al. Persistent deterioration of visuospatial performance in spaceflight. Sci. Rep. 11, 9590 (2021).
Perrin, F., Pernier, J., Bertrand, O. & Echallier, J. F. Spherical splines for scalp potential and current density mapping. Electroencephalogr. Clin. Neurophysiol. 72, 184–187 (1989).
Oostenveld, R., Fries, P., Maris, E. & Schoffelen, J.-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 1–9 (2011).
Huang, Y., Parra, L. C. & Haufe, S. The New York Head—A precise standardized volume conductor model for EEG source localization and tES targeting. Neuroimage 140, 150–162 (2016).
Gramfort, A., Papadopoulo, T., Olivi, E. & Clerc, M. OpenMEEG: opensource software for quasistatic bioelectromagnetics. Biomed. Eng. OnLine 9, 45 (2010).
Pascual-Marqui, R. D. Discrete, 3D distributed, linear imaging methods of electric neuronal activity. Part 1: exact, zero error localization. Preprint at http://arxiv.org/abs/0710.3341 (2007).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002).
Mormann, F., Lehnertz, K., David, P. & E. Elger, C. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Phys. Nonlinear Phenom. 144, 358–369 (2000).
Garcés, P., Martín-Buro, M. C. & Maestú, F. Quantifying the test-retest reliability of magnetoencephalography resting-state functional connectivity. Brain Connect. 6, 448–460 (2016).
Bloomberg, J. J. & Mulavara, A. P. Changes in walking strategies after spaceflight. IEEE Eng. Med. Biol. Mag. 22, 58–62 (2003).
Riemann, B. L. & Lephart, S. M. The sensorimotor system, part II: The role of proprioception in motor control and functional joint stability. J. Athl. Train. 37, 80–84 (2002).
Moon, K. M. et al. Proprioception, the regulator of motor function. BMB Rep. 54, 393–402 (2021).
Macaulay, T. R. et al. Developing proprioceptive countermeasures to mitigate postural and locomotor control deficits after long-duration spaceflight. Front. Syst. Neurosci. 15, 658985 (2021).
Tsuchimoto, S. et al. Resting-state fluctuations of EEG sensorimotor rhythm reflect BOLD activities in the pericentral areas: A simultaneous EEG-fMRI study. Front. Hum. Neurosci. 11, 356 (2017).
Boisgueheneuc, F. D. et al. Functions of the left superior frontal gyrus in humans: a lesion study. Brain 129, 3315–3328 (2006).
Van Noordt, S. J. R. & Segalowitz, S. J. Performance monitoring and the medial prefrontal cortex: A review of individual differences and context effects as a window on self-regulation. Front. Hum. Neurosci. https://doi.org/10.3389/fnhum.2012.00197 (2012).
Ellison, A. An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain 127, 2307–2315 (2004).
Jillings, S. et al. Prolonged microgravity induces reversible and persistent changes on human cerebral connectivity. Commun. Biol. 6, 46 (2023).
Marušič, U., Meeusen, R., Pišot, R. & Kavcic, V. The brain in micro- and hypergravity: The effects of changing gravity on the brain electrocortical activity. Eur. J. Sport Sci. 14, 813–822 (2014).
Jaušovec, N. & Jaušovec, K. Resting brain activity: Differences between genders. Neuropsychologia 48, 3918–3925 (2010).
Schneider, S., Robinson, R., Smith, C., Von Der Wiesche, M. & Goswami, N. Gender specific changes in cortical activation patterns during exposure to artificial gravity. Acta Astronaut. 104, 438–443 (2014).
Acknowledgements
EEG data analysis (A.Q.L., M.S.G., P.C., S.P., R.B., P.F., M.F., F.M.) was in part supported by a grant [NASA Cooperative Agreement NNX16AO69A] from the Translational Research Institute for Space Health (TRISH) in Houston, TX. Obtaining the original EEG data, here used for comparison, from several ISS expeditions was funded by the Belgian Federal Science Police Office (A.C., G.C.), the European Space Agency (ESA), (AO-2004, 118 for A.C., G.C.), the Belgian National Funds for Scientific Research (G.C.), the research funds of the Université Libre de Bruxelles (A.C., G.C.), and the Université de Mons-Hainaut (G.C.).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
A.Q.L., M.S.G.: methodology, data processing, statistical analysis, manuscript—original draft preparation and figures preparation. P.C.: methodology, data processing, manuscript—review and editing. S.P.: methodology, statistical analysis, manuscript—review and editing. R.B.: methodology, manuscript -review and editing. P.F.: funding acquisition, data processing, manuscript—review and editing. A.C., G.C.: manuscript—review and editing. M.F., F.M.: funding acquisition, manuscript—review and editing. All present authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quivira-Lopesino, A., Sevilla-García, M., Cuesta, P. et al. Changes of EEG beta band power and functional connectivity during spaceflight: a retrospective study. Sci Rep 15, 13399 (2025). https://doi.org/10.1038/s41598-025-96897-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96897-5
Keywords
This article is cited by
-
Brain power comparison between microgravity and head-down tilt bed rest: an electroencephalography approach
Scientific Reports (2025)
-
Power spectral density and peak alpha frequency modulation related to the shooting process in archers
Experimental Brain Research (2025)