Abstract
This study aims to evaluate the association between the dietary inflammatory index (DII) and stroke risk in hypertensive patients. Data were sourced from the National Health and Nutrition Examination Survey (NHANES) spanning 1999–2020, including 23,712 hypertensive patients. DII scores were calculated based on dietary intake data, and stroke diagnoses were determined through self-reported physician diagnoses. The relationship between DII and stroke risk was assessed using multivariable logistic regression models. Dose–response relationships and subgroup differences were explored through stratified analysis and restricted cubic spline (RCS) methods. Key dietary factors associated with stroke were identified using least absolute shrinkage and selection operator (LASSO) regression and incorporated into a risk prediction nomogram model. The model’s discriminatory ability for stroke was evaluated using receiver operating characteristic (ROC) curves. After adjusting for confounding factors, the highest DII quartile was associated with an adjusted odds ratio (OR) of 1.44 (95% CI 1.19, 1.74) for stroke compared to the lowest quartile, and each unit increase in DII was associated with an OR of 1.08 (95% CI 1.04–1.13) for stroke prevalence. The RCS curve demonstrated a nonlinear relationship between DII and stroke, with a turning point at 0.29. The nomogram model based on key dietary factors identified by LASSO regression had an area under the curve (AUC) of 70.93% (95% CI 69.81%–72.06%). There is a nonlinear relationship between DII and stroke risk in hypertensive patients. Given the inherent limitations of a cross-sectional study design, further research is needed to establish causality.
Similar content being viewed by others
Introduction
Hypertension affects over 1.13 billion people worldwide and is a significant contributor to cardiovascular diseases (CVD) such as stroke, heart failure, and myocardial infarction1. Effective management of hypertension is crucial for reducing these conditions. Stroke, which results from a disruption in the brain’s blood supply, can be ischemic (due to blood clots) or hemorrhagic (due to ruptured blood vessels). It is a leading cause of death and long-term disability globally, placing substantial burdens on individuals, families, and healthcare systems2. Survivors often face pronounced physical and cognitive impairments, increasing healthcare costs and reducing quality of life3. Recognizing and addressing modifiable risk factors, such as diet, is essential for stroke prevention.
Diet plays a critical role in cardiovascular health, significantly influencing the risk of developing CVD. Poor dietary habits—such as high intake of saturated fats, trans fats, and refined sugars—can lead to atherosclerosis, hypertension, and obesity, all major risk factors for CVD4. Conversely, diets rich in fruits, vegetables, whole grains, and healthy fats, such as the Mediterranean diet, have been shown to reduce cardiovascular risk5. Inflammation is a key factor in the development and progression of CVD. Chronic systemic inflammation contributes to the pathogenesis of atherosclerosis, causing plaque formation and instability, which can result in heart attacks and strokes. Biomarkers such as C-reactive protein (CRP) and interleukin-6 (IL-6) are commonly used to measure systemic inflammation6, and elevated levels of these markers are associated with an increased risk of cardiovascular events7.
The dietary inflammatory index (DII) is a tool designed to assess the inflammatory potential of an individual’s diet by evaluating the intake of various dietary components known to influence inflammation. It assigns scores to these components, with higher scores indicating a pro-inflammatory diet and lower scores reflecting an anti-inflammatory diet. The DII was developed through an extensive literature review to identify dietary factors that affect inflammatory markers, followed by validation studies to ensure its reliability and accuracy8. Research has established a strong link between the DII and multiple health outcomes. High DII scores have been associated with increased inflammatory markers and higher risk of chronic diseases such as diabetes, cancer, and CVD9. Specific studies have demonstrated that diets with high inflammatory potential, as indicated by higher DII scores, are associated with greater risk of cardiovascular events, including myocardial infarction and stroke10. These findings suggest that the DII may serve as a valuable indicator of diet-related inflammation, which, when combined with other clinical and dietary factors, could contribute to the overall risk assessment of CVD in at-risk populations.
While existing studies highlight the link between dietary inflammation and CVD, comprehensive research on the specific impact of the DII on stroke risk in hypertensive patients remains limited. Therefore, we aim to address this gap by examining the association between DII and stroke risk in hypertensive patients. Understanding this relationship is vital for developing effective preventive strategies. This study’s primary objective is to investigate the association between DII and stroke risk, with secondary objectives to assess the predictive value of DII and develop a predictive model. We hypothesize that higher DII scores are associated with increased stroke risk, and that this association remains significant after adjusting for confounding factors.
Methods
Study design and population
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative survey of the U.S. population that employs a complex, stratified, multistage probability design. It includes household interviews, physical examinations conducted either at home or in mobile examination centers (MECs), and laboratory tests. The survey is conducted biennially, and its detailed sampling and data collection procedures have been previously published. NHANES is administered by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention, with approval from the NCHS Institutional Review Board; all participants provided written informed consent11. This study adheres to ethical protocols #98–12, #2005–06 (and their continuations), as well as #2011–17 (and its continuation). The NHANES data used in this analysis are publicly available at https://www.cdc.gov/nchs/nhanes.
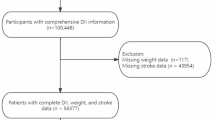
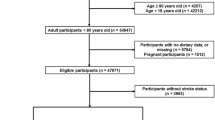
This study is a national cross-sectional analysis focusing on respondents with hypertension from the NHANES surveys conducted between 1999 and 2020. Hypertension is defined as a systolic blood pressure (SBP) ≥ 140 mmHg and/or a diastolic blood pressure (DBP) ≥ 90 mmHg, the use of antihypertensive medication, or a self-reported diagnosis of hypertension12. After excluding individuals under 18 years of age, those without hypertension, and participants with missing DII, weight, or stroke data, a total of 23,712 hypertensive individuals from the NHANES dataset were included in this analysis (Fig. 1).
Definition of DII
The DII is a tool designed to assess the inflammatory potential of an individual’s diet by evaluating the consumption of various dietary components and their established relationships with inflammatory biomarkers. The DII was developed through an extensive review of scientific literature, which identified 45 food parameters—including nutrients, flavonoids, and food groups—that influence inflammation13. Dietary data from NHANES participants were obtained via 24-h dietary recalls, providing detailed information on all foods and beverages consumed on the previous day. Each food parameter was assigned an inflammatory score based on its effect on biomarkers such as CRP, IL-6, and tumor necrosis factor-alpha (TNF-α). The overall DII score for each participant was calculated by summing the standardized scores of these parameters, with higher scores indicating a more pro-inflammatory diet and lower scores indicating an anti-inflammatory diet14. In this study, the DII was calculated using 25 specific nutrients: carbohydrates, vitamins B12, D, iron, vitamin E, protein, cholesterol, magnesium, vitamin B2, folic acid, caffeine, niacin, zinc, polyunsaturated fats (PUFAs), vitamin A, alcohol, monounsaturated fats (MUFAs), selenium, dietary fiber, vitamin B1, total fat, vitamin C, saturated fat, energy, and vitamin B6. Notably, the DII calculation remains accurate and feasible even when fewer than 30 nutrients are included8.
Diagnosis of stroke
The diagnosis of stroke in this study was based on participants’ responses to a self-administered questionnaire during NHANES data collection. Specifically, participants were asked, “Has a physician or other health professional ever told you that you had a stroke?” Based on their responses, individuals were classified into either the “Stroke” or “non-Stroke” group.
Covariates
Race/ethnicity was categorized according to the survey design into Mexican American, Non-Hispanic Black, Non-Hispanic White, and Other Race. Educational level was dichotomized into “high school or above” and “less than high school.” Marital status was classified as either “married” or “other.” The poverty income ratio (PIR) is an index of income relative to the federal poverty line, adjusted for economic inflation and family size. Smoking status was categorized as never, former, or current smokers, and alcohol consumption was divided into five levels—never, former, mild, moderate, and heavy—based on daily intake and the frequency of binge drinking15.
Data on smoking, alcohol consumption, history of diabetes, history of coronary heart disease (CHD), and medication use were obtained via self-reported questionnaires. Blood pressure, weight, and height were measured using standard procedures at mobile examination centers, with body mass index (BMI) calculated as weight divided by height squared. Clinical indicators—including fasting plasma glucose (FPG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (SCR), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C)—were measured in NHANES laboratories. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula16.
Statistical analysis
NHANES is a multistage, stratified, probability-based survey that oversamples certain groups17. To account for unequal selection probabilities and non-response, all respondent data were weighted using NHANES-recommended weights. Continuous variables are presented as mean ± standard deviation (SD) and compared using the student’s t-test, while categorical variables are expressed as frequencies (percentages) and compared using the chi-square test. DII scores were divided into four quartiles (Q1: DII < 0.43; Q2: 0.43 ≤ DII < 1.95; Q3: 1.95 ≤ DII < 3.09; Q4: DII ≥ 3.09), with the first quartile (Q1) serving as the reference.
Multivariable logistic regression models were used to assess the association between DII and stroke among hypertensive patients while adjusting for potential confounders. The analysis included demographic characteristics and traditional risk factors associated with both DII and stroke. Odds ratios (ORs) were calculated using three models: an unadjusted model (Model 1), a model adjusted for age, sex, race, education level, marital status, and PIR (Model 2), and a fully adjusted model (Model 3) that further included smoking status, alcohol use, diabetes, CHD, eGFR, ALT, TG, TC, HDL-C, LDL-C, BMI, and the use of antihypertensive drugs, hypoglycemic agents, lipid-lowering drugs, and antiplatelet drugs. Additionally, restricted cubic splines (RCS) regression with three knots was applied to examine the nonlinear relationship between DII and stroke among hypertensive patients. Subgroup analyses were also conducted based on clinical characteristics—including sex, age, race, BMI, smoking status, alcohol consumption, diabetes, and CHD—with interaction P-values calculated for these groups.
To identify the most significant dietary predictors of stroke and address multicollinearity among variables, we employed the Least Absolute Shrinkage and Selection Operator (LASSO) regression model. In this model, coefficients of variables contributing minimally to the overall model are shrunk to zero, thereby enhancing predictive performance18. Cross-validation was used to evaluate model performance and optimize parameter selection, dividing the dataset into 10 subsets and iteratively training and testing the model. We selected a λ value slightly larger than the λ with the minimum deviation observed during cross-validation to stabilize the model, prevent overfitting, and improve its generalizability to new data. Furthermore, we developed a risk prediction nomogram based on several key stroke-related variables, and its discriminatory ability to predict stroke risk was validated using receiver operating characteristic (ROC) curves. All statistical analyses were performed using R software version 4.4.1 (http://www.R-project.org, R Foundation for Statistical Computing, Vienna, Austria). Missing covariate data were addressed using multiple imputation methods specifically designed for survey datasets19. Statistical significance was defined by a two-tailed P-value of less than 0.05.
Results
Baseline characteristics of study participants
The baseline characteristics of the 23,712 respondents, categorized by stroke status, are summarized in Table 1. Compared to non-stroke participants, those with a history of stroke were older, more likely to be female, and more often Non-Hispanic White. Additionally, stroke participants had higher prevalences of diabetes and CHD and were more likely to be smokers and alcohol users. They also exhibited lower levels of eGFR, ALT, AST, TG, TC, HDL-C, and LDL-C, but higher levels of FPG, SCR, and DII scores. With the exception of BMI, all these differences were statistically significant (P < 0.05).
Association between DII and stroke prevalence
Logistic regression analysis revealed a significant association between higher DII levels and increased stroke prevalence among hypertensive patients (Table 2). Compared to the Q1 group, the Q4 group had significantly higher odds of stroke, with ORs (95% CIs) of 2.05 (1.71–2.46), 1.65 (1.36–1.99), and 1.44 (1.19–1.74) in Models 1, 2, and 3, respectively, with trend P-values of less than 0.0001 across all models. Moreover, the continuous model indicated that each unit increase in DII was associated with an OR of 1.08 (1.04–1.13) for stroke prevalence after adjusting for confounders (P < 0.001). These findings underscore the significant impact of dietary inflammation on stroke risk.
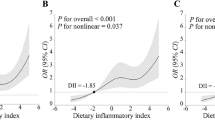
Using logistic regression models with RCS functions, we further examined the relationship between DII and stroke prevalence among hypertensive patients. A nonlinear dose–response relationship was observed (nonlinear P < 0.001, Fig. 2), with an inflection point at a DII of 0.29. Below this threshold, DII was not significantly associated with stroke prevalence (P = 0.84, OR = 1.02, 95% CI = 0.84–1.23). However, above this threshold, each unit increase in DII corresponded to a 17% increase in stroke prevalence (OR = 1.17, 95% CI = 1.09–1.25, P < 0.0001) after adjustment for various factors.
Stratified analyses
Multivariable logistic regression analysis, adjusting for potential confounders, was used to explore the association between DII and stroke within subgroups defined by age, sex, BMI, race, smoking status, alcohol consumption, diabetes, and CHD (Fig. 3). In most subgroups, an increase in DII was associated with higher ORs for stroke, ranging from 1.062 to 1.156 per unit increase in DII. However, this association was not statistically significant (P > 0.05) among participants under 60 years old, males, Mexican Americans, individuals with BMI ≥ 30 kg/m2, never smokers, mild and heavy drinkers, and those with CHD. Furthermore, no significant interactions were observed across the subgroups (P > 0.05). These results indicate that a higher DII is a significant risk factor for stroke, independent of age, sex, BMI, race, smoking status, alcohol consumption, diabetes, and CHD.
Identifying key dietary components influencing stroke risk
This study employed LASSO regression to identify the dietary components within the DII that most significantly affect stroke risk in hypertensive patients. LASSO regression improves upon ordinary least squares regression by incorporating an L1 regularization term, which shrinks some coefficients to zero, thereby effectively selecting the most important features. This method helps prevent overfitting, enhances the model’s generalizability, and aids in identifying dietary parameters closely associated with stroke (Fig. 4). Specifically, LASSO regression minimizes a combination of the loss function and the L1 regularization term, forcing some coefficients to zero and excluding the corresponding features (Fig. 4A). To validate the model’s stability, cross-validation was performed (Fig. 4B). Initially, variables that were significant in Model 3 (age, PIR, smoking, DM, CHD, eGFR, ALT, antihypertensive drugs, lipid-lowering drugs, and antiplatelet drugs) along with 26 dietary components were included in the model. Through LASSO regression, 12 variables with non-zero coefficients were identified to construct the risk prediction nomogram: age, PIR, eGFR, protein intake, dietary fiber, total saturated fat, vitamin A, beta-carotene, total folate, vitamin D, selenium, and alcohol. Ultimately, the final model was based on six variables that were statistically significant predictors of stroke risk: age, PIR, eGFR, dietary fiber, total saturated fat, and total folate. The model’s predictive performance for stroke was validated using ROC curve analysis, demonstrating high predictive accuracy (Area Under the Curve, AUC = 70.93%, 95% CI 69.81%–72.06%) (Fig. 5).
Development and validation of a stroke risk prediction model. (A) Nomogram model based on age, PIR, eGFR, and nine key dietary factors associated with stroke identified by LASSO regression. (B) ROC curve for evaluating the predictive ability of the nomogram model for stroke. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Main findings
In our study of 23,712 NHANES hypertensive participants, we observed that the average DII score of stroke patients was significantly higher than that of non-stroke patients. Based on this observation, we applied RCS analysis to confirm a nonlinear relationship between DII and stroke risk among hypertensive patients in the United States, independent of various confounding factors. This association was further reaffirmed using multiple logistic regression models. Subgroup analyses demonstrated that the association remained robust across different subgroups after adjusting for numerous covariates. Among the 26 dietary components used to calculate the DII, we identified three factors most strongly associated with stroke risk in hypertensive patients: dietary fiber, total saturated fat, and total folate. By integrating these key dietary factors with age, PIR, and eGFR, we developed a nomogram model that exhibited strong performance in predicting stroke risk.
Interpretation of results and comparison with existing literature
Our study demonstrates a significant association between higher DII scores and increased stroke risk among hypertensive patients, suggesting that the inflammatory potential of the diet is an important independent risk factor for stroke. Specifically, patients with higher DII scores—indicative of a more pro-inflammatory diet—exhibited an elevated risk of stroke, underscoring the potential of dietary modifications to reduce stroke risk by mitigating dietary inflammation. Moreover, our analysis revealed a nonlinear relationship between DII and stroke risk: below a certain threshold, DII does not significantly impact stroke risk; however, once this threshold is exceeded, the risk increases markedly. This finding implies that while moderate dietary inflammatory potential may not pose a significant risk, higher levels can substantially elevate stroke risk.
Our results align with existing literature, reinforcing the validity of the DII as a predictor of chronic disease risk. Multiple studies have consistently demonstrated the role of inflammation in CVD, including stroke. For example, research has shown that higher DII scores are associated with an increased risk of CVD, highlighting the inflammatory potential of diet as a critical factor8. This foundational study paved the way for understanding how pro-inflammatory diets contribute to cardiovascular risks. Additional research has directly linked dietary inflammatory potential to systemic inflammation markers such as CRP and IL-6, which are established predictors of cardiovascular risk20. Supporting our findings, a large cohort study observed that higher DII scores are correlated with increased inflammatory markers and a higher incidence of stroke over an extended period21. Furthermore, another significant longitudinal study found that a pro-inflammatory diet, as indicated by higher DII scores, was associated with an increased risk of cardiovascular events, including stroke, across diverse populations22. Finally, research focusing on ischemic stroke demonstrated that higher DII scores were significantly linked to greater stroke risk, underscoring the importance of dietary interventions in stroke prevention23. Our study builds upon these findings by leveraging a large sample size and extensive multivariate adjustments, thereby providing robust and nuanced evidence of the relationship between DII and stroke risk.
Mechanisms linking diet and stroke risk
The relationship between diet and stroke, particularly ischemic stroke, is closely linked to chronic inflammation—a well‐established risk factor for various CVD. Diets with high inflammatory potential, as indicated by higher DII scores, can exacerbate systemic inflammation and thereby increase stroke risk. Different dietary patterns exert distinct effects on immune responses and inflammation. For instance, Western diets—typically high in processed foods, red meat, and refined sugars—are associated with elevated levels of inflammatory markers such as CRP and IL-6. These pro-inflammatory diets contribute to endothelial dysfunction and atherosclerosis, ultimately increasing stroke risk24. Endothelial dysfunction impairs blood flow regulation and promotes clot formation, both critical in the development of ischemic stroke. Moreover, such diets elevate oxidative stress by creating an imbalance between free radical production and the body’s antioxidant defenses, leading to cellular damage and further inflammation25. In contrast, the Mediterranean diet—rich in fruits, vegetables, whole grains, nuts, and olive oil—has been shown to reduce inflammation and lower stroke risk. This diet is associated with decreased inflammatory markers and improved endothelial function. Its anti-inflammatory effects are likely mediated through improved lipid profiles, enhanced antioxidant status, and better glycemic control26. Specifically, omega-3 fatty acids found in fish and flaxseeds inhibit the production of pro-inflammatory eicosanoids, cytokines, and reactive oxygen species27. Polyphenols present in fruits, vegetables, tea, and wine scavenge free radicals and inhibit inflammatory enzymes28, while dietary fiber from whole grains, fruits, and vegetables can reduce CRP levels and improve gut health, thereby modulating systemic inflammation29. Several studies have demonstrated that these dietary components work synergistically to enhance cardiovascular health and reduce the incidence of stroke30. In summary, dietary patterns play a crucial role in modulating systemic inflammation and influencing stroke risk. While pro-inflammatory diets elevate inflammation and stroke risk, anti-inflammatory diets—particularly the Mediterranean diet—offer protective effects.
Impact of specific dietary components on stroke risk
Current literature demonstrates that three dietary components—dietary fiber, total saturated fat, and total folate—significantly influence cardiovascular health via various mechanisms. Dietary fiber, for instance, is well-documented for its cardiovascular benefits. Increased fiber intake is associated with lower levels of inflammatory markers, improved lipid profiles, and better glycemic control. Specifically, higher dietary fiber consumption has been linked to reduced CRP levels, an established inflammatory marker associated with cardiovascular disease risk29. Furthermore, multiple epidemiological studies have confirmed the protective effects of dietary fiber against stroke, revealing an inverse relationship between fiber intake and stroke incidence31. Total saturated fat intake remains a contentious issue in cardiovascular health. Saturated fats are known to elevate LDL-C levels, a major risk factor for atherosclerosis and stroke. Research suggests that substituting saturated fats with polyunsaturated fats not only reduces the risk of CHD but may also lower stroke risk32. Additionally, higher saturated fat consumption is correlated with increased inflammatory marker levels, further exacerbating the risk of ischemic events33. Folate, a B-vitamin crucial for homocysteine metabolism, also plays an important role. Elevated homocysteine levels are linked to increased stroke risk due to their contributions to endothelial dysfunction and thrombogenesis. Adequate folate intake can reduce homocysteine levels, thereby potentially lowering stroke risk. Indeed, higher folate consumption is associated with a reduced risk of stroke, underscoring its importance in stroke prevention34. Moreover, dietary folate has been shown to improve endothelial function, which may help mitigate the progression of atherosclerosis35. Overall, the analysis of these dietary components—dietary fiber, total saturated fat, and total folate—provides valuable insight into their roles in modulating stroke risk among hypertensive patients. While increased dietary fiber and folate intake appear to exert protective effects, higher saturated fat consumption seems to elevate risk. These findings underscore the critical importance of dietary modifications as a strategy for stroke prevention, particularly in hypertensive populations.
Clinical significance and applications
The findings of this study hold important clinical implications for managing hypertensive patients. Physicians can utilize DII scores to assess stroke risk in these individuals and offer tailored dietary recommendations aimed at risk mitigation. The nomogram model developed in this study serves as a practical tool for precisely predicting individual stroke risk based on specific dietary and health factors. From a public health perspective, promoting anti-inflammatory diets could markedly reduce stroke incidence among hypertensive populations. Public health policies should, therefore, focus on educating the public about the link between diet, inflammation, and stroke risk, thereby encouraging dietary changes that support cardiovascular health.
Strengths and potential limitations of the study
The study exhibits significant strengths, notably the application of LASSO regression analysis and the construction of a nomogram model, which enhance the accuracy and practical utility of stroke risk prediction in hypertensive patients. However, several limitations should be acknowledged. First, the cross-sectional design restricts the ability to establish a causal relationship between the DII and stroke risk, as it captures only a single point in time. Second, the reliance on self-reported dietary data may introduce biases and inaccuracies, potentially leading to misclassification of dietary exposures. Third, the DII in this study includes only 25 food parameters, meaning that several nutrients with known anti-inflammatory properties—such as certain spices and flavonoids—were not considered. This may result in an incomplete assessment of the overall inflammatory potential of the diets and even an overestimation of their pro-inflammatory effects. Fourth, the diagnosis of stroke is based solely on self-report, lacking confirmation from imaging or other objective criteria, which could further introduce diagnostic inaccuracies. Fifth, the temporal relationship between dietary intake and stroke occurrence remains unclear. In this study, the dietary and nutritional indicators were based on the meal on the day before the visit, but the exact timing of stroke events is unknown. Considering that an individual’s diet may vary over time and often changes following the onset of a major disease, it is difficult to ascertain whether the current diet is causally related to past stroke events or if adopting a low DII diet would indeed reduce future stroke risk. Additionally, the lack of differentiation between stroke types and the influence of age variations among participants might affect the findings.
Conclusions
This study demonstrates a significant association between DII and stroke risk in hypertensive patients. Future research should prioritize longitudinal studies—and ideally collect dietary data from patients at the time of acute stroke—to establish causality and investigate additional confounding factors, thereby deepening our understanding of the relationship between diet, inflammation, and stroke risk.
Data availability
The raw datasets can be accessed on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm).
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under the curve
- BMI:
-
Body Mass Index
- CDC:
-
Centers for Disease Control and Prevention
- CHD:
-
Coronary Heart Disease
- CI:
-
Confidence interval
- CKD-EPI:
-
Chronic kidney disease epidemiology collaboration
- CRP:
-
C-reactive protein
- DBP:
-
Diastolic blood pressure
- DII:
-
Dietary inflammatory index
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration rate
- FPG:
-
Fasting plasma glucose
- HDL-C:
-
High-density lipoprotein cholesterol
- IL-6:
-
Interleukin-6
- LASSO:
-
Least absolute shrinkage and selection operator
- LDL-C:
-
Low-density lipoprotein cholesterol
- NCHS:
-
National Center for Health Statistics
- NHANES:
-
National Health and Nutrition Examination Survey
- OR:
-
Odds ratio
- PIR:
-
Poverty income ratio
- RCS:
-
Restricted cubic splines
- ROC:
-
Receiver operating characteristic
- SBP:
-
Systolic blood pressure
- SCR:
-
Serum creatinine
- SE:
-
Standard error
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- TNF-α:
-
Tumor necrosis factor-alpha
References
Forouzanfar, M. H. et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA 317, 165–182 (2017).
Donnan, G. A., Fisher, M., Macleod, M. & Davis, S. M. Stroke. Lancet 371, 1612–1623 (2008).
Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396:1204–1222.
Mozaffarian, D., Appel, L. J. & Van Horn, L. Components of a cardioprotective diet: new insights. Circulation 123, 2870–2891 (2011).
Estruch, R. et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 368, 1279–1290 (2013).
Ridker, P. M., Rifai, N., Stampfer, M. J. & Hennekens, C. H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101, 1767–1772 (2000).
Libby, P., Ridker, P. M. & Maseri, A. Inflammation and atherosclerosis. Circulation 105, 1135–1143 (2002).
Shivappa, N. et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 17, 1825–1833 (2014).
Shivappa, N., Wirth, M. D., Hurley, T. G., Hébert, J. R. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and Nutrition Examination Survey-1999–2002. Mol. Nutr. Food Res. 61 (2017).
Ruiz-Canela, M. et al. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvención con DIeta MEDiterránea) trial. Br. J. Nutr. 113, 984–995 (2015).
Johnson, C. L. et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital. Health Stat. 2, 1–24 (2013).
Chobanian, A. V. et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 42, 1206–1252 (2003).
Shivappa, N., Steck, S. E., Hurley, T. G., Hussey, J. R. & Hébert, J. R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 17, 1689–1696 (2014).
Matsumoto, Y. et al. Change in dietary inflammatory index score is associated with control of long-term rheumatoid arthritis disease activity in a Japanese cohort: the TOMORROW study. Arthrit. Res. Ther. 23, 105 (2021).
Rattan, P. et al. Inverse association of telomere length with liver disease and mortality in the US population. Hepatol. Commun. 6, 399–410 (2022).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Chen, T. C. et al. National health and nutrition examination survey: Estimation procedures, 2011–2014. Vital. Health Stat. 2, 1–26 (2018).
Rajaratnam, B., Roberts, S., Sparks, D. & Dalal, O. Lasso regression: Estimation and shrinkage via the limit of gibbs sampling. J. R. Stat. Soc. Ser. B Stat. Methodol. 78, 153–174 (2015).
Zhang, Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann. Transl. Med. 4, 30 (2016).
Cavicchia, P. P. et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 139, 2365–2372 (2009).
Szypowska, A., Regulska-Ilow, B., Zatońska, K., Szuba, A. Comparison of intake of food groups based on dietary inflammatory index (DII) and cardiovascular risk factors in the middle-age population of lower Silesia: Results of the PURE Poland Study. Antioxidants (Basel) 12 (2023).
Tabung, F. K. et al. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: Results from the Women’s Health Initiative. Cancer Causes Control 26, 399–408 (2015).
Wirth, M. D. et al. Construct validation of the dietary inflammatory index among African Americans. J. Nutr. Health Aging 21, 487–491 (2017).
Fung, T. T. et al. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am. J. Clin. Nutr. 73, 61–67 (2001).
Kastorini, C. M. et al. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 57, 1299–1313 (2011).
Trichopoulou, A., Costacou, T., Bamia, C. & Trichopoulos, D. Adherence to a mediterranean diet and survival in a Greek population. N. Engl. J. Med. 348, 2599–2608 (2003).
Calder, P. C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 83, 1505s–1519s (2006).
Scalbert, A., Manach, C., Morand, C., Rémésy, C. & Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 45, 287–306 (2005).
Ma, Y. et al. Association between dietary fiber and serum C-reactive protein. Am. J. Clin. Nutr. 83, 760–766 (2006).
Estruch, R. et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 378, e34 (2018).
Threapleton, D. E. et al. Dietary fiber intake and risk of first stroke: A systematic review and meta-analysis. Stroke 44, 1360–1368 (2013).
Jakobsen, M. U. et al. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 89, 1425–1432 (2009).
Mozaffarian, D., Micha, R. & Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 7, e1000252 (2010).
Wang, X. et al. Efficacy of folic acid supplementation in stroke prevention: A meta-analysis. Lancet 369, 1876–1882 (2007).
Bazzano, L. A. et al. Dietary intake of folate and risk of stroke in US men and women: NHANES I epidemiologic follow-up study. National Health and Nutrition Examination Survey. Stroke 33, 1183–1188 (2002).
Acknowledgements
We extend our appreciation to the researchers, staff, and participants of the National Health and Nutrition Examination Survey for their invaluable contributions, which made this analysis possible. This study did not receive any specific funding from public, commercial, or non-profit funding agencies.
Funding
This work was supported by the Shantou Medical and Health Category Science and Technology Plan Project (NO.220811205271420) and Baoshan Science and Technology Planning Project (NO.2024bskyjlzd007).
Author information
Authors and Affiliations
Contributions
J.F., Y.Y., Y.J.Y., and D.R. were pivotal in conceptualizing the research and overseeing its design. J.F., Y.Y., Y.J.Y., and D.R. spearheaded the data analysis and interpretation. S.G., M.L., and Y.W. took responsibility for creating the figures and tables. J.F. and D.R. drafted the initial version of the manuscript. Y.W., along with other contributors, reviewed and approved the final manuscript draft. Y.F. and C.Z., as the corresponding authors, ensured the integrity and accuracy of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study adhered to ethical protocols #98-12, #2005-06 and its continuation, and #2011-17 and its continuation. All participants provided written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fan, J., Yang, Y., Yang, Y. et al. Predictive role of the dietary inflammatory index on stroke risk among hypertensive patients. Sci Rep 15, 13602 (2025). https://doi.org/10.1038/s41598-025-96908-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96908-5
Keywords
This article is cited by
-
Association of the dietary inflammation index with the prevalence of stroke in patients with diabetes
Scientific Reports (2025)