Abstract
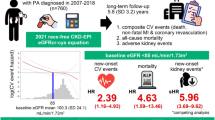
Although the connection between decreased kidney function and hypertension is commonly acknowledged, there is insufficient research examining the relationship between renal hyperfiltration (higher-than-normal estimated glomerular filtration rate (eGFR)) and the incidence risk of hypertension. Therefore, through a nationwide longitudinal study, our research aimed to explore the relationship between the eGFR and the incidence risk of hypertension in the general population. This research used the cohort records for the National Health Insurance Service in Korea, analyzing records from 1,873,550 individuals between the ages of 20 and 79 who underwent health check-ups between 2010 and 2011. The eGFR levels, determined by applying the 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, were employed to evaluate the renal function. An incidence of hypertension was confirmed when a diagnosis of (primary or secondary) hypertension (International Classification of Diseases (ICD)-10 codes I10-I11) was noted at least once per year during outpatient or inpatient care with a prescription for antihypertensive medication or at least one more surpassing 140/90 mmHg from a health examination after the index date after excluding diagnosis of secondary hypertension. The mean age of subjects was 46.03 ± 11.24 years. The 411,029 (21.9%) hypertension cases were identified over a median follow-up of 9.53 years. In the multivariable Cox regression analysis, compared with the 5th decile, the 10th eGFR deciles (≥ 115.58 mL/min/1.73 m²) (hazard ratio (HR): 0.87, 95% confidence interval (CI)(0.85–0.88), p < 0.001) demonstrated a significant association with a reduced incidence of hypertension. Moreover, an eGFR exceeding 120 mL/min/1.73 m² was linked to a lowered likelihood of hypertension (HR: 0.78, 95% CI (0.76–0.80), p < 0.001) compared to normal eGFR levels (90 ~ 120 mL/min/1.73 m²). In contrast, in the subgroup analysis of ages over 70 years old, renal hyperfiltration was not associated with a reduced incidence of hypertension. In our study, renal hyperfiltration were associated with a reduced risk of hypertension, and this association was particularly significant in those younger than 70 years old. The association between renal hyperfiltration and a lower risk of hypertension incidence was likely to vary with age.
Similar content being viewed by others
Introduction
Impaired kidney function is associated with cerebrovascular and cardiovascular diseases or with risk factors such as atrial fibrillation, heart failure, myocardial infarction, and stroke1,2. A standard indicator of chronic kidney disease (CKD) is a diminished estimated glomerular filtration rate (eGFR), and this decreased eGFR level significantly correlates with the likelihood of developing cardiovascular disease1,3,4. In addition to low eGFR, an unusually higher-than-normal eGFR can also be associated with multiple health issues. Although a higher-than-normal eGFR is typically noted as a regular physiological condition or better renal function, it might signal an underlying abnormal kidney function. For instance, in individuals with high blood pressure, it could indicate early signs of damage to the kidney’s filtering units or suggest the presence of preclinical kidney disease in diabetic patients5. Renal hyperfiltration (Higher-than-normal eGFR) is closely linked with prediabetes, diabetes, and obesity, which could contribute to the occurrence of cerebrovascular or cardiovascular events6.
Hypertension is a significant health issue globally, with projections estimating that it will affect about 1.4 billion people worldwide by 20257. Given its potent correlation with cardiovascular disease, strokes, and mortality, exploring effective preventative methods to prohibit the onset of hypertension is crucial8,9,10. A range of environmental elements, including lifestyle choices and genetic susceptibilities, heavily influence the occurrence of hypertension. Modifiable lifestyle factors, such as excessive salt consumption, insufficient intake of potassium, weight gain, and a sedentary lifestyle, elevate the chances of developing hypertension. However, addressing these factors alone does not entirely decrease the risk of hypertension. Hence, it is essential to continue research efforts to identify additional correlating factors or risk factors to enhance preventative strategies against hypertension11.
Since renal hyperfiltration is often perceived as an indicator of fine renal function, the population displaying this elevated eGFR may experience a decreased likelihood of developing hypertension. Conversely, if a renal hyperfiltration is an early sign of kidney disease, it might imply a greater risk of hypertension. However, currently, there is limited research on whether a renal hyperfiltration may be related to the onset of hypertension. Therefore, our research sought to examine the link between renal hyperfiltration and the incidence risk of hypertension in the general population by conducting a nationwide longitudinal study.
Methods
Data source
This study sourced data from the National Health Insurance Service-Health Screening Cohort (NHIS-HEALS) database, a subset of the Korean NHIS. The NHIS, a government program, provides health insurance to nearly 97% of the Korean population. The Medical Aid program, an affiliate of the NHIS, attends to the 3% of the population that the NHIS does not cover12,13. The NHIS encourages participants to undergo standardized health check-ups every two years to aid in the early identification and prevention of diseases. The NHIS-HEALS database collects various information, including demographic details, socioeconomic background, health screening results, recorded diagnoses, and treatment specifics. These screenings involve assessments such as height, weight, blood pressure, lab tests, and evaluations of lifestyle behaviors14,15,16,17,18,19.
Study population
The study’s cohort from the NHIS-HEALS database included 2,708,874 individuals aged between 20 and 79 who participated in health screenings from 2010 to 2011 (under the dataset identifier: NHIS-2022-01-313)13,20,21. From this group, we excluded individuals previously diagnosed with end-stage renal disease (ESRD), which totaled 34,138 individuals. Moreover, 53,474 individuals had demographic and laboratory missing data and were excluded. Additionally, 747,712 individuals with a history of hypertension and secondary hypertension were excluded at the commencement of the study. Finally, the study’s analysis involved a sample of 1,873,550 participants (Fig. 1).
Definitions and variables
The starting point for tracking each participant’s outcome, referred to as the index date, was established based on the date of their health evaluation. To determine the eGFR, serum creatinine levels from the health check-up were used along with the formulas provided by the 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) (Supplementary Methods)22. Baseline characteristics, including age, gender, body mass index, waist circumference, and household income, were evaluated on the index date. Details on habits, such as smoking, drinking alcohol, and regular exercise, were collected through questionnaires. Participants’ smoking habits were classified as non-smoker, past smoker, or current smoker. Both alcohol intake and consistent physical activity were noted in terms of their weekly occurrence. Proteinuria was confirmed if the urine dipstick test showed a result of ≥ + 1. Comorbidities, including diabetes mellitus, dyslipidemia, myocardial infarction, heart failure, cardiomyopathy, valvular heart disease, hyperthyroidism, and congenital heart disease, were recognized using specific criteria from January 2009 to the index date (refer to Supplementary Methods). Diagnostic codes were categorized according to the International Classification of Diseases (ICD)-10, following methodologies from prior research23,24,25,26,27,28. The incidence of hypertension was stipulated as having at least one annual outpatient or inpatient claim diagnosis (primary or secondary) for hypertension, as indicated by International Classification of Diseases (ICD)-10 codes I10-I11, coupled with a prescription for antihypertensive medication. Alternatively, it was identified by the presence of at least one systolic blood pressure (SBP) reading from a health examination conducted after the index date that surpassed 140 mmHg or diastolic blood pressure (DBP) surpassing 90 mmHg27,29,30. If an individual had an ICD-10 code (I12, I15, E21, E24, E26, E27, C74.1, or D35) corresponding to secondary hypertension at the time of meeting the inclusion criteria for hypertension, they were not considered to have developed the outcome.
Statistical analysis
The data are presented either as mean ± standard deviation or as numbers and percentages. To investigate the association between eGFR and the incidence of hypertension, participants were stratified into eGFR deciles or classified following eGFR ranges (< 30, 30–60, 60–90, 90–120, and > 120 mL/min/1.73m2). The reference group comprised the fifth decile and ranged from 90 to 120 mL/min/1.73 m², respectively. The distribution of eGFR for the overall population and across different age groups was accessed with Shapiro-Wilk test, the results indicated no significant deviation from a normal distribution (p > 0.05), confirming that eGFR levels followed a normal distribution regardless stratified age groups. The examination of this relationship utilized Kaplan–Meier survival curves, and distinctions between eGFR deciles and ranges were assessed through log-rank tests. The hazard ratio (HR) and 95% confidence intervals (CIs) for the correlation between eGFR and the incidence of hypertension were calculated using Cox proportional hazard models. Multivariable regression analysis was employed to adjust for potential confounding factors which were closely related with hypertension, including variables such as sex, age, body mass index, waist circumference, income levels, smoking, alcohol consumption, regular physical activity, proteinuria, diabetes mellitus, dyslipidemia, heart failure, myocardial infarction, valvular heart disease, cardiomyopathy, hyperthyroidism, congenital heart disease, and Charlson comorbidity index. Subgroup analyses were conducted, considering age and body mass index, as these variables are closely linked to eGFR levels. In the context of a sensitivity analysis, additional assessment was conducted by calculating eGFR levels using the Modification of Diet in Renal Disease (MDRD) study equation31. Statistical analyses were performed using SAS software (version 9.2, SAS Institute, Cary, NC, USA), and the significance was established with a p-value < 0.05.
Ethical approval statement
This study received approval from the Ewha Womans University College of Medicine’s Institutional Review Board, which also granted a waiver for consent (approval number: SEUMC 2022-02-018). This was because the NHIS allows researchers unrestrained use of anonymous data for research objectives.
Results
The mean age of the subjects in this study was 46.03 ± 11.24 years, with men forming 50.4% of the subjects. The prevalence of current smokers, diabetes mellitus, and dyslipidemia was 25.0%, 3.9%, and 10.2%, respectively. In terms of eGFR categories, the distribution of individuals with eGFR levels < 30, 30–60, 60–90, 90–120 (used as the reference), and > 120 mL/min/1.73m2 were 0.1%, 2.0%, 41.4%, 51.2%, and 5.3%, respectively (Table 1).
Considering higher-than-normal eGFR, individuals with eGFR > 120 mL/min/1.73m2 appeared to have a lower mean BMI and smaller waist circumferences and were younger than the other groups (Supplementary Table 1). Moreover, a lower prevalence of proteinuria, diabetes mellitus, and dyslipidemia was noted in individuals with an eGFR > 120 mL/min/1.73 m2 (Supplementary Table 1).
Over a median follow-up period of 9.53 years (interquartile range: 9.12–10.02 years), 411,029 (21.9%) hypertension cases were identified. Kaplan–Meier survival analysis demonstrated a significant association between the decreased risk of hypertension and elevated eGFR in the 10th decile (≥ 115.58 mL/min/1.73m2) (p < 0.001), and renal hyperfiltration group (> 120mL/min/1.73m2) (p < 0.001). Considering the eGFR range, an eGFR > 120mL/min/1.73m2 was also associated with a decreased risk of hypertension (Fig. 2A and B).
In the multivariable analysis, compared with the 5th decile, the 10th eGFR deciles (≥ 115.58 mL/min/1.73m2) (HR: 0.87, 95% CI (0.85–0.88), p < 0.001) were significantly associated with a decreased incidence of hypertension (Table 2, Supplementary Table 2). Moreover, an eGFR > 120mL/min/1.73m2 was associated with a reduced risk of hypertension (HR: 0.78, 95% CI (0.76–0.80), p < 0.001) (Table 2 and Supplementary Table 3). In both the decile and range groups, as the eGFR increased, the hazard ratio plot illustrated a decrease in the hazard ratio for hypertension (Fig. 3).
Hazard ratios for hypertension as determined by the estimated glomerular filtration rate (Fig. 3A: deciles, Fig. 3B: ranges).
The hazard ratios, depicting the correlation between renal function and hypertension incidence, are presented as (a) decile groups or (b) eGFR ranges. The solid blue line illustrates the multivariate-adjusted hazard ratios with corresponding 95% confidence intervals for each group, while the dashed lines denote a hazard ratio of 1. The red line signifies restricted cubic spline curves. The hazard ratios were calculated using the multivariable Cox model outlined in Table 2.
In the subgroup analysis, the relationship between higher-than-normal eGFR and a reduced risk of hypertension incidence was consistently noted in the age < 70 group. However, in the age ≥ 70 group, there was no association between the 10th eGFR decile and the incidence of hypertension, compared to the 5th decile (HR: 0.97, 95% CI (0.93–1.01), p = 0.180) (Supplementary Tables 4, 5).
Regarding body mass index, in obese participants (body mass index ≥ 25), compared to the 5th decile, the 10th eGFR decile had a significant association with a decreased incidence of hypertension (HR: 0.92, 95% CI (0.90–0.94), p < 0.001). Furthermore, an eGFR > 120mL/min/1.73m2 was related to a decreased risk of hypertension in obese participants (HR: 0.86, 95% CI (0.83–0.90), p < 0.001) compared to normal kidney function (eGFR 90 ~ 120) (Supplementary Table 6). Additionally, the association between an eGFR > 120mL/min/1.73m2 and a decreased risk of incidence for hypertension was consistently shown irrespective of the patient’s history of diabetes mellitus (p for interaction = 0.341). Regardless of whether eGFR was measured using the MDRD method, the correlation between higher-than-normal eGFR levels and risk for incidence of hypertension remained consistently evident in the sensitivity analyses (Supplementary Table 7). Moreover, the association of renal hyperfiltration with decreased risk of incidence of hypertension also noted regardless of age groups and sex except ≥ 70 years group (Supplementary Tables 4, 5, and 8 ~ 20).
Discussion
The main findings from our investigation were that individuals with a 10th decile eGFR (≥ 115.58 ml/min/1.73 m²) or those falling with an eGFR > 120mL/min/1.73m2 had a decreased risk of hypertension, except in patients aged ≥ 70 years.
A higher-than-normal eGFR, also called glomerular hyperfiltration, has been considered a clinical marker of early kidney impairment, especially in individuals with diabetes and hypertension32,33,34. A meta-analysis of 780 type I diabetes mellitus patients revealed that those with hyperfiltration had an elevated likelihood of developing microalbuminuria and nephropathy progression35. A study monitoring 502 young-to-middle-aged Italian patients with stage I hypertension also found that after 8 years of follow-up, hyperfiltration was an independent predictor of urinary albumin excretion33. Another previous study analyzed 99,140 prediabetes and prehypertension Japanese people and observed that glomerular hyperfiltration increased as the stages of prediabetes and prehypertension progressed36. Hyperfiltration, demonstrating an association with components of the so-called metabolic syndrome37, has also been recognized to predict cardiovascular outcomes independently. According to a recent study, which followed 8794 patients for 6.2 years, eGFR and adverse cardiovascular outcomes had a U-shaped relationship38.
Meanwhile, according to data from 2,645,042 individuals in the Korean NHIS, hyperfiltration demonstrated an inverse association with atrial fibrillation risk39. Furthermore, according to a study analyzing health checkup data from 3124 individuals presented in Italy, the association between various elements of metabolic syndrome (blood pressure, fasting blood glucose, lipid profile, waist circumference, etc.) and hyperfiltration varied depending on the method used to measure the eGFR, with some cases identifying a relationship and others not40. Therefore, the inconsistent results regarding the association between a renal hyperfiltration and hypertension may be attributed to differences in the study’s design or population being used.
Amidst the inconsistent findings in earlier research, our study found no significant relationship between glomerular hyperfiltration and the onset of hypertension in the ≥ 70 age group. Although we cannot clearly explain this result, several possible explanations exist. In the general population, the eGFR naturally decreases at an average rate of around 1 mL per minute per year after age 40 to 50 years41. Therefore, in the group aged 70 or older, even if glomerular hyperfiltration occurs, the absolute eGFR may be measured as low. This factor can mask the prevalence of hypertension in individuals who are already in the stages of whole-kidney-GFR decline. Several studies have found that patients with specific clinical conditions are prone to developing glomerular hyperfiltration. Among these factors, being male, younger, and having a higher body mass index were found to be significant contributors to glomerular hyperfiltration in both diabetic and hypertensive patients33. Therefore, even in the presence of glomerular hyperfiltration, the association with hypertension may not be significant if the aforementioned clinical factors do not coexist. It is possible that these results were derived from the healthy participant effect, as the eGFR > 120 group had a relatively lower BMI than the normal eGFR group and included relatively more individuals with good overall health. In fact, statistical significance for the association of renal hyperfiltration with incidence risk of hypertension was absent in the aged (≥ 70 years old) population, therefore, caution should be used in interpreting our results.
A renal hyperfiltration might be linked to dysfunction in the renin–angiotensin system through vascular inflammation, increased arterial stiffness, and endothelial dysfunction since these are the main components that lead to cardiovascular disease and a higher risk of death42,43,44,45. Conversely, in our study, there was a reduced risk of hypertension in the group with a renal hyperfiltration. Although previous studies have focused on the detrimental effect of renal hyperfiltration on long-term kidney function, it is necessary to reiterate that a renal hyperfiltration is an adaptive mechanism to efficiently manage salt and fluid excretion in response to increased metabolic demands. When the kidney experiences early stress, hyperfiltration can enhance the clearance of sodium and metabolic waste products, thereby helping to maintain extracellular fluid balance and preventing the salt retention and volume expansion that are known drivers of CKD-inducing hypertension46. This adaptive response is analogous to ischemic preconditioning observed in myocardial tissue—short, controlled episodes of stress that render the organ more resistant to subsequent insults47. Similarly, early renal hyperfiltration may confer a protective effect against minor kidney injuries caused by glomerular hypertension, such as new-onset diabetes mellitus or atrial fibrillation48,49.
Our study demonstrated that renal hyperfiltration in the general population may be associated with a lower risk of developing hypertension, likely due to the predominance of younger and relatively healthy individuals in our study population. In younger individuals without underlying health issues, maintaining good renal function over time may contribute to a lower risk of hypertension, suggesting that renal hyperfiltration could potentially serve as a preventive factor in this group. However, this association was not observed in individuals aged 70 years or older, indicating that renal hyperfiltration does not necessarily confer a lower hypertension risk in the elderly population. Therefore, our findings should not be overinterpreted. In older individuals, even when renal hyperfiltration is present, maintaining a healthy lifestyle—through dietary modifications, regular physical activity, and blood pressure monitoring—remains essential for hypertension prevention, regardless of eGFR levels.
This study has some limitations that need to be recognized. Firstly, our results might be influenced by potential ethnic bias, potentially restricting the broader relevance of our conclusions to other demographic groups. It is important to conduct further studies across various racial and ethnic groups. Secondly, our study only analyzed eGFR in a cross-sectional manner and did not consider measurements of cystatin C, as these were not available in the NHIS database. Thirdly, our dataset cannot provide important factors for hypertension development, such as dietary habits or sodium intake. Moreover, our dataset does not collect information on socio-economic status, access to health care, etc. as they are personal information and therefore cannot be adjusted. Also, lifestyle habits may change, but it is difficult for our dataset to adjust for these aspects. Fourthly, a chance exists that we may have overestimated the kidney function in individuals with an eGFR > 90 mL/min/1.73m2, which might affect the reliability of our results. In addition, individuals with high eGFR may simply reflect low muscle mass, which should be clearly distinguished from true glomerular hyperfiltration. Nevertheless, our dataset does not provide information on muscle mass. Therefore, it should be noted that muscle mass was not considered when interpreting our results. Finally, despite our research being a comprehensive, nationwide longitudinal study, its retrospective design makes it challenging to determine cause-and-effect relationships.
Conclusion
In this study, renal hyperfiltration were associated with a lower risk of incidence of hypertension, with particular significance for this association noted only in those younger than 70 years. The association between a renal hyperfiltration and a lower risk of incidence of hypertension is likely to vary according to age.
Data availability
The data used in this study are available in the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) database; however, restrictions apply to the public availability of these data used under license for the current study. Requests for access to the NHIS data can be made through the National Health Insurance Sharing Service homepage (http://nhiss.nhis.or.kr/bd/ab/bdaba021eng.do). For access to the database, a completed application form, research proposal, and application for approval from the Institutional Review Board should be submitted to the inquiry committee of research support at the NHIS for review.
References
Song, T. J. et al. Distribution of cerebral microbleeds determines their association with impaired kidney function. J. Clin. Neurol. 10, 222–228. https://doi.org/10.3988/jcn.2014.10.3.222 (2014).
Deferrari, G., Cipriani, A. & La Porta, E. Renal dysfunction in cardiovascular diseases and its consequences. J. Nephrol. 34, 137–153. https://doi.org/10.1007/s40620-020-00842-w (2021).
Gansevoort, R. T. et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 382, 339–352. https://doi.org/10.1016/S0140-6736(13)60595-4 (2013).
Liu, Y. et al. Relationship between lipoprotein (a), renal function indicators, and chronic kidney disease: evidence from a large prospective cohort study. JMIR Public. Health Surveillance. 10, e50415 (2024).
Helal, I., Fick-Brosnahan, G. M., Reed-Gitomer, B. & Schrier, R. W. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 8, 293–300. https://doi.org/10.1038/nrneph.2012.19 (2012).
Kanbay, M. et al. Renal hyperfiltration defined by high estimated glomerular filtration rate: A risk factor for cardiovascular disease and mortality. Diabetes Obes. Metab. 21, 2368–2383. https://doi.org/10.1111/dom.13831 (2019).
Mills, K. T. et al. Global disparities of hypertension prevalence and control: A systematic analysis of Population-Based studies from 90 countries. Circulation 134, 441–450. https://doi.org/10.1161/CIRCULATIONAHA.115.018912 (2016).
Collaborators, G. B. D. R. F. Global, regional, and National comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 392, 1923–1994. https://doi.org/10.1016/S0140-6736(18)32225-6 (2018).
Forouzanfar, M. H. et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm hg, 1990–2015. JAMA 317, 165–182. https://doi.org/10.1001/jama.2016.19043 (2017).
Shu, T. et al. Assessing global, regional, and National time trends and associated risk factors of the mortality in ischemic heart disease through global burden of disease 2019 study: Population-Based study. JMIR Public. Health Surveillance. 10, e46821 (2024).
Carey, R. M., Muntner, P., Bosworth, H. B. & Whelton, P. K. Prevention and control of hypertension: JACC health promotion series. J. Am. Coll. Cardiol. 72, 1278–1293. https://doi.org/10.1016/j.jacc.2018.07.008 (2018).
Chang, Y., Woo, H. G., Park, J., Lee, J. S. & Song, T. J. Improved oral hygiene care is associated with decreased risk of occurrence for atrial fibrillation and heart failure: A nationwide population-based cohort study. Eur. J. Prev. Cardiol. 27, 1835–1845. https://doi.org/10.1177/2047487319886018 (2020).
Chang, Y., Lee, J. S., Lee, K. J., Woo, H. G. & Song, T. J. Improved oral hygiene is associated with decreased risk of new-onset diabetes: a nationwide population-based cohort study. Diabetologia 63, 924–933. https://doi.org/10.1007/s00125-020-05112-9 (2020).
Kim, D. et al. Association of gamma-glutamyl transferase variability with risk of osteoporotic fractures: A nationwide cohort study. PLoS One. 18, e0277452. https://doi.org/10.1371/journal.pone.0277452 (2023).
Chang, Y., Lee, H. & Song, T. J. Association of gamma-glutamyl transferase variability with risk of venous thrombosis. Sci. Rep. 13, 7402. https://doi.org/10.1038/s41598-023-34368-5 (2023).
Kim, D., Kim, J. H. & Song, T. J. Total cholesterol variability and the risk of osteoporotic fractures: A nationwide Population-Based cohort study. J. Pers. Med. 13 https://doi.org/10.3390/jpm13030509 (2023).
Chang, Y., Kim, S. H., Jeon, J., Song, T. J. & Kim, J. Oral health and risk of retinal vascular occlusions: A nationwide cohort study. J. Pers. Med. 13 https://doi.org/10.3390/jpm13010121 (2023).
Park, J. H., Kim, J. W., Lee, H., Hong, I. & Song, T. J. Better Oral Hygiene Is Associated with a Decreased Risk of Meniere’s Disease: A Nationwide Cohort Study. J. Pers. Med. 13 https://doi.org/10.3390/jpm13010080 (2022).
Park, J. H., Chang, Y., Kim, J. W. & Song, T. J. Improved oral health status is associated with a lower risk of venous thromboembolism: A nationwide cohort study. J. Pers. Med. 13 https://doi.org/10.3390/jpm13010020 (2022).
Park, M. S., Jeon, J., Song, T. J. & Kim, J. Association of periodontitis with microvascular complications of diabetes mellitus: A nationwide cohort study. J. Diabetes Complications. 36, 108107. https://doi.org/10.1016/j.jdiacomp.2021.108107 (2022).
Seong, S. C. et al. Cohort profile: the National health insurance Service-National health screening cohort (NHIS-HEALS) in Korea. BMJ Open. 7, e016640. https://doi.org/10.1136/bmjopen-2017-016640 (2017).
Tent, H. et al. Performance of MDRD study and CKD-EPI equations for long-term follow-up of nondiabetic patients with chronic kidney disease. Nephrol. Dial Transpl. 27 (Suppl 3), iii89–95. https://doi.org/10.1093/ndt/gfr235 (2012).
Song, T. J., Kim, J. W. & Kim, J. Oral health and changes in lipid profile: A nationwide cohort study. J. Clin. Periodontol. 47, 1437–1445. https://doi.org/10.1111/jcpe.13373 (2020).
Woo, H. G., Chang, Y., Lee, J. S. & Song, T. J. Association of Tooth Loss with New-Onset Parkinson’s Disease: A Nationwide Population-Based Cohort Study. Parkinsons Dis. 2020 (4760512). https://doi.org/10.1155/2020/4760512 (2020).
Chang, Y., Woo, H. G., Lee, J. S. & Song, T. J. Better oral hygiene is associated with lower risk of stroke. J. Periodontol. 92, 87–94. https://doi.org/10.1002/JPER.20-0053 (2021).
Lee, K. et al. Oral health and Gastrointestinal cancer: A nationwide cohort study. J. Clin. Periodontol. 47, 796–808. https://doi.org/10.1111/jcpe.13304 (2020).
Kim, J., Kim, H. J., Jeon, J. & Song, T. J. Association between oral health and cardiovascular outcomes in patients with hypertension: a nationwide cohort study. J. Hypertens. 40, 374–381. https://doi.org/10.1097/HJH.0000000000003022 (2022).
Song, T. J., Chang, Y., Jeon, J. & Kim, J. Oral health and longitudinal changes in fasting glucose levels: A nationwide cohort study. PLoS One. 16, e0253769. https://doi.org/10.1371/journal.pone.0253769 (2021).
Lee, H., Park, M. S., Kang, M. K. & Song, T. J. Association between proteinuria status and risk of hypertension: A nationwide Population-Based cohort study. J. Pers. Med. 13 https://doi.org/10.3390/jpm13091414 (2023).
Woo, H. G., Chang, Y., Lee, J. S. & Song, T. J. Tooth loss is associated with an increased risk of hypertension: A nationwide population-based cohort study. PLoS One. 16, e0253257. https://doi.org/10.1371/journal.pone.0253257 (2021).
Levey, A. S. et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin. Chem. 53, 766–772. https://doi.org/10.1373/clinchem.2006.077180 (2007).
Tonneijck, L. et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J. Am. Soc. Nephrology: JASN. 28, 1023 (2017).
Palatini, P. et al. Glomerular hyperfiltration predicts the development of microalbuminuria in stage 1 hypertension: the HARVEST. Kidney Int. 70, 578–584 (2006).
Amin, R. et al. The relationship between microalbuminuria and glomerular filtration rate in young type 1 diabetic subjects: the Oxford regional prospective study. Kidney Int. 68, 1740–1749 (2005).
Magee, G. M. et al. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52, 691–697 (2009).
Okada, R. et al. Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol. Dialysis Transplantation. 27, 1821–1825 (2012).
Bystad, E. W., Stefansson, V. T., Eriksen, B. O. & Melsom, T. The association between metabolic syndrome, hyperfiltration, and long-term GFR decline in the general population. Kidney Int. Rep. 8, 1831–1840 (2023).
Reboldi, G. et al. Glomerular hyperfiltration is a predictor of adverse cardiovascular outcomes. Kidney Int. 93, 195–203 (2018).
Kang, M. K. et al. Association of high estimated glomerular filtration rate with risk of atrial fibrillation: a nationwide cohort study. Front. Med. 10, 1207778 (2023).
Monami, M. et al. Glomerular hyperfiltration and metabolic syndrome: results from the FIrenze-BAgno A Ripoli (FIBAR) study. Acta Diabetol. 46, 191–196 (2009).
Coresh, J., Astor, B. C., Greene, T., Eknoyan, G. & Levey, A. S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: third National health and nutrition examination survey. Am. J. Kidney Dis. 41, 1–12 (2003).
Reboldi, G. et al. Glomerular hyperfiltration is a predictor of adverse cardiovascular outcomes. Kidney Int. 93, 195–203. https://doi.org/10.1016/j.kint.2017.07.013 (2018).
Cherney, D. Z. et al. Renal hyperfiltration and arterial stiffness in humans with uncomplicated type 1 diabetes. Diabetes Care. 33, 2068–2070. https://doi.org/10.2337/dc10-0767 (2010).
Cherney, D. Z. et al. Hyperfiltration and effect of nitric oxide Inhibition on renal and endothelial function in humans with uncomplicated type 1 diabetes mellitus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R710–718. https://doi.org/10.1152/ajpregu.00286.2012 (2012).
Cui, C. et al. Arterial stiffness and obesity as predictors of diabetes: longitudinal cohort study. JMIR Public. Health Surveillance. 10, e46088 (2024).
Noronha, I. L. et al. Glomerular filtration in the aging population. Front. Med. 9, 769329 (2022).
Tomai, F., Crea, F., Chiariello, L. & Gioffrè, P. A. Ischemic preconditioning in humans: models, mediators, and clinical relevance. Circulation 100, 559–563 (1999).
Kim, M. J., Kang, M. K., Hong, Y. S., Leem, G. H. & Song, T. J. Association of renal hyperfiltration with incidence of New-Onset diabetes mellitus: A nationwide cohort study. J. Clin. Med. 5 https://doi.org/10.3390/jcm13175267 (2024).
Kang, M. K. et al. Association of high estimated glomerular filtration rate with risk of atrial fibrillation: a nationwide cohort study. Front. Med. (Lausanne). 10 https://doi.org/10.3389/fmed.2023.1207778 (2023).
Author information
Authors and Affiliations
Contributions
HJH, MKK, and JHK contributed to data interpretation and manuscript drafting. HJH, JHK, and TJS participated in data analysis and interpretation. Y-HC and TJS contributed to the conception, design, data acquisition, interpretation, and critical revision of the manuscript. All authors provided final approval and agreed to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional Information
The authors declare no potential conflicts of interest with respect to authorship and/or publication of this article. This work was supported by Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea government(MSIT) (No. 2022-0-00621, RS-2022-II220621, Development of artificial intelligence technology that provides dialog-based multi-modal explainability). This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2023-00262087 to TJS). This study was funded by the National Research Foundation of Korea (grant number: NRF-2020R1A5A2019210433 to YHC). The funding source had no role in the design, conduct, or reporting of this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ha, HJ., Kang, M.K., Kim, J.H. et al. Renal hyperfiltration is associated with a reduced incidence of hypertension in individuals younger than 70. Sci Rep 15, 12573 (2025). https://doi.org/10.1038/s41598-025-97023-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97023-1
Keywords
This article is cited by
-
Shear wave elastography as a reliable tool in the prediction of renal histopathological abnormalities
BMC Medical Imaging (2026)