Abstract
Deep Brain Stimulation (DBS) is a critical intervention for various neurological disorders. While effective, the traditional local infiltration anesthesia used in DBS surgeries often hinders electrophysiological recording quality and patient cooperativeness. The research aims to evaluate the impact of local infiltration versus scalp block anesthetic methods on electrophysiological signal quality and patient cooperativeness during DBS surgeries. This study involved patients who participated in an intraoperative task during the bilateral subthalamic nucleus DBS surgery for Parkinson’s Disease between Jan 2020 and Dec 2022. Patients were either administered the traditional local infiltration anesthesia or the modified scalp block anesthesia. Intraoperative electrophysiological recording data and anesthetic data was collected. Spike sorting was performed to evaluate the recording stability. Patient cooperativeness and intraoperative experience was assessed and compared. The patients under scalp block anesthesia exhibited shorter pre-acquisition time, longer stable recording time, higher number of tasks per site, higher number of neurons recorded per task (all ps < 0.05). In behavior, patients under scalp block anesthesia showed higher accuracy in tasks (p < 0.05), while the response time was comparable. The overall satisfaction of anesthesia was also higher in scalp block, as revealed by the visual analogue scale, Likert scale and mean arterial pressure (all ps < 0.05). The modified scalp block anesthetic method offers considerable advantages over traditional local infiltration anesthesia in DBS surgeries. It helps to improve both patient comfort and cooperation during the surgery, and thereby enhancing the overall quality of neurological data and efficacy of DBS procedures.
Similar content being viewed by others
Introduction
Deep Brain Stimulation (DBS) has emerged as a transformative intervention in the treatment of a spectrum of neurological disorders, providing relief to patients burdened by conditions such as Parkinson’s disease (PD), dystonia, essential tremor and treatment-resistant psychiatric disorders1,2. Beyond its clinical efficacy, the significance of DBS extends into the realm of cutting-edge neuroscience, positioning itself as a powerful tool for research on the function of brain3,4. Psycho-physiological research performed during the DBS surgery is a unique and reliable application of DBS surgery as a research platform, allowing researchers to delve into the dynamic interplay of neural activity and psychological behavior5,6.
Whether in the clinical setting of DBS surgery or in research applications, there is a high demand for effective anesthesia due to the requirement that patients can complete electrical stimulation testing or cooperate with researchers in the behavior tasks during the surgery. The traditional anesthetic method for awake DBS surgery is local infiltration anesthesia, which is still most widely used in most DBS centers7. This simple anesthetic method can provide effective pain management and allow for patient cooperation during the surgery. However, with advancements in medical practices, a modified approach involving neural block anesthesia has emerged as an alternative8. This modified scalp block method boasts the advantage of offering more comprehensive anesthesia coverage throughout the surgical process. Scalp block anesthesia may contribute to a more stable and controlled surgical environment, reducing interference and noise that could compromise the fidelity of electrophysiological data. Beyond its impact on signal quality, the choice of anesthesia can also influence the patient’s level of cooperativeness during the procedure. While local anesthesia allows for some degree of patient interaction, the modified scalp block anesthesia holds promise in potentially fostering increased patient cooperation8. The depth and scope of anesthesia achieved through neural block techniques may alleviate patient discomfort and anxiety, promoting a more conducive environment for patients to actively engage in psychological tasks during the surgery9.
Nevertheless, the effects of anesthetic methods on electrophysiological recordings in combination with behavioral task during DBS surgery have never been investigated. In this study, we evaluated the impact of the traditional local infiltration anesthesia and the modified scalp block anesthesia on signal quality in electrophysiological recordings and the level of patient cooperativeness during intraoperative tasks. This study provides valuable insights that may inform best practices in the field.
Materials and methods
Ethics approval and patient population
This was a single-center, prospective, observational cohort study which was performed in accordance with the Declaration of Helsinki and all procedures were approved by the local IRB of Beijing Tiantan Hospital (No. KY2019-097-05). The patients were recruited for a bilateral DBS surgery through the clinics of Beijing Tiantan Hospital between Jan 2020 and Dec 2022. The inclusion criteria for the cohort study were as follows: (1) aged between 50 and 75 years; (2) diagnosed with idiopathic PD; (3) indicated for bilateral Subthalamic Nucleus (STN) DBS surgery; (4) agreement to participate in the intraoperative task and provision of written informed consent signed by the patient or their legal guardian. The patients whose preoperative Mini-mental State Examination (MMSE) score was less than 24, or Montreal Cognitive Assessment (MoCA) score was less than 1810, and those patients who could not go through with the task either in the training or in the real surgery, or whose intraoperative electrophysiological signal quality was unacceptable were excluded. Baseline demographic and clinical characteristics were collected for all enrolled patients, including age, gender, disease duration, Hoehn and Yahr stage, levodopa equivalent daily dose (LEDD), MMSE scores, MoCA scores, Hamilton Anxiety Rating Scale (HAMA) scores, Hamilton Depression Rating Scale (HAMD) scores, and preoperative movement disorder society-sponsored unified parkinson’s disease rating scale (MDS-UPDRS) scores (part III, in the medication-on state). Postoperative MDS-UPDRS III scores were also assessed in both the medication-on and stimulation-on states. All patients included provided their ethic approval for the study.
Anesthetic protocols
For patients anesthetized with the traditional method, 1% lidocaine together with 0.5% ropivacaine was infused surrounding the straight incisions on the frontal part of the head where the entry point of DBS leads located. On the other hand, participants using the modified scalp block anesthetic method underwent scalp nerve blockade with 0.5% ropivacaine to block the bilateral supraorbital, auriculo-temporal and supratrochlear nerves before the operation. Local infiltration of 1% lidocaine around the incisions was conducted to reinforce anesthesia8 (Fig. 1).
Schematic diagrams of anesthesia methods. The modified scalp block anesthesia method includes scalp nerve blockade with ropivacaine to block the bilateral supraorbital, ototemporal and supratrochlear nerves and local infiltration of lidocaine around the incisions (A), while the traditional local infiltration anesthesia method is only local infiltration of lidocaine around the incisions (C). The area covered by these anesthesia methods was shown on the right side (B, D). SO supraorbital nerve, AT auriculo-temporal nerve, ST supratrochlear nerve.
Surgical procedures
The surgical procedures were described in previous reports11,12. All patients underwent a bilateral, frame-based STN DBS surgery. Stereotactic planning was carried out with preoperative 3.0T MRI merged with stereotactic CT scan obtained on the day of surgery using the SurgiPlan software (Elekta, Sweden). Coordinates of the DBS targets and the entry points were calculated using the direct targeting method as described elsewhere13,14. Briefly, after anesthesia and disinfection, incisions were made on the scalp near the entry point of DBS trajectories. A 1.5-cm burr hole was made on each side of the skull. Microelectrode recording (MER) was conducted with a single tungsten microelectrode (400–700 kOhm, Neuroprobe, Alpha Omega, Israel) driven through the central passage on the electrode holder (Bengun, Alpha Omega, Israel) into the target area by a microdrive (Drive Headstage, Alpha Omega, Israel) with a stepsize of 100–200 μm. The dura puncture technique used on all patients has been described elsewhere15. STN signals were recorded using the Neuro Omega recording system (Alpha Omega, Israel), as described elsewhere12,16. The intraoperative tasks were performed when the neuronal firing signal of STN was continuously recorded, as mentioned below. After the task, the DBS electrodes (Model 3389 S, Medtronic, US; L 301, Pins, China) were implanted, with the lowest contact of the electrode being placed near the bottom of STN according to the microelectrode recording findings. Stimulation effectiveness and side effects were tested using the DBS electrode by neurosurgeons using an external stimulator, followed by implantation of the rest parts of the DBS system if stimulation effectiveness was confirmed and no side effects were elicited. The locations of the DBS electrodes, reconstructed with Lead-DBS software17, were independently checked by neurosurgeons (J.Z. and L.S.).
Intraoperative task
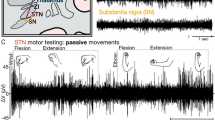
During the electrophysiological recording process, an intraoperative task was performed whose detailed description can be found in previous work16,18. Briefly, when the microelectrode read typical STN signal, the stepsize of the microdrive was lowered to 20–50 μm to decrease the thrust of the microelectrode towards the brain. When a site with active neuronal firing was found, the microdrive was stopped for at least 2 min to wait for the stabilization of the signal. If the neuronal firing signal faded away, the microelectrode was advanced to seek for another site with good signal. If the signal was still stable after 2 min, the intraoperative task was initiated. The task was a working memory task which consisted of a series of 108 images chosen from an online image database19, 54 of these images being unique and others being repeated. Each image belonged to one of three categories (evenly distributed among animals, landscapes, and fruits). The images were shown on a liquid crystal display screen placed in front of the patient. Each trial started with a fixation cross shown for 0.5–1.0 s (randomized). Afterwards, an image was shown for 1.5 s. After image offset, a second fixation cross was shown for 0.5 s, followed by a question to categorize each image as seen or unseen with a response pad (RB-844, Cedrus Inc, UK) by pressing the red or green button on the pad. The question remained on the screen until the patient made a response or 5 s lapsed (Fig. 2). The task was implemented in Matlab (R2021a, Mathworks, US)20. Each participant completed 2–8 independent sessions of this working memory task in all site with stable signal depending on intraoperative cooperation, with fully randomized and non-overlapping image sets across sessions to avoid learning effects. On the day before surgery, the patient was trained with a shortened version of the task. The patients who could not understand the task or who did not wish to coordinate could quit before or during surgery.
Outcome measures
To assess the effects of anesthesia, the pain level was assessed using visual analogue scale. Blood pressure and bispectral index were recorded. Mean arterial pressure was calculated using the diastolic pressure plus one third of the pulse pressure21. To reflect the dynamic variations through the surgery, all these variables were determined at the following five time points (Supplementary Fig. 1), including entering the operation room (T0), before surgery started (T1), before task was initiated (T2), after task was finished (T3), and after surgery (T4). The overall surgical experience satisfaction was evaluated using the seven-point Likert scale22. The number of adverse events was recorded throughout the surgery, including unstoppable tremor, spiking of blood pressure, extremely nervous or neurotic conditions of the patients, obvious dyspnea, and other conditions that greatly hinder the continuation of surgery.
Aiming to estimate the neuronal firing stability, the locations where each task was performed, the pre-acquisition time (the period of looking for a stable firing site before each task was initiated), the longest stable recording time (the time length between task initiation and the moment that signal got lost) were collected. The normalized root mean square(nRMS), a parameter of the electrode recorded sampling signal to evaluate signal intensity23, was exported from the Neuro Omega recording system. Besides, single-unit activity in the electrophysiological data was sorted using the Osort software (an open-source semi-automatic template-matching algorithm, version 4.0)24. Clusters that represented putative single neurons with stable firing were identified using criteria described in a previous study25,26, as shown in Fig. 3. The number of clusters for each task was counted according to the spike sorting results. In addition, the behavioral results including task accuracy rate and average response time of the patients were calculated.
An exemplary single unit in subthalamic nucleus. (A) Notch filtered raw data showing multi-unit firing. The spikes that pass the red lines are counted and further processed; (B) Overlapped waveform of all spikes assigned to a neuron; (C) Distribution of the inter-spike intervals; (D) Spike amplitude and firing rate.
Sample size and statistical analyses
The sample size was calculated using the longest stable recording time. According to our preliminary study, the longest stable recording time during DBS in PD patient without nerve block procedure is approximately 15 min(900s). We calculated the required number of patients using PASS 11 software with an alpha error of 0.05 and power of 95% for a 5-minute difference in longest stable recording time, and determined that 37 patients would need to be included in each group. Considering the dropout rate of 10%, we defined a sample size of 82 participants.
For continuous quantitative data, descriptive statistics were presented as mean ± standard deviation or median [P25, P75], and comparisons between groups were performed using the t-test or the rank-sum test, as appropriate. For categorical data, frequencies and percentages were used for description, and intergroup comparisons were conducted using the chi-square test or Fisher’s exact probability test. For repeated measures data, the generalized estimating equation model was implemented using the geepack package in R to estimate intergroup differences. The correlation structure was set as “exchangeable,” and adjusted for age, gender, and course. Additionally, the false discovery rate method was applied to correct P-values for intergroup comparisons at different time points. To account for potential confounding factors between groups, 1:1 propensity score matching was performed using the MatchIt package in R. Propensity scores were calculated using a binary logistic model with a caliper value of 0.1, and the included confounding variables were age, gender, and course. The same analyses were repeated for the matched data after PSM. Furthermore, inverse probability weighting (IPW) was conducted using the ipw package in R to control for confounding factors, with the same set of variables included as in the PSM analysis. The balance between groups before and after matching or weighting was evaluated using the standardized mean difference, and an SMD value of less than 0.2 was considered indicative of good intergroup balance. All statistical analyses were conducted with R 4.4.227. Significance was recognized if p value \(\:\le\:\) 0.05.
Results
Data overview
Between Jan 2020 and Dec 2022, 82 patients were included in the original psychological research performed during the DBS surgery. Of the 82 patients, 74 whose data was complete provided approval for the current study, with 39 anesthetized with the modified scalp block method and 35 anesthetized with the traditional local infiltration method (Supplemental Fig. 2). Patient characteristics were balanced between groups (Supplemental Table 1). The locations of the task-side DBS electrodes, shown on the postoperative CT images which were co-registered to the preoperative MRI images, were all placed accurately as preoperatively planned. There were no significant differences in both the preoperative and the postoperative scores of MDS-UPDRS III between the two groups. After propensity score matching or inverse probability weighting, the standardized mean differences for age, gender, and course in both groups decreased to approximately 0.1 or lower, indicating improved balance between the groups (Supplemental Figs. 3–4).
Neuronal firing stability and behavioral results of the intraoperative task
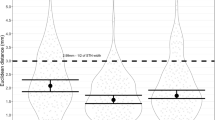
During DBS surgery, the modified group completed a mean of 6.9 ± 1.2 working memory tasks in all site with stable signal, compared to 6.1 ± 1.2 tasks completed by the traditional group. During the working memory task, the modified anesthesia group exhibited shorter pre-acquisition time (439.9 ± 42.5 s vs. 536.2 ± 54.3 s; p < 0.001) and longer stable recording time (1276.8 ± 410.9 s vs. 839.3 ± 382.3 s, p < 0.001). Additionally, this group completed more tasks per site (2.3 ± 0.8 tasks vs. 1.5 ± 0.5 tasks, p < 0.001), comparing with the traditional group. Single unit sorting results further indicated that the number of neurons recorded per task was significantly higher in the modified group than in the traditional group (1.3 ± 0.4 vs. 1.1 ± 0.3, p = 0.044). These findings collectively suggest superior neuronal firing stability in the modified anesthesia group during the working memory task (Fig. 4 and Supplemental Table 2). After propensity score matching or inverse probability weighting, we repeated the aforementioned analyses and found results consistent with those obtained previously (Supplemental Tables 3–4).
Neuronal firing stability and behavioral results of the intraoperative task. (A) Pre-acquisition time; (B) average number of tasks per site; (C) max number of tasks per site; (D) longest stable recording time; (E) number of neurons per site; (F) task response time; (G) task accuracy; (H) mean nRMS per task. MA modified anesthesia; nRMS normalized root mean square, TA traditional anesthesia. **p < 0.05; ***p < 0.001.
Furthermore, as shown in Fig. 4, the modified group demonstrated higher task accuracy (0.9 ± 0.03) compared to the traditional group (0.8 ± 0.03, p < 0.001), while response times were similar (1.0 ± 0.4 s vs. 1.3 ± 0.7 s, p = 0.107). The means of nRMS per task were 2.2 ± 0.2 in the modified group and 1.3 ± 0.7 in the traditional group, with no significant differences between the groups (p = 0.107). Together, these results highlight enhanced task performance in the modified group, which, to some extent, reflects the increase in patient cooperation during surgery. The subsequent analyses conducted after applying propensity score matching and inverse probability weighting yielded results that were consistent with our earlier findings (Supplemental Tables 3–4).
Assessments of anesthetic management in the DBS surgery
As shown in Fig. 5 and Supplemental Table 5, after controlling for confounding factors such as age, gender, and course, the results from the generalized estimating equation model showed that the modified anesthesia group reported lower pain levels at all timepoints during the surgery (T1–T4), as measured by the visual analogue scale (all p < 0.05). After implementing propensity score matching and inverse probability weighting approaches, we performed the same analytical procedures and obtained results that aligned with our previous observations (Supplemental Tables 6–7). They also exhibited lower mean arterial pressure (all p < 0.05). Patient satisfaction, assessed via the Likert scale, was significantly higher in the modified group than in the traditional group (5.6 ± 1.1 vs. 4.3 ± 1.5, p < 0.001). Moreover, fewer adverse events were recorded in the modified group (0.5 ± 1.1) as compared with the traditional group (1.1 ± 1.0, p = 0.008). These results suggested that patients with modified anesthesia had a better surgical experience and less adverse events during the surgery.
Discussion
This prospective observational cohort study aimed to investigate the effect of scalp nerve block combined with local anesthesia on the electrophysiological signal quality and patient cooperativeness during DBS surgery in patients with PD. We found that patients who underwent the modified anesthesia reported higher electrophysiological signal quality and better cooperation. In addition, after adjusting for confounding factors by implementing multiple approaches including propensity score matching and inverse probability weighting, our results remained largely consistent with the previous maintenance. These findings indicate that the modified anesthesia may be an effective intervention to enhance electrophysiological signal quality and patient cooperativeness during DBS surgery.
Scalp nerve block and local anesthesia are common anesthetic techniques used in awake neurosurgical procedures such as DBS surgery. However, there is still debate over which method is more appropriate28. Under traditional anesthesia, patients often experience pain and anxiety. In response to pain, the body activates the sympathetic nervous system, triggering the release of epinephrine and norepinephrine, which accelerate heart rate, induce vasoconstriction, and subsequently elevate blood pressure29. This physiological response may increase the risk of hemorrhage and other complications, particularly in PD patients, who are generally older and have relatively poor vascular conditions8. Furthermore, postoperative opioid dependence for pain relief is more common, potentially leading to adverse events such as nausea, respiratory depression, and drug dependence30,31. Previous studies have shown that the scalp nerve block can provide perioperative analgesia and reduce complications related to intraoperative and postoperative pain, which aligns with our research where the modified technique of combining scalp nerve block with local anesthesia, as compared to traditional local anesthesia, significantly reduced intraoperative mean arterial pressure, decreased the incidence of adverse events, and alleviated pain and anxiety32,33,34,35. Additionally, it may lower the occurrence of hemorrhage and other blood pressure-related complications, providing greater overall benefits for patients. Additionally, some patients opt for general anesthesia for DBS surgery due to anxiety, tension, or other psychological factors. However, general anesthesia has certain drawbacks. For instance, sedatives such as propofol may significantly attenuate MER signals36. Moreover, intraoperative stimulation cannot be performed under general anesthesia, immediate response of clinical effects and adverse effects associated with stimulation cannot be assessed during the STN DBS surgery37. Furthermore, general anesthesia may be associated with a higher risk of symptomatic intracranial hemorrhage38. Our technique offers better patient comfort and overall satisfaction and, in certain cases, can serve as an alternative to general anesthesia. This approach facilitates intraoperative testing and MER while also providing opportunities for studying human behavior.
It is accepted that behavior is the result of brain function and brain processes govern how we feel, act, learn, and remember39. Although modern technologies such as structural and functional MRI have achieved excellent spatial resolution, and techniques like EEG and MEG offer high temporal resolution, the spatial and temporal resolution of these imaging modalities remains far below the level required to record individual neurons and their small-scale neural assemblies40,41. Understanding the behavior and function of individual neurons during tasks is not only a prerequisite for establishing brain-behavior connections but also critical for unraveling the pathological mechanisms underlying various diseases42,43. Intraoperative MER during DBS offers a unique opportunity to study the function of subcortical brain structures in humans, since it provides several attractive features for research: both single-unit activity, representing the activity of individual neurons, and local field potentials (LFPs), representing the combined activity of local populations of cells, can be recorded along the electrode trajectory44. This provides an opportunity to develop more precise therapeutic interventions and may even help identify early biomarkers of pathological activity. Therefore, obtaining high-quality single-neuron electrophysiological data is essential for advancing neuroscience research. Patients, with their heads immobilized in stereotactic frames, actively engage in tasks while microelectrodes capture neural signals, including spike firing and local field potentials in the basal ganglia. To acquire signal with good quality, neurosurgeons need to proceed the microelectrode very cautiously in 10 to 50 μm steps and wait for hundreds of seconds for the stabilization of the signal. The difficulties in securing stable neural recordings during such instances can lead to suboptimal signal quality, thereby hampering the success of the research6,45. Instability in recordings can necessitate repeated adjustments, prolonging surgery and increasing patient risk16. Under traditional anesthesia, increased tremors or involuntary movements caused by pain can lead to microelectrode displacement relative to the target nucleus, resulting in frequent signal loss. The findings of our study highlight a significant advantage of the modified anesthetic method in improving signal quality during DBS surgery, enhancing the overall quality of intraoperative research involving behavioral tasks. Additionally, the increased satisfaction provided to patients led to greater endurance and a stronger willingness to cooperate, allowing them to engage in necessary intraoperative tasks for a longer duration (Fig. 6).
Treatment with DBS is promising and it is already applied or is being considered for the treatment of various other conditions, like obsessive-compulsive disorder, chronic pain, Alzheimer’s disease, Tourette syndrome, autism, or other psychiatric disorders46,47,48,49,50. Clinically, the improved signal stability and patient cooperation directly contribute to the precision of electrode placement, which is critical for the effectiveness of DBS. Academically, Intraoperative electrophysiological studies during DBS offer a unique opportunity to access various brain regions, allowing us to explore the dynamic relationship between neural activity and behavior5,6. Therefore, patient cooperation and surgical precision are crucial to successful research outcomes, highlighting the need for interdisciplinary collaboration in optimizing DBS as both a clinical treatment and a research tool.
Our study has limitations. The major limitation is the observational nature of the study, which failed to balance the number of patients in the two groups. To strengthen future research, a randomized controlled trial would provide more robust evidence by minimizing bias and enhancing the reliability of the results in assessing the efficacy of anesthesia methods in deep brain stimulation surgery.
In conclusion, this study offers valuable insights into the optimization of anesthetic strategies in DBS surgeries. It opens avenues for further research and potential modifications in clinical practice, aiming to enhance both the quality of neuroscientific research and patient experience during DBS surgery.
Data availability
Data is available upon reasonable request to the corresponding authors after the approval of the local IRB.
Abbreviations
- DBS:
-
Deep brain stimulation
- MMSE:
-
Mini-mental state examination
- MoCA:
-
Montreal cognitive assessment
- PD:
-
Parkinson’s disease
- STN:
-
Subthalamic nucleus
- MDS-UPDRS:
-
Movement disorder society-sponsored unified parkinson’s disease rating scale
- nRMS:
-
Normalized root mean square
References
Mayberg, H. S. et al. Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660 (2005).
Cagnan, H., Denison, T., McIntyre, C. & Brown, P. Emerging technologies for improved deep brain stimulation. Nat. Biotechnol. 37, 1024–1033 (2019).
Khan, I. S., D’Agostino, E. N., Calnan, D. R., Lee, J. E., & Aronson, J. P. Deep brain stimulation for memory modulation: A new frontier. World Neurosurg. 126 (2019).
Schulder, M. et al. Advances in technical aspects of deep brain stimulation surgery. Stereotact. Funct. Neurosurg. 101, 112–134 (2023).
Kiminski, J. et al. Novelty-Sensitive dopaminergic neurons in the human substantia Nigra predict success of declarative memory formation. Curr. Biol. CB 28 (2018).
Mosher, C. P. et al. Cellular classes in the human brain revealed in vivo by Heartbeat-Related modulation of the extracellular action potential waveform. Cell. Rep. 30 (2020).
Lettieri, C. et al. Deep brain stimulation: subthalamic nucleus electrophysiological activity in awake and anesthetized patients. Clin. Neurophysiol. Off J. Int. Fed. Clin. Neurophysiol. 123, 2406–2413 (2012).
Krauss, P., Marahori, N. A., Oertel, M. F., Barth, F., & Stieglitz, L. H. Better hemodynamics and less antihypertensive medication: comparison of scalp block and local infiltration anesthesia for Skull-Pin placement in awake deep brain stimulation surgery. World Neurosurg. 120 (2018).
Sung, C. H. et al. Scalp block is associated with improved recurrence profiles in patients undergoing primary glioma resection surgery. J. Neurosurg. Anesthesiol. 33, 239–246 (2021).
Mini-Mental State. Examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI) - PubMed. https://pubmed.ncbi.nlm.nih.gov/34313331/
Kocabicak, E. & Temel, Y. Deep brain stimulation of the subthalamic nucleus in Parkinson’s disease: surgical technique, tips, tricks and complications. Clin. Neurol. Neurosurg. 115, 2318–2323 (2013).
Koirala, N. et al. Mapping of subthalamic nucleus using microelectrode recordings during deep brain stimulation. Sci. Rep. 10, 19241 (2020).
Mirzadeh, Z. et al. Parkinson’s disease outcomes after intraoperative CT-guided ‘asleep’ deep brain stimulation in the globus pallidus internus. J. Neurosurg. 124, 902–907 (2016).
Li, B., Jiang, C., Li, L., Zhang, J. & Meng, D. Automated segmentation and reconstruction of the subthalamic nucleus in Parkinson’s disease patients. Neuromodulation J. Int. Neuromodulation Soc. 19, 13–19 (2016).
Fan, S. et al. Comparison of dural puncture and dural incision in deep brain stimulation surgery: A simple but worthwhile technique modification. Front. Neurosci. 16 (2022).
Shi, L. et al. Microstimulation is a promising approach in achieving better lead placement in subthalamic nucleus deep brain stimulation surgery. Front. Neurol. 12, 683532 (2021).
Rodrigues, R. B. et al. Lead-DBS: an additional tool for stereotactic surgery. Rev. Assoc. Med. Bras. 67 (2021).
Shi, L. et al. Comparison of cognitive performance between patients with Parkinson’s disease and dystonia using an intraoperative recognition memory test. Sci. Rep. 11, 20724 (2021).
Tkačik, G. et al. Natural images from the birthplace of the human eye. PloS One. 6, e20409 (2011).
Niehorster, D. C., Andersson, R., Nyström, M. & Titta A toolbox for creating PsychToolbox and psychopy experiments with tobii eye trackers. Behav. Res. Methods. 52, 1970–1979 (2020).
Hamzaoui, O. & Shi, R. Microcirculation and mean arterial pressure: friends or foes? Ann. Transl Med. 8, 803 (2020).
Dourado, G. B. et al. Likert scale vs visual analog scale for assessing facial pleasantness. Am. J. Orthod. Dentofac. Orthop. Off Publ Am. Assoc. Orthod. Its Const. Soc. Am. Board. Orthod. 160, 844–852 (2021).
Xie, S. et al. Choice of anaesthesia in microelectrode recording-guided deep-brain stimulation for Parkinson’s disease (CHAMPION): study protocol for a single-centre, open-label, non-inferiority randomised controlled trial. BMJ Open. 13, e071726 (2023).
Wu, Y. et al. An unsupervised real-time Spike sorting system based on optimized OSort. J. Neural Eng. 20 (2023).
Rutishauser, U., Mamelak, A. N. & Schuman, E. M. Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron 49, 805–813 (2006).
Sisterson, N. D. et al. Electrocorticography during deep brain stimulation surgery: safety experience from 4 centers within the National Institute of neurological disorders and stroke research opportunities in human consortium. Neurosurgery 88, E420–E426 (2021).
R: A Language and Environment for Statistical Computing. (2012).
Chen, Y. J. et al. Monitored anesthetic care combined with scalp nerve block in awake craniotomy: an effective attempt at enhanced recovery after neurosurgery. World Neurosurg. 154, e509–e519 (2021).
Saccò, M. et al. The relationship between blood pressure and pain. J. Clin. Hypertens. Greenwich Conn. 15, 600–605 (2013).
Duda, T. et al. Systematic review and Meta-Analysis of randomized controlled trials for scalp block in craniotomy. Neurosurgery 93, 4–23 (2023).
Patel, K. S. et al. Selective scalp block decreases short term post-operative pain scores and opioid use after craniotomy: A case series. J. Clin. Neurosci. Off J. Neurosurg. Soc. Australas. 93, 183–187 (2021).
Watson, R. & Leslie, K. Nerve blocks versus subcutaneous infiltration for stereotactic frame placement. Anesth. Analg. 92, 424–427 (2001).
Effect of Scalp Nerve Block Combined with Intercostal Nerve Block on the Quality. Of recovery in patients with Parkinson’s disease after deep brain stimulation: protocol for a randomized controlled Trial - PubMed. https://pubmed.ncbi.nlm.nih.gov/36009070/
Regional Anesthesia to Scalp for Craniotomy. Innovation With Innervation - PubMed. https://pubmed.ncbi.nlm.nih.gov/26083426/
Regional scalp block for. postcraniotomy analgesia: a systematic review and meta-analysis - PubMed. https://pubmed.ncbi.nlm.nih.gov/23477962/
Raz, A., Eimerl, D., Zaidel, A., Bergman, H. & Israel, Z. Propofol decreases neuronal population spiking activity in the subthalamic nucleus of parkinsonian patients. Anesth. Analg. 111, 1285–1289 (2010).
Park, H. R. et al. Bilateral subthalamic nucleus deep brain stimulation under general anesthesia: literature review and single center experience. J. Clin. Med. 9, 3044 (2020).
Holewijn, R. A. et al. General anesthesia vs local anesthesia in microelectrode Recording-Guided Deep-Brain stimulation for Parkinson disease: the GALAXY randomized clinical trial. JAMA Neurol. 78, 1212–1219 (2021).
Notterman, J. M. The brain, neurons, and behavior. Science 306, 1683 (2004).
Gupta, P., Balasubramaniam, N., Chang, H. Y., Tseng, F. G. & Santra, T. S. A Single-Neuron: current trends and future prospects. Cells 9, 1528 (2020).
Fried, I. Neurons as will and representation. Nat. Rev. Neurosci. 23, 104–114 (2022).
Romo, R. & Salinas, E. Flutter discrimination: neural codes, perception, memory and decision making. Nat. Rev. Neurosci. 4, 203–218 (2003).
Kubska, Z. R. & Kamiński, J. How human Single-Neuron recordings can help Us understand cognition: insights from memory studies. Brain Sci. 11, 443 (2021).
Sanchez, A. M. A., Roberts, M. J., Temel, Y. & Janssen, M. L. F. Invasive neurophysiological recordings in human basal ganglia. What have we learned about non-motor behaviour? Eur. J. Neurosci. 60, 6145–6159 (2024).
Ramayya, A. G., Zaghloul, K. A., Weidemann, C. T., Baltuch, G. H. & Kahana, M. J. Electrophysiological evidence for functionally distinct neuronal populations in the human substantia Nigra. Front. Hum. Neurosci. 8, 655 (2014).
Deep Brain Stimulation for Obsessive-Compulsive Disorder and. Depression - PubMed. https://pubmed.ncbi.nlm.nih.gov/37018916/
Tan, H., Yamamoto, E. A., Elkholy, M. A. & Raslan, A. M. Treating chronic pain with deep brain stimulation. Curr. Pain Headache Rep. 27, 11–17 (2023).
Liu, Z., Shu, K., Geng, Y., Cai, C. & Kang, H. Deep brain stimulation of fornix in Alzheimer’s disease: from basic research to clinical practice. Eur. J. Clin. Invest. 53, e13995 (2023).
Marini, S., D’Agostino, L., Ciamarra, C. & Gentile, A. Deep brain stimulation for autism spectrum disorder. World J. Psychiatry. 13, 174–181 (2023).
Figee, M. et al. Deep brain stimulation for depression. Neurother. J. Am. Soc. Exp. Neurother. 19, 1229–1245 (2022).
Acknowledgements
We thank the patients and families for their participation. Also, we are grateful for all those who have been devoted in the study but are not listed as authors.
Funding
This research was supported by the National Natural Science Foundation of China (82471490), Beijing Natural Science Foundation (JQ23038), Beijing Hospitals Authority Clinical Medicine Development of special funding support (YGLX202321) and Bethune Charitable Foundation (bnmr-2023-009).
Author information
Authors and Affiliations
Contributions
S.X. and L.S. conceived and designed the study. S.X., Y.L., R.H., J.Z, A.Y., F.M., C.J. and L.S. performed the study and collected data. H.F., Y.L. and S.X. investigated the data and performed the statistical analysis. S.X., Y.L., H. F. and L.S. wrote the manuscript, and all authors reviewed the manuscript. All authors met the requirements for authorship. All authors believe the manuscript represents honest work, and approved the submission of the final version of the manuscript.# S.X. and Y.L. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The current study was performed in accordance with the Declaration of Helsinki and all procedures were approved by the institutional review board of Beijing Tiantan Hospital (No. KY2019-097-02). The patients included in the study were recruited through the Functional Neurosurgery Department of Beijing Tiantan Hospital between Jan 2020 and Dec 2022.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xie, S., Liu, Y., Yang, A. et al. Scalp block improves electrophysiological stability and patient cooperation during deep brain stimulation surgery. Sci Rep 15, 12596 (2025). https://doi.org/10.1038/s41598-025-97141-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97141-w