Abstract
Membranous nephropathy (MN) is one of the most common glomerular diseases. Although the diagnostic method based on serum PLA2R antibodies has gradually been applied in clinical practice, only 52–86% of PLA2R-associated MN patients show positive anti-PLA2R antibodies. Therefore, renal biopsy remains the gold standard for diagnosing MN. However, the renal biopsy procedure is highly complex and involves multiple steps, including tissue sampling, fixation, dehydration, embedding, sectioning, PAS staining, Masson trichrome staining, and silver staining. Each step requires precise technique from laboratory personnel, as any error can affect the quality of the final tissue sections and, consequently, the diagnosis. As a result, there is an urgent need to develop a method that enables rapid diagnosis after renal biopsy. Previous studies have shown that Raman spectroscopy offers promising results for diagnosing MN, exhibiting high sensitivity and specificity when applied to human serum and urine samples. In this study, we propose a rapid diagnostic method combining Raman spectroscopy of mouse kidney tissue with a CNN-BiLSTM deep learning model. The model achieved 98% accuracy, with specificity and sensitivity of 98.3%, providing a novel auxiliary tool for the pathological diagnosis of MN.
Similar content being viewed by others
Introduction
Chronic kidney disease is becoming an increasingly severe global public health issue, with membranous nephropathy (MN) being a major subtype. MN is characterized by diffuse thickening of the glomerular basement membrane (GBM), subepithelial immune complex deposits, and granular deposits of IgG along the glomerular capillary loops. MN accounts for 30% of cases of glomerular disease, with an incidence of 1.7 per 100,000 per year. A multi-center study conducted by our team in Xinjiang Uygur Autonomous Region, which analyzed 10,684 renal biopsies, revealed that 1503 cases were diagnosed with MN, accounting for 17.61% of biopsies, making it one of the top three pathological types of primary glomerular diseases1.
MN primarily affects podocytes, which play a crucial role in regulating the permeability of various molecules, including proteins, in the kidney. In healthy individuals, large proteins such as serum albumin are not filtered by the kidney, but in MN and other podocyte injury-related diseases, large amounts of protein are excreted in the urine, leading to massive proteinuria and hypoalbuminemia, sometimes accompanied by edema or hypertension. Clinically, MN is the most common glomerular disease associated with nephrotic syndrome in adults. The disease’s pathogenesis involves the formation and deposition of in-situ or circulating immune complexes on the subepithelial side of the GBM, which triggers immune responses through complement activation2. MN is categorized as primary MN or secondary MN, depending on the presence of secondary causes such as systemic lupus erythematosus, malignancies, or hepatitis B virus infection3.
In recent decades, significant advances in understanding the molecular pathogenesis of MN have been made, inspired by animal models such as Heymann nephritis and cationic bovine serum albumin (cBSA)-induced MN models. These animal models have been instrumental in studying the disease mechanism and testing new therapies. Heymann nephritis identified podocyte antigen megalin as a target antigen for in-situ immune complex formation, while cBSA-induced MN is a model of exogenous antigens. In this model, cBSA deposits on the subepithelial side of the GBM, where circulating antibodies form immune complexes with the deposited cBSA, activating the complement pathway and causing glomerular damage and proteinuria4.
Renal biopsy is the gold standard for diagnosing MN; however, the procedure is technically demanding, involving steps such as tissue sampling, fixation, dehydration, embedding, sectioning, and various staining techniques (including hematoxylin–eosin (HE) staining, periodic acid-Schiff (PAS) staining, Masson trichrome staining, and silver staining). Each stage requires meticulous attention to detail from laboratory personnel, as even minor errors can compromise the quality of the tissue sections and, consequently, the diagnostic outcome. In summary, renal biopsy is a complex, time-consuming, and labor-intensive process.
Due to its high chemical specificity, Raman spectroscopy has emerged as a novel and efficient tool for disease diagnosis. In a previous study, our team developed three deep learning models to classify serum data from MN patients, achieving a classification accuracy of 1.0 and a discrimination score above 0.85 for urine data5. Raman spectroscopy provides a highly sensitive and specific method for identifying molecules in biological samples without the need for labels, offering a rapid and precise diagnostic approach. Portable Raman spectrometers further enable on-site rapid detection, eliminating the need for costly equipment and complex procedures. When combined with deep learning, Raman spectroscopy’s data processing capabilities are significantly enhanced, making it increasingly promising for clinical applications6.
Advancements in deep learning have enabled neural network models to automatically extract complex features and patterns from datasets, achieving unprecedented accuracy and efficiency in areas such as image recognition, speech processing, and natural language understanding. These models also exhibit exceptional generalization capabilities7.
In this study, we propose a rapid diagnostic method for MN based on Raman spectroscopy of mouse kidney tissue combined with deep learning, utilizing a CNN-BiLSTM model. The model achieved an accuracy of 98%, with specificity and sensitivity of 98.3%, offering a novel approach to complement the pathological diagnosis of MN.
The main contributions of this study can be summarized as follows: (1) A CNN-BiLSTM model framework was proposed for the diagnosis of mouse MN. This framework integrates Raman spectroscopy data to provide a more comprehensive understanding of molecular properties and characteristics, thereby enhancing diagnostic accuracy for mouse MN and offering new insights into the diagnosis of human MN. (2) The model first uses a Convolutional Neural Network (CNN) for local feature extraction, followed by a BiLSTM that captures global sequence interactions to mine global information, thereby improving the accuracy and robustness of predictions.
Materials and methods
Ethical approval
This study was conducted in accordance with the International Guiding Principles for Biomedical Research Involving Animals, animal welfare regulations, and relevant laboratory operating procedures.
All methods are reported in accordance with ARRIVE guidelines.
The study was approved by the Animal Ethics Committee of Xinjiang Medical University (IACUC-20230627-19).
Animals
Due to the excellent discriminative ability of Raman spectroscopy in MN5, combined with the cost-effectiveness of constructing MN animal model, thirty 6-week-old male BALB/c mice at SPF grade, weighing 22 ± 5 g, were purchased from the Animal Experimental Center of the First Affiliated Hospital of Xinjiang Medical University (license: SCXK (Xin) 2018-0003). The mice were housed under standard conditions (23 ± 3 °C, relative humidity 40–60%).
Induction of MN in mouse by cBSA
The MN mouse model was established following previous studies8,9. Mice were randomly assigned into two groups: the cBSA-induced MN group (n = 20) and the control group (n = 10), using the Random Number Table method. Each experimental unit was a single animal. The MN group received 0.1 mL of an emulsion containing cBSA (0.1 mg, #9058, Chondre) mixed with an equal volume of Freund’s complete adjuvant (#7008, Chondrex) via tail injection. Two weeks later, the MN group was administered intravenous injections of 100 µg cBSA every other day, with subsequent doses increased to 13 mg/kg, three times a week for four weeks. The control group received an equivalent dose of saline. After 4 weeks, 24-h urine samples were collected in metabolic cages, and proteinuria levels above 15 mg/day were considered indicative of successful model establishment. Mice were then euthanized, and blood and kidney samples were collected for further analysis. Raman spectroscopy data were analyzed by personnel who were blinded to the group allocation, ensuring that the analysis was conducted without any bias based on group assignment.
Biochemical analysis
Blood was collected from the retro-orbital sinus using sterile, enzyme-free EP tubes. After clotting at room temperature, samples were centrifuged at 3000 rpm for 10 min at 4 °C. The serum was separated from the supernatant and stored at – 80 °C until analysis. Biochemical parameters, including serum creatinine (SCr), urea (UREA), triglycerides (TG), total cholesterol (TC), and albumin (ALB), were measured using an automated biochemical analyzer.
Histology
Following blood collection, the mice were euthanized by cervical dislocation. The kidneys were promptly removed and rinsed with saline. The left kidney was fixed in 4% paraformaldehyde for histopathological examination, while the right kidney was snap-frozen and stored at – 80 °C. Paraffin-embedded tissues were sectioned and stained with HE and PAS stains to confirm successful model establishment.
Immunofluorescence
Paraffin sections were deparaffinized, followed by antigen retrieval and blocking. The sections were incubated overnight at 4 °C with FITC-conjugated goat anti-mouse IgG (#ab6785, Abcam). Cell nuclei were stained with DAPI, and images were captured using a confocal laser scanning microscope (Leica, Germany).
Raman spectral acquisition
Twenty sections from the MN group and ten sections from the control group were placed in an oven at 65 °C for 2 h and subsequently deparaffinized by immersing them in deparaffinizing solutions I and II for 20 min each. Raman spectra were acquired using a high-resolution confocal Raman spectrometer (LabRAM HR Evolution, Gora Raman Spectroscopy, Ideaoptics, China), with a spectral range of 500–2000 cm−1. The excitation wavelength was set to 785 nm, with an integration time of 15 s and a laser power of 160 mW. Data acquisition was conducted in continuous mode, and the laser beam was focused on the sample surface using a 10× objective lens. To minimize errors during spectral acquisition, three spectra were recorded from different locations of each sample, and the average spectrum of each sample was used for further analysis. In total, 150 Raman spectra from the tissue samples were collected.
Due to the influence of paraffin slides on the Raman spectral data of the kidney tissue, the excitation of paraffin under Raman light illumination generates fluorescence, which can mask or interfere with the tissue’s Raman signal. Therefore, the spectral range from 1273.65 to 1485.85 cm⁻¹ was excluded, and the remaining spectral bands were analyzed5. Detailed information about the specific samples can be found in Table 1.
Data preprocessing
Due to scattering and interference issues during the processing of Raman spectral data, an adaptive iteratively reweighted penalized least squares algorithm (airPLS) was employed to subtract background noise, thereby improving the signal-to-noise ratio (SNR) and facilitating the analysis and interpretation of the experimental results. After background subtraction, baseline drift unrelated to the wavelength was removed. The data smoothing process was performed using the Smooth algorithm, which locally fits the data using polynomials. The window size for fitting and the polynomial order were determined as part of this process; typically, the window size is an odd number. For each data point, a polynomial fit is applied to the data within the selected window to generate the smoothed value for that point, thereby producing the processed data. All preprocessing steps were performed in MATLAB R2022a.
In the experiment, the sample data were divided into categories with an 8:2 ratio, ensuring that each spectral data point was assigned either to the training or testing set. To address the challenges of small sample classification problems, a five-fold stratified cross-validation method was applied to randomly split the data into training and testing subsets. The final model performance was determined by calculating the average of the five test results.
All quantitative data are presented as mean ± standard deviation. Data visualization and statistical analysis were performed using GraphPad Prism 9.0 software. Normality was assessed using the Shapiro–Wilk test, and homogeneity of variance was tested with Bartlett’s test. For data meeting both normality and homogeneity of variance, an unpaired two-tailed Student’s t-test was used to compare two groups, while ANOVA followed by post hoc t-tests was used for multiple comparisons. If these conditions were not met, the nonparametric Kruskal–Wallis test was applied. A P value of < 0.05 was considered statistically significant.
Results
Generation and basic characterization of cBSA-induced MN mouse
We established a cBSA-induced mouse model of MN and conducted comprehensive biochemical and pathological analyses. The results showed significantly elevated levels of urinary protein (25.26 ± 3.56 vs. 2.66 ± 0.61, P < 0.0001), serum creatinine (17.73 ± 10.21 vs. 9.30 ± 3.74, P < 0.05), urea (9.28 ± 3.69 vs. 4.69 ± 1.32, P < 0.001), and triglycerides (1.20 ± 0.55 vs. 0.75 ± 0.24, P < 0.05) in the MN group compared to the control group (Fig. 1a–f). Histopathological evaluation using HE and PAS staining revealed diffuse thickening of the glomerular capillary walls in the MN mice. Immunohistochemical staining for IgG further demonstrated granular deposits along the glomerular basement membrane (Fig. 1g–i), indicating subepithelial immune complex deposition. These findings confirm the successful establishment of the MN model.
Biochemical and pathological images of mouse models. (a) Urinary protein levels in mouse at 24 h; (b–f) Blood biochemical results; (g) Hematoxylin and eosin (HE) staining results; (h) Periodic Acid-Schiff (PAS) staining results; (i) Immunofluorescence staining results (×400). ALB albumin, H&E hematoxylin–eosin, MN membranous nephropathy, PAS periodic acid-Schiff, SCr serum creatinine, TC total cholesterol, TG triglycerides, UREA urea.
Raman spectral analysis
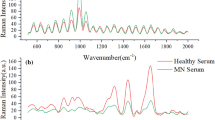
Figure 2a,b show Raman spectrograms of MN mice and healthy mice. Common Raman peaks are observed at 620, 850, 991, 1120, 1520, 1667 and 1860 cm− 1. Table 2 lists some biomolecules corresponding to these characteristic Raman peaks. Figure 2c illustrates the difference in Raman spectra between MN mice and healthy mice.
The Raman peak at 620 cm−1 shows significant differences, corresponding to C–C twisting of aromatic rings. The peak at 850 cm−1 also exhibits noticeable differences, corresponding to polysaccharides. The peak at 991 cm−1 corresponds to phenylalanine, an essential amino acid for protein synthesis. The peak at 1667 cm−1 is associated with proteins. The peak at 1120 cm−1 is related to cell proliferation, differentiation, and apoptosis. The peak at 1520 cm−1 corresponds to carotenoids. The Raman peak at 1860 cm−1 is notably different and is associated with the C=C bond10. MN is a disease characterized by the deposition of immune complexes formed by the reaction between antigens such as PLA2R and antibodies on the glomerular basement membrane11. The PLA2R is a type I transmembrane glycoprotein related to the C-type animal lectin family that includes the mannose receptor. In the development and progression of MN, increased protein and lipid deposition in kidney tissues are common pathological changes, reflecting the kidney’s response to immune damage, oxidative stress, and metabolic disorders. These changes are closely associated with the immunological mechanisms of the disease, glomerular injury, and tubular-interstitial pathology12,13. These findings provide a foundation for subsequent classification and identification of the most sensitive spectral segments.
Deep learning model design
Local information feature extraction
In the study of MN, Raman spectroscopy was utilized to capture information on molecular vibrations and rotations within the renal tissue of mice. These spectral data provide sensitive insights into the chemical composition and microstructural differences of renal tissue. This research introduces an innovative data processing approach that integrates the strengths of a multilayer perceptron (MLP) and a convolutional neural network (CNN).
Specifically, the Raman spectral data from mice were initially preprocessed using a MLP. As a fully connected artificial neural network, the MLP performs nonlinear mapping to extract initial feature information from the spectral data. These processed features then serve as inputs for the CNN, leveraging the integration of the fully connected MLP layers with the locally connected, weight-sharing properties of the CNN layers.
The MLP module facilitates feature mapping by adapting the input data to the convolution operation through dimensionality reduction followed by dimensionality enhancement, thereby improving the model’s expressiveness and generalization ability. It comprises a hidden layer and an output layer, enhancing model performance by weighting and integrating outputs from multiple branches. This process strengthens the model’s capacity to learn complex feature representations, which is particularly advantageous for subsequent CNN and bidirectional long short-term memory (BiLSTM) layers to capture deeper patterns.
The CNN effectively extracts local features from the Raman spectral data through its multilayer structure. By hierarchically abstracting these features, the CNN identifies complex patterns and characteristics within the spectral data, further enhancing the model’s predictive capabilities.
In this study, the CNN was employed to extract intricate features from the Raman spectra of the glomerular basement membrane in MN. By utilizing a series of convolutional and pooling layers, the network effectively learned and integrated local spectral features into higher-level representations. A key strength of the CNN lies in its ability to detect subtle variations within the spectral data, which are intricately linked to the manifestation of MN in mice.
As illustrated in Fig. 3, which depicts the CNN-BiLSTM model architecture, the preprocessed Raman spectral data from mice were first input into the MLP layer. Serving as a feature extractor, the MLP layer performed initial feature extraction from the spectral data. The process is mathematically represented by the following formula:
where\(\:{\:\text{H}}_{\text{m}\text{l}\text{p}}\)is the output of the MLP layer, \(\:{\text{H}}_{\text{m}\text{l}\text{p}}\)and \(\:{\text{b}}_{\text{m}\text{l}\text{p}}\:\)are the weight and bias terms of the MLP layer, respectively, and \(\:X\) is the input Raman spectral data of the mice.
When applying a CNN to extract local features from the Raman spectral data of mice renal tissue14, the convolutional kernel traverses the sequence axis of the input data, computing the dot product between the kernel and localized regions of the input data. This process involves the following operations:
where \(\:I\) is the input Raman spectral data sequence of the mice, \(\:K\) is the convolutional kernel, \(\:i\) is the position of the kernel on the sequence, and \(\:m\) is the index of the convolutional kernel. The convolution operation involves applying a specific set of convolutional kernels \(\:K\) that slide over the data sequence \(\:I\) at designated positions with corresponding index \(\:m\). The multilayer structure of the CNN enables the network to learn hierarchical features within the data, ranging from basic edge and texture information in the initial layers to more complex biomolecular patterns in deeper layers.
Through its multilayer structure, the CNN progressively learns hierarchical features of the data, beginning with shallow attributes such as edges and textures and advancing to deeper, more complex patterns. The convolutional layers perform key feature extraction via convolution operations, generating feature maps that are then transformed using the ReLU activation function. This step introduces nonlinearity, enhancing the network’s capacity to learn and represent complex features.
Following this, a pooling layer is incorporated into the network to reduce the dimensionality of the feature maps. This approach not only lowers the computational demands on subsequent layers but also improves the spatial and temporal invariance of the features. By selecting the maximum values of local features, max pooling becomes instrumental in capturing spectral patterns reflective of variations in microstructure and chemical composition. In this study, the max pooling operation can be mathematically expressed as:
where \(\:P\) is the pooled feature sequence, \(\:F\) is the output of the pooling layer, and \(\:window\left(i\right)\) is the index set of the window centered at \(\:i\). After the convolutional and pooling layers extract features and reduce the dimensionality of the input data, the fully connected layer integrates the learned local feature representations and transforms them into high-level abstract representations tailored for classification and diagnostic tasks. In this layer, each input neuron is connected to every output neuron through weight connections, forming a dense neural network structure that enables comprehensive feature synthesis. This operation can be mathematically expressed as:
where \(\:O\) is the output feature, \(\:W\) is the weight matrix, \(\:F\) is the output feature from the pooling layer, and \(\:b\) is the bias term. By leveraging a deep learning architecture consisting of convolutional layers, pooling layers, and fully connected layers, the CNN automatically uncovers deep feature representations in the Raman spectral data. This process enables efficient feature abstraction and selection, significantly improving the model’s diagnostic classification performance. The integration of these layers optimizes both diagnostic accuracy and computational efficiency. As a result, the CNN is capable of learning complex features from the Raman spectra, facilitating effective feature extraction and enabling more accurate and efficient diagnoses15.
Global information feature interaction
Bidirectional Long Short-Term Memory Network (Bi-LSTM) is an advanced recurrent neural network architecture that captures both forward and backward sequential information at each time step16. When combined with CNN, this model efficiently processes spectral information, enhancing its ability to capture and interpret spatiotemporal features in the data, thus improving feature utilization and predictive accuracy.
In this study, Bi-LSTM is employed for deep learning analysis on time series data, with the goal of improving the accuracy of medical diagnosis. As illustrated in the CNN-BiLSTM model architecture in Fig. 3, the Bi-LSTM considers both the evolution of MN and the latest developments in the condition during disease diagnosis. The Bi-LSTM sends the same time series into two independent LSTM networks: one forward LSTM network analyzes changes from the onset of the disease to the current time, while the other backward LSTM network processes the data in reverse, from the current time to the onset. At each time step, both LSTM networks update their hidden states, which are then merged, typically by concatenating the forward and backward output vectors, to form the comprehensive output of the Bi-LSTM. The prediction vector generated by the CNN-BiLSTM model provides a precise assessment for MN diagnosis in mice.
The key advantage of Bi-LSTM lies in its ability to handle long-term and short-term dependencies, effectively addressing the vanishing or exploding gradient problems that traditional Recurrent Neural Networks (RNN) face when processing long sequences. The bidirectional learning strategy further enhances the model’s ability to analyze time-series data, significantly improving both prediction accuracy and reliability. This method enables the model to comprehensively consider both past and future information, offering clinicians more precise and complete data, which supports more scientifically informed medical decisions. The mathematical operation for this process is represented as:
The advantages of Bi-LSTM in sequence prediction tasks are not only due to its ability to capture bidirectional context but also its efficient processing of long sequences, which makes it particularly effective in prediction tasks. These characteristics make Bi-LSTM an ideal choice for diagnosing MN in mice17.
By combining CNNs, which excel in extracting local features from data, with Bi-LSTM, which handles sequential data and captures long-term dependencies, this model can focus on both local features and global contextual information. This approach improves diagnostic accuracy. The CNN-BiLSTM architecture, designed for processing time-series data, first extracts local features through CNN and then captures long-term dependencies through Bi-LSTM. Initially, the feature dimension is 267, and after dimensionality reduction via the MLP, it is reduced to 80. The second dimension is then expanded to 80 to adapt to the convolution operation. The CNN performs the convolution operation, and the output is flattened to 567. The flattened output is passed through a fully connected (FC) layer, reducing the dimensionality to 80. After concatenating features, the dimension becomes 160, which is fed into the Bi-LSTM for decision-making. Finally, the Bi-LSTM outputs the disease prediction probabilities. This CNN-BiLSTM combination enhances the model’s ability to capture complex patterns in both time-sequential and spatially localized data, leading to improved prediction performance. Therefore, the integration of CNN and Bi-LSTM offers a powerful tool for diagnosing MN in mice based on tissue analysis, increasing diagnostic accuracy and the effectiveness of tissue-based screening.
Data analysis
Deep learning architecture
MLP, a classic deep learning architecture, offers significant advantages in medical diagnosis, particularly in spectral data analysis, due to its simple structure and ease of implementation. The MLP excels in identifying complex patterns and latent relationships within spectral data through its multi-layer nonlinear transformation capabilities, leading to exceptional performance in feature extraction and classification accuracy. In medical diagnosis, this ability enables the MLP to detect subtle differences in spectral data, which is critical for early detection and accurate disease diagnosis. Furthermore, the strong generalization ability of MLP ensures model robustness when handling complex and variable biomedical data, providing solid technical support for applying spectral techniques in medical diagnostics18.
In the field of deep learning, CNN and Bi-LSTM are two widely used neural network architectures. CNN effectively extracts local features from images through convolutional and pooling layers, which are crucial for recognition and classification tasks15.
However, CNN has limitations in capturing global information due to constraints imposed by the size of convolutional kernels and the network depth. On the other hand, Bi-LSTM is particularly suited for processing sequential data, as it captures long-term dependencies through both forward and backward LSTM networks. Bi-LSTM’s strength lies in its ability to handle contextual information in time series data, making it ideal for tasks requiring the capture of long-term dependencies19. In this study, we combine the local feature extraction capabilities of CNN with the global sequence modeling capabilities of Bi-LSTM to construct a more powerful and comprehensive model, enabling more accurate and efficient diagnosis of MN in mice.
Model evaluation indexes
The model evaluation was based on six standard metrics: accuracy, precision, sensitivity, specificity, F1 score, and AUC, as detailed in the following formulas.
Accuracy reflects the overall correctness of the model’s predictions, while precision measures the proportion of true positives among the predicted positive cases. Sensitivity indicates the proportion of actual positives correctly predicted, whereas specificity refers to the proportion of actual negatives correctly predicted. The F1 score, the harmonic mean of precision and recall, is used to assess the model’s robustness. The AUC represents the area under the ROC curve, evaluating the model’s performance across different thresholds; a larger AUC indicates stronger classification capability.
Experimental results and discussion
To demonstrate the effectiveness of the proposed CNN-BiLSTM model, nine baseline models were selected for comparative binary classification experiments using the mouse MN dataset. The selected models included: Latent Dirichlet Allocation (LDA)20, Decision Trees (DT)21, Logistic Regression (LR)22, Random Forest (RF)23, Long Short-Term Memory (LSTM)24, Deep Neural Networks (DNN)25, AlexNet26,ResNet27 and DenseNet28. The results, summarized in Table 3, highlight the binary classification performance of these models.
While advanced deep convolutional models such as DenseNet, ResNet, and AlexNet demonstrate strong performance, their high model complexity and parameter counts pose challenges in data-limited scenarios. In contrast, the CNN-BiLSTM model, with a parameter count of just 0.26 M, significantly outperforms classical deep convolutional models in situations with restricted sample sizes, making it more suitable for such datasets.
The CNN-BiLSTM model achieved an accuracy and precision of 98%, with sensitivity, specificity, and AUC reaching 98.33%. From the experimental results of the loss and accuracy curves for the testing phase of CNN-BiLSTM, as illustrated in Fig. 4, the loss and accuracy after five-fold cross-validation tend to stabilize. These results highlight its robustness in delivering reliable diagnostic outcomes, even with the limited sample size of the mouse MN dataset, where data scarcity can hinder parameter learning and generalization in more complex models.
Classical models such as LDA, DT, LR, and RF demonstrated reasonable performance; however, the integration of CNN’s local feature extraction capability with BiLSTM’s sequence modeling ability in the CNN-BiLSTM model led to enhanced diagnostic accuracy. This architecture enables the model to capture subtle changes in spectral data, facilitating early disease detection, reducing misdiagnoses, and supporting timely intervention and treatment. Specifically, CNN extracts local features from Raman spectral data of mouse tissues, identifying subtle spectral variations closely associated with the pathological state of MN. Bi-LSTM then processes these extracted features, leveraging its capacity for global sequence interactions to exploit temporal patterns within the spectral data for a deeper analysis of MN-related signatures.
This deep learning model, which integrates time-series analysis, enhances both the accuracy and reliability of diagnosis. Experimental validation demonstrated that the CNN-BiLSTM model not only improved diagnostic accuracy but also provided robust support for enhancing the effectiveness of tissue-based diagnostic experiments. This achievement represents a significant technological breakthrough in medical auxiliary diagnosis, offering new insights and methodologies for the early diagnosis and treatment of a broader range of diseases in the future.
Despite the promising results, the cBSA-induced mouse MN model used in this study—while effectively recapitulating key pathological features of human MN, such as glomerular capillary wall thickening and subepithelial immune complex deposition—cannot fully substitute for human MN due to inherent species differences. These differences, including variations in disease progression and immune responses, highlight the need for further validation in human tissues. To address this, we plan to analyze renal biopsy specimens from MN patients, aiming to bridge the gap between experimental models and clinical relevance, and to ensure the robustness and translational potential of our findings.
Conclusion
In this study, we present a rapid diagnostic method for MN by integrating mouse tissue Raman spectroscopy with deep learning algorithms, specifically employing a CNN-BiLSTM model. This approach offers an efficient and accurate solution for MN diagnosis. As technology evolves, diagnostic methods based on tissue Raman spectroscopy and deep learning are poised to play an increasingly important role in clinical practice.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to data privacy laws, but are available from the corresponding author on reasonable request.
References
Duan, Y. et al. Analysis of pathological data and epidemiological characteristics of 10 684 cases of renal biopsy in Xinjiang Uygur autonomous region. Chin. J. Nephrol. 37, 490–498 (2021).
Cravedi, P. et al. Immune-monitoring disease activity in primary membranous nephropathy. Front. Med. 6, 241 (2019).
Couser, W. G. Primary membranous nephropathy. Clin. J. Am. Soc. Nephrol. CJASN. 12, 983–997 (2017).
Feng, Z. et al. How does herbal medicine treat idiopathic membranous nephropathy? Front. Pharmacol. 11, 994 (2020).
Zhang, X. et al. Rapid diagnosis of membranous nephropathy based on serum and urine Raman spectroscopy combined with deep learning methods. Sci. Rep. 13, 3418 (2023).
Devitt, G., Howard, K., Mudher, A. & Mahajan, S. Raman spectroscopy: an emerging tool in neurodegenerative disease research and diagnosis. ACS Chem. Neurosci. 9, 404–420 (2018).
Bakator, M. & Radosav, D. Deep learning and medical diagnosis: a review of literature. Multimodal Technol. Interact. 2, 47 (2018).
Wu, H. H., Chen, C. J., Lin, P. Y. & Liu, Y. H. Involvement of prohibitin 1 and prohibitin 2 upregulation in cBSA-induced podocyte cytotoxicity. J. Food Drug Anal. 28, 183–194 (2020).
Chen, J. S. et al. Mouse model of membranous nephropathy induced by cationic bovine serum albumin: antigen dose-response relations and strain differences. Nephrol. Dial Transpl. Off Publ Eur. Dial Transpl. Assoc. - Eur. Ren. Assoc. 19, 2721–2728 (2004).
Yvon, H. J. Raman spectroscopy for analysis and monitoring. Horiba Jobin Yvon Raman Appl. Note 1–2 (2017).
van de Logt, A. E., Fresquet, M., Wetzels, J. F. & Brenchley, P. The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int. 96, 1292–1302 (2019).
Hanasaki, K. & Arita, H. Phospholipase A2 receptor: A regulator of biological functions of secretory phospholipase. Prostaglandins Other Lipid Mediat. 68–69, 71–82 (2002).
Su, H. et al. Lipid deposition in kidney diseases: interplay among redox, lipid mediators, and renal impairment. Antioxid. Redox Signal. 28, 1027–1043 (2018).
He, K., Gkioxari, G., Dollar, P., Girshick, R. & Mask, R-C-N-N. IEEE Trans. Pattern Anal. Mach. Intell. 42, 386–397 (2020).
Alzubaidi, L. et al. Review of deep learning: concepts, CNN architectures, challenges, applications, future directions. J. Big Data. 8, 53 (2021).
Shahid, F., Zameer, A. & Muneeb, M. Predictions for COVID-19 with deep learning models of LSTM, GRU and bi-LSTM. Chaos Solitons Fractals. 140, 110212 (2020).
Ranawat, N. S., Prakash, J., Miglani, A. & Kankar, P. K. Performance evaluation of LSTM and bi-LSTM using non-convolutional features for blockage detection in centrifugal pump. Eng. Appl. Artif. Intell. 122, 106092 (2023).
Taud, H. & Mas, J. F. Multilayer perceptron (MLP). In Geomatic Approaches for Modeling Land Change Scenarios (eds Camacho Olmedo, M. T., Paegelow, M., Mas, J. F. & Escobar, F.) 451–455. https://doi.org/10.1007/978-3-319-60801-3_27. (Springer International Publishing, 2018).
Siami-Namini, S., Tavakoli, N. & Namin, A. S. The performance of LSTM and BiLSTM in forecasting time series. In IEEE International Conference on Big Data (Big Data) 3285–3292. https://doi.org/10.1109/BigData47090.2019.9005997 (IEEE, 2019).
Jelodar, H. et al. Latent dirichlet allocation (LDA) and topic modeling: models, applications, a survey. Multimed. Tools Appl. 78, 15169–15211 (2019).
Kotsiantis, S. B. Decision trees: a recent overview. Artif. Intell. Rev. 39, 261–283 (2013).
LaValley, M. P. Logistic regression. Circulation 117, 2395–2399 (2008).
Rigatti, S. J. Random forest. J. Insur. Med. 47, 31–39 (2017).
Yu, Y., Si, X., Hu, C. & Zhang, J. A review of recurrent neural networks: LSTM cells and network architectures. Neural Comput. 31, 1235–1270 (2019).
Zhang, J., Zheng, Y., Qi, D., Li, R. & Yi, X. DNN-based prediction model for spatio-temporal data. In Proceedings of the 24th ACM SIGSPATIAL International Conference on Advances in Geographic Information Systems 1–4. https://doi.org/10.1145/2996913.2997016 (Association for Computing Machinery, 2016).
Krizhevsky, A., Sutskever, I. & Hinton, G. E. ImageNet classification with deep convolutional neural networks. Commun. ACM. 60, 84–90 (2017).
Xu, W., Fu, Y. L. & Zhu, D. ResNet and its application to medical image processing: research progress and challengesresnet. Comput. Methods Progr. Biomed. 240, 107660 (2023).
Hemalatha, J., Roseline, S. A., Geetha, S., Kadry, S. & Damaševičius, R. An efficient DenseNet-based deep learning model for malware detection. Entropy. 23, 344 (2021).
Acknowledgements
We sincerely thank the First Affiliated Hospital of Xinjiang Medical University and Xinjiang University for their contributions to this work.
Funding
This research was partially supported by the Leading Talents in Science and Technology Innovation Project (Grant No. 2022TSYCLJ0022), Xinjiang Uygur Autonomous Region Regional Collaborative Innovation Special Project (Grant No.2023E01020) and the Major Scientific Research Project Cultivation Program of Xinjiang Medical University (Grant No. XYD2024ZX05).
Author information
Authors and Affiliations
Contributions
G.Z. were responsible for the experimental design and some of the papers, H.S. and X.Z. were responsible for the literature research and some of the papers, C.C. and M.S. were responsible for guiding the experimental design and some of the papers, S.W. and G.A. were responsible for the literature research and experimental guidance, L.Z., S.W. and W.Y. were responsible for some of the papers, C.L. was responsible for the experimental guidance and paper revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was conducted in accordance with the International Guiding Principles for Biomedical Research Involving Animals, animal welfare regulations, and relevant laboratory operating procedures. All methods are reported in accordance with ARRIVE guidelines. The study was approved by the Animal Ethics Committee of Xinjiang Medical University (IACUC-20230627-19).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, G., Shadekejiang, H., Zhang, X. et al. Rapid diagnosis of membranous nephropathy based on kidney tissue Raman spectroscopy and deep learning. Sci Rep 15, 13038 (2025). https://doi.org/10.1038/s41598-025-97351-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97351-2