Abstract
This study evaluated the effects of ZnO and green-synthesized ZnO nanoparticles (ZnO-NPs) from Sargassum ilicifolium extract on the biochemical interactions between tomato leaves and the destructive pest Tuta absoluta. The average size and stability of ZnO-NPs were characterized using DLS, X-RD, and FE-SEM. Peaks in the FT-IR spectrum of the extract of the algae and ZnO-NPs, indicated the presence of carboxyl groups, amines, alkynes and alcohols. Treated tomato leaves exhibited increased total chlorophyll and anthocyanin contents, particularly at 100 and 50 ppm, compared to the control. A significant increase in total flavonoid content was observed at 100 ppm, while total phenolic content was also enhanced at 50 ppm. In T. absoluta larvae, exposure to 50 and 100 ppm ZnO-NPs led to a reduction in α-amylase, total protease, total protein, and TAG levels, alongside increased catalase activity. Additionally, G6PD and ALT activities decreased at 50 ppm compared to the control. Lactate, MDA, and the RSSR/RSH increased at 100 ppm. Furthermore, ZnO-NPs at 50 and 100 ppm elevated larval aldolase activity, while AST and γ-GT activities declined. These findings indicate that ZnO-NPs enhance tomato defense mechanisms and mitigate larval damage, supporting their potential use in integrated pest management strategies against T. absoluta.
Similar content being viewed by others
Introduction
In recent years, nanotechnology in agriculture enhances crop yield and sustainability by improving nutrient uptake, increasing stress resistance, and enabling intelligent pesticide delivery, while also reducing chemical use and pollution1,2,3. Zinc oxide nanoparticles (ZnO-NPs) stand out due to their high surface energy, large surface-to-volume ratio, exceptional adsorption capacity, and catalytic efficiency4. Among metal-based NPs, ZnO-NPs are particularly valued for their notable physical, optical, and antimicrobial properties, as well as their biocompatibility, low toxicity, and cost- effectiveness5. The green synthesis of ZnO-NPs enhances their efficacy and environmental impact by using biological sources like plant extracts, microorganisms, or natural compounds4,6. This method reduces energy consumption, relies on inexpensive raw materials, and minimizes toxic waste production6,7. Green-synthesized NPs are stable and less toxic to non-target organisms, making them a promising solution for sustainable agriculture. Additionally, they have diverse applications beyond agriculture, including water purification, medical treatments, solar power generation, and electronic sensors7,8,9,10.
While seaweeds are frequently used to synthesize gold and silver NPs due to their rich content of essential trace elements, their use in ZnO-NP production has been relatively limited11,12. Both the ZnO-NPs and Zn-based nano- fertilizers have potential to enhance germination, growth, biomass production, and antioxidant enzyme activity in various plants13,14. Nanotechnology also improves the efficiency of micronutrient uptake, with better crop growth and yield15. However, the production of seaweed-derived ZnO-NPs faces challenges, including the complexity of biological extracts, variability among seaweed species, and the need for standardized production processes16,17. Standardizing extraction methods can help ensure uniformity in NPs quality and composition. Additionally, identifying and utilizing specific seaweed species with superior biosynthetic capabilities can enhance production efficiency18. Despite these challenges, the eco-friendly nature of seaweed-derived ZnO-NPs presents a compelling case for further research19,20.
Seaweeds have applications in nutrition, medicine, and as bio- fertilizers; their use in green ZnO-NP synthesis has been shown to enhance crop yield and seed quality, using several genera, including Sargassum, Caulerpa, Gracilaria, Pterocladia, and Arthrospira21,22. Additionally, ZnO-NPs synthesized from the brown seaweed Turbinaria ornata have been found to improve seed quality and yield in rice23. Brown seaweed extracts contain key phytocompounds such as polysaccharides, betaines, phenols, and carotene, which enhance the effectiveness of ZnO-NPs in horticultural crops by improving nutrient uptake, promoting plant growth, and increasing stress resilience24. ZnO-NPs derived from red and green seaweed exhibit therapeutic potential due to their antimicrobial, antioxidant, and antitumor properties, as well as their ability to remove organic dyes from aqueous solutions17,25.
Tomatoes (Lycopersicon esculentum L., Solanaceae) are a nutrient-rich food, containing high levels of proteins, calcium, iron, potassium, magnesium, vitamins A and C, antioxidants, and carotenoids, making them a valuable food source26. Zinc is a micronutrient particularly important for improving tomato yield and quality27. Insufficient uptake of micronutrient fertilizers, including zinc, can cause considerable yield losses in various crops27. Zinc deficiency in plants leads to reduced plant growth, small leaves and stunted internodes, leaf chlorosis, delayed maturity and tissue death, resulting in lower crop yields and increased susceptibility to environmental stress28,29. It interferes with enzyme activity, reduces protein synthesis and stunts stems and roots28. Zinc is important for plant growth, enzymes, growth hormones, gene expression, protein synthesis, antioxidant activity and cell membrane function, and contributes to fertility, hormone stimulation and overall development28,30. Micronutrient deficiencies can also increase plant susceptibility to insect damage31.
The tomato leaf miner, Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae), is a major pest in tomato cultivation, causing significant economic damage. Due to its rapid spread and ability to persist in various climates throughout the year, extensive control efforts are required32,33. However, widespread pesticide use has led to issues with resistance development and crop residue34. According to FAO35, global pesticide usage nearly doubled from 1990 to 2021, increasing from 1.7 to 3.5 million tonnes. Nanotechnology offers a promising and safer alternative for integrated pest management in agriculture, with the potential to reduce negative impacts on non-target plant tissues and the environment36.
Plants activate their antioxidant defense systems to protect against pests, which can influence the survival, nutrition, and reproduction of herbivores37. Insects rely on antioxidant enzymes, such as glutathione peroxidase (GPX), ascorbate peroxidase (APX), peroxidase (POX), catalase (CAT), and glucose 6-phosphate dehydrogenase (G6PD), to mitigate damage and support overall growth38. These enzymes respond to reactive oxygen species (ROS) and host plant oxidants that can damage lipids, proteins, and nucleic acids, resulting in oxidative stress38. Superoxide radicals (O₂⁻) are converted to hydrogen peroxide (H₂O₂) by superoxide dismutases (SODs), which is subsequently broken down into water by CAT and POX enzymes39. Oxidative stress, arising from an imbalance between oxidants and antioxidants, can lead to lipid peroxidation, protein oxidation, and cell death40. Understanding these antioxidant systems is essential for grasping the ecological interactions between insects and their host plants, particularly how oxidative stress influences insect growth, survival, reproduction, and lifespan37.
The NPs enhance crop development and yield, offering promising avenues for sustainable agriculture41. High concentrations of NPs in agriculture can disrupt plant growth, cause oxidative stress and damage microorganisms42. To mitigate the risks, appropriate concentrations and environmentally friendly synthesis methods should be used. Research, standardization and education on targeted nano- fertilizers, -pesticides and -fungicides are essential for sustainable use in agriculture42,43. Green synthesized ZnO-NPs have the potential to improve the nutritional status and resistance of plants under greenhouse conditions44,45. This study examines the dual role of S. ilicifolium as both a rich natural resource for ZnO-NP synthesis and an agent for enhancing plant health. Laboratory analyses confirm that this seaweed is a valuable source of hydrocolloids, sodium alginate, fucoxanthin, chlorophyll a and c, and carotenoids46. By investigating the interaction between ZnO-NPs derived from S. ilicifolium and tomato plants, this research aims to promote plant health and manage tomato leafminer infestations in greenhouses and open fields.
Materials and methods
Seaweed gathering and preparation
Sargassum ilicifolium was collected during the months of November to January from the coast of the Oman Sea in Iran (24.7143° N, 58.7374° E). The seaweed was cleaned thoroughly before being air dried. Then, the samples were ground with powder and filtered to obtain a homogeneous material. The powdered seaweed was stored under refrigeration until used to obtain ZnO-NPs extract.
Synthesis of ZnO-NPs using S. ilicifolium
ZnO-NPs were synthesized by reacting zinc acetate dihydrate [Zn(OAc)₂·2H₂O], 0.1 M with an aqueous seaweed extract in a shaking incubator. The resulting white precipitate was separated by centrifugation at 4000 rpm for 15 min, then washed with distilled water and dried in oven at 100 °C for 24 h. The dried powder was subsequently heated in a muffle furnace at 450 °C for 5 h.
Dynamic light scattering (DLS) and zeta potential measurements were performed using a Horiba-Jobin Yvon SZ-100Z device (France). The samples underwent surface morphology, shape, and size analysis through field emission scanning electron microscopes (FE-SEM) (MIRA3 FEG-SEM, Tescan, Czech Republic).
To investigate the surface chemistry of the synthesized ZnO-NPs, Fourier Transform Infrared )FT-IR( spectroscopy was performed (Agilent Technologies, Cary 630 FT-IR, Germany). For the FT-IR analysis, the ZnO-NPs were uniformly dispersed in a dry potassium bromide (KBr) matrix and then compressed into transparent disks to create samples. These KBr pellets served as the standard for the analysis. Functional groups present on the NPs’ surface were identified within the spectral range of 400–4000 cm-1.
X-ray diffraction (XRD) analysis was conducted to determine the crystalline nature and particle size of the samples. Nanoparticle dimensions were calculated using the Scherrer equation:
where, D is the crystal size, λ the wavelength, β the peak width at half height, θ the diffraction angle in degrees, and K the constant 0.9.
Preparation of ZnO-nano-priming solution and tomato seed priming
Tomato seeds (cv. 4129) were purchased from the Mazra Danesh Company, Shiraz, Iran. They were sterilized with 3% hydrogen peroxide and then rinsed with deionized water. ZnO-NPs and bulk zinc oxide (B-ZnO) were prepared at varying concentrations (5, 10, 25, 50, and 100 ppm) by mixing in deionized water, followed by soaking and air drying. The duration of seed soaking in ZnO-NPs and B-ZnO solutions at different concentrations was 24 h. The various NP treatments were then ultrasonically dispersed (40 kHz; Hielscher Ultrasonics UP50H, Germany) in deionized water for 20–30 min before testing. Deionized water served as the hydro priming control. Prior to assays, the seeds were rinsed again with distilled water. Seedlings were planted in germination trays and watered weekly. After 25 days, they were transferred to plastic pots and maintained in a greenhouse at 25 ± 5 °C, 65 ± 5% relative humidity, and a 14:10 (light: dark) photoperiod. The soil used had the following features: 38.51%, 30.94% silt, 30.54% san 8.1, electrical conductivity 1.374 dS m-1, 5.82% nitrogen, 1.09% organic carbon, 11.43 mg·kg-1 available phosphorus, 625 mg·kg-1 exchangeable potassium, 09 mg·L-1 sodium, and 121.4 mg·L-1 calcium. The soil biological activity metrics was 0.521 mg CO₂·g-1·day-1 basal respiration, 1.19 mg CO2 h-1 kg-1 substrate- induced respiration, and microbial population density of 1.25 × 10⁷ per g soil dry weight.
Scanning electron microscopy
The ZnO- Nanoprimed tomato seeds were washed with distilled water to remove unabsorbed NPs and air-dried at room temperature. The seeds were transferred to carbon-coated copper grids, gold covered, and analyzed with a Field Emission scanning electron microscope (MIRA3 FEG-SEM, Tescan, Czech Republic).
ZnO- nanopriming on seed germination
The germination of tomato seeds was tested using ZnO-NPs, bulky NPs (Zinc acetate), and ZnO-NPs. The test involved 50 experimental and control seeds in an incubator at 25 °C and 90% relative humidity, with four replicates.
Biochemical analyses
To evaluate the influence of various concentrations of B-ZnO and ZnO-NPs on the levels of secondary metabolites, protein and starch content in tomato leaves, we conducted a completely randomized design assay. This assay involved individual plants at the five- to six-leaf stage and included four replicates for analysis.
Pigments content
To extract protein from leaf tissue, 800 µL of cold acetone and 200 µL of sodium phosphate buffer (50 mM and pH 7.2) were added to a micro tube. The samples were centrifuged at 13,000×g in a D-37520 Osterode am Harz, 1-14 K, centrifuge and the supernatant evaluated a spectrophotometer at of 663 nm, 645 nm, and 470 nm wavelengths to determine the chlorophyll content using pure acetone as a control and expressed in mg g−1 of fresh weigh (FW)47.
Secondary metabolites content
Tomato leaves (5 g) were ground, transferred to 50 mL of 80% ethanol, filtered, and centrifuged at 13,000 × g for five minutes. The supernatant was used for subsequent assays.
The total anthocyanin content (TAC) of the leaves was determined using the method48. The leaf extract was stored in tubes in the dark at 25 °C for 24 h and the absorbance measured at 550 nm wavelength and was expressed in mg QE g-1 of fresh weight. .
The total phenolic content (TPC) was measured according to Slinkard and Singleton49. Briefly, 2.5 mL of 10% Folin-Ciocalteu reagent was added to 0.5 mL of each extract (1 mL extract diluted to 10 mL with 80% methanol). Then, the samples were incubated for 30 min with 2.5 mL of 7.5% sodium carbonate, following absorbance measurement at 720 nm wavelength. The TPC was expressed in mg GAE g-1 of fresh weight and compared to a standard curve (expressed as gallic acid equivalents, GAE).
The total flavonoid content (TFC) was determined with colorimetric aluminum chloride method50. The extract (0.5 mL) was mixed with 1.5 mL methanol, 0.1 mL 10% AlCl₃, 0.1 mL 1 M potassium acetate, and 2.8 mL distilled water. The absorbance was then measured at 415 nm wavelength with a spectroscopy device (UNICO S 2100 SUV, USA). For the analysis of the total flavonoid content the standard as quercetin equivalent (QE) is used and was expressed in mg QE g-1 of fresh weight.
Protein and starch contents
The total protein content in the tomato leaf extract was determined according to Lowry et al.51, using bovine serum albumin as standard and expressed in mg g−1 of fresh weigh (FW).
The plant sample was purified using ethanol, and the remaining precipitate was used to measure starch. The sediment was dried, distilled water added, and insoluble sugars detected using phenol and sulphuric acid methods. A standard curve was established using glucose solutions with specific concentrations and expressed in mg g−1 of dry weigh (DW)52.
Biochemical analyses in T. absoluta larvae
A colony of the tomato leaf miner Tuta absoluta was established by collecting infested leaves from various tomato greenhouses in Ardabil City, Iran. To eliminate the influence of previous host feeding, larvae were reared on tomato plants treated with different concentrations (0, 5, 10, 25, 50, and 100 ppm) of bulk ZnO (B- ZnO) and ZnO in ventilated cages for two generations prior to the start of the experiments.
To obtain cohorts of larvae aged 12–16 h, 10–15 couples of moths were confined on the same tomato plants for oviposition for 24 h. For the main test, 10 larvae were randomly selected from each repeat of treatment and homogenized with a hand pummeler in 1 mL of ice-cold distilled water. The samples were then centrifuged at 13,000 × g for 15 min at 4 °C. The resulting supernatant was collected and stored at -20 °C for subsequent biochemical analyses, which were conducted in quadruplicate53,55.
Digestive enzymes
The α-amylase activity was measured according to Bernfeld56, with dinitrosalicylic acid (DNS) as the colorimetric agent and 1% starch as the substrate. After incubating the larvae extract (10 µL) with soluble starch and a universal buffer [2-(N-Morpholino) ethanesulfonic acid, Glycine and Succinate 0.02 M)] for 10 min, 80 µL of DNS was added, the mixture heated, and absorbance measured at 545 nm wavelength and expressed in Unit mg−1 protein.
Total protease activity was measured according to Elpidina et al.57. Briefly, a reaction mixture with 2% azocasein substrate (80 µL), 20 mM universal buffer at pH 8.0 (80 µL), and insect extracts (20 µL) was incubated for 60 min. Then, the reaction was stopped with 30% trichloroacetic acid (100 µL) at 4 °C for 5 min and centrifuged at 13,000 g for 5 min. Finally, 1 M NaOH was added, and the absorbance was measured at 450 nm wavelength and expressed in Unit mg−1 protein.
Fat body triacylglycerides and protein contents
A diagnostic kit for measuring triglycerides (TAG) was provided by Pars Azmoon Co. (Tehran, Iran). The reagent solution contained 50 mM phosphate buffer (pH 7.2), 4 mM 4-chlorophenol, 2 mM adenosine triphosphate, 15 mM magnesium sulfate, 0.4 kU/L glycerol kinase, 2 kU/L peroxidase, 2 kU/L lipoprotein lipase, 0.5 mM 4-aminoantipyrine, and 0.5 kU/L glycerol-3-phosphate oxidase. Following the method58, a 10 µL larval extract was incubated with 10 µL of distilled water and 70 µL of reagent solution for 20 min at 25 °C. Then, the absorbance was measured at 546 nm to determine the TAG concentration with the equation:
where, OD is the optical densities of the sample and of the standard triacylgliceride and expressed in mg mL−1.
The protein content was determined using the Lowry et al.51 method using bovine serum albumin as standard and expressed in mg mL−1.
Enzymes and content
A chlorometric assay method was used to quantify the enzymes aspartate (AST) and alanine (ALT) aminotransferase. After independent incubation, the enzyme solution (10 mL) was added to Reagents A and B. At 340 nm and expressed in Unit mg−1 protein59.
The \(\gamma\)-Glutamyl Transferase (γ-GT) activity was measured according to Szasz60 with Biorex Fars Co. reagents. The reaction mixture was 50 µL of buffer reagent, 20 µL of substrate reagent (L-γ-glutamyl-3-carboxy-4-nitroanilide), and 10 µL of the sample, incubated for three minutes at room temperature. The absorbance was measured at 405 nm wavelength and expressed in Unit mg−1 protein.
Aldolase (ALDA) activity was determined according to Pinto et al.61. The reaction mixture contained 50 µL of buffer reagent, 25 µL of substrate reagent (fructose-1, 6-diphosphate), 10 µL of cofactor reagent (NADH), and 20 µL of the sample. After five minutes of incubation, absorbance was measured at 340 nm wavelength and expressed in Unit mg−1 protein.
Oxidative stress
Catalase activity (CAT) was quantified following the method39. In this assay, 500 µL of insect extract was combined with 500 µL of 1% hydrogen peroxide, and the mixture was incubated for 10 min at 28 °C. Changes in absorbance were recorded and converted to optical density, reflecting the enzyme’s activity. The measurement was taken at a wavelength of 240 nm and expressed in Unit mg−1 protein.
The ascorbate peroxidase (APX) activity was determined according to Addy and Goodman62. The reaction mixture included 100 µL insect extract, 500 µL 1% hydrogen peroxide, and 500 µL of buffered pyrogallol (0.05 M pyrogallol in 0.1 M phosphate buffer, pH 7.0). Absorbance at 430 nm was measured using a spectrophotometer, with readings taken at 30 s intervals for two minutes and expressed in Unit mg−1 protein.
The peroxidase (POX) activity was assessed following Chance and Maehly63. The reaction mixture consisted of 1400 µL of 100 mM potassium phosphate buffer pH = 7, 750 µL of 10 mM aqueous guaiacol solution, 100 µL of 70 mM H2O2 solution in 100 mM potassium phosphate pH = 7, and 750 µL of sterilized double distilled water. This reaction mixture, along with 20 µL of the insect extract, was transferred into a 3 mL glass cuvette and POX activity was measured at 470 nm wavelength of over a reaction time of 180 s and expressed in Unit mg−1 protein.
The activity of glucose-6-phosphate dehydrogenase (G6PDH) was assessed using the method64. The reaction mixture included 50 µL of 100 mM Tris–HCl buffer (pH 8.2), 50 µL of 2 mM NADP, 50 µL of 0.1 M MgCl₂, 50 µL of water, 50 µL of 6 mM glucose-6-phosphate, and 50 µL of insect extract. Absorbance was measured at 340 nm wavelength and expressed in Unit mg−1 protein.
Assay of MDA, oxidised/reduced thiols (RSSR/RSH) and lactate
Malondialdehyde (MDA) levels were determined as an indicator of lipid peroxidation, following the protocol described by Bar-Or et al.65. The procedure involved mixing 500 µL of insect extract with 250 µL of 20% trichloroacetic acid. After centrifugation with 15,000 g for 10 min, the mixture was incubated with 250 µL of 8% thiobarbituric acid for 60 min at 80 °C. Absorbance was then measured at 535 nm using a spectrophotometer and expressed in μM mg−1 protein.
The concentrations of oxidized and reduced thiols were determined according to Khramtsov et al.66. Briefly, 50 µL insect extract, 100 µL water and 100 µL trichloroacetic acid (1 M) were incubated at room temperature for 15 min and then centrifuged at 13,000 rpm for 15 min. The supernatant was then removed and 100 µL of 20 mM phosphate buffer, pH 7.0 and 0.1% 5, 5-dithio-bis-2-nitrobenzoic acid (DTNB) were added. The mixture was incubated at 37 °C for 5 min. Then, 50 µL of the sample, 100 mM sodium phosphate buffer pH 7.0 and 50 µl of DTNB were incubated for 15 min at room temperature. Then the absorbance was measured at 405 nm wavelength to determine the RSSR/RSH ratio.
Lactate concentration was measured using Biorex diagnostic reagents according to the manufacturer’s instructions. A 0.5 mL insect sample was incubated with the reagents for 30 to 45 min. Absorbance was measured at 546 nm, and expressed in m mol L−1 ref.67.
Statistics analyses
On the basis of FE-SEM image, size distribution and the average size of the NPs were estimated with the assistance of Image J and Origin Pro. The FT-IR cures draw by using Origin Pro software 2018. The X’Pert High Score program version 3 was utilized to analyze the XRD peaks and calculate the crystal size of the particles.
The bioassays were conducted using a completely randomized design with four replicates for each treatment. The Kolmogorov–Smirnov test was employed to assess the normality of the data. Subsequently, the data were analyzed using one-way analysis of variance (ANOVA) and Tukey’s post hoc Honestly Significant Difference (HSD) test (P < 0.05) with MINITAB software version 16. Graphs were created using Excel software.
The different concentrations of B-ZnO and ZnO-NPs were categorized into resistant, extremely susceptible, and susceptible groups using Ward’s approach and hierarchical cluster analysis in the form of a dendrogram in SPSS 16 software.
Results and discussion
DLS and zeta potential analysis
DLS indicated that the ZnO-NPs had a hydrodynamic diameter of 163.7 nm and a polydispersity index of 0.297, suggesting a relatively monodisperse distribution. In this research, ZnO-NPs made from S. ilicifolium extract, a brown alga, had a zeta potential of -5.3 mV, which shows that bioactive compounds had an effect16. The main bioactive compounds include fucoidans and phlorotannins which have anti-inflammatory and antioxidant properties16,19. Alginic acid, a polysaccharide found in brown seaweed, is useful for sustainable agriculture because it improves soil structure, nutrient absorption, plant resilience, and bioremediation by eliminating heavy metals68. The negative zeta potential indicates stability problems that could be mitigated by stabilizing agents in the seaweed extract19. Consistent with previous findings that NPs with zeta potentials in the range of + 10 to −10 mV are generally considered stable and exhibit enhanced biological properties69,70,71. Additionally, studies have suggested that bioactive molecules present in brown alga extract may influence the zeta potential of ZnO-NPs72.
FE-SEM analysis
The formation of ZnO-NPs was confirmed through visual evaluation and demonstrated morphology at different resolutions (Fig. 1A-G). The color of the reaction mixture changed from dark brown to pale white during the reaction, indicating the synthesis of ZnO-NPs. The FE-SEM image of Fig. 1 H demonstrated the particle size and its distribution that the pure ZnO-NPs formed with hexagonal structures and particle sizes ranging from 5 to 65 nm with some deviations (with mean = 25.22 nm; Fig. 2A, B). Green-synthesized ZnO-NPs derived from Lobelia leschenaultiana displayed both spherical and hexagonal shapes, ranging in size from 20 to 65 nm73. Brown macroalgae, Sargassum wightii, and brown seaweed, Turbinaria ornate, produce spherical NPs with varying sizes, with some exhibiting rod-shaped morphology and 15 to 62 nm average size23,74. Green-synthesized NPs from plant sources typically have a spherical shape, consistent with previous research75,76.
Field emission scanning electron micrographs of ZnO-NPs showing their morphology at eight different resolutions. Scanning electron micrographs of Sargassum ilicifolium- mediated ZnO-NPs. SEM of ZnO-NPs at 250× (A), 500× (B), 1500× (C), 2500× (D), 5000× (E), 10,000× (F), 30,000× (G), and 50,000× (H).
FT-IR analysis
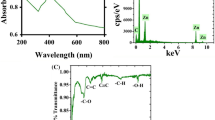
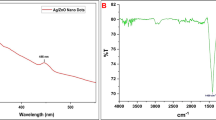
The functional molecules involved in the stabilization of the S. ilicifolium extract-fabricated ZnO-NPs are found by FT-IR spectroscopic analysis in the range 400- 4000 cm-1 (Fig. 3). The peaks recognized from FTIR spectrum analysis were located at 3896.06, 3849.21, 3434.99, 2349.44, 2058.16, 1633.69, 1277.17, and 666.21 cm-1 (Fig. 3). The FT-IR spectrum of S. ilicifolium extract and ZnO-NPs green synthesis showed distinct stretching and transition peaks, with a broad absorption band at 3434.99 cm−1 can be attributed to the OH hydroxyl group and N–H stretching, indicative of H bound in alcohol or phenol groups. Functional O–H and N–H groups have a significant influence on the nucleation and growth rates of NPs, especially in coordination complexes with Zn2⁺ ions77,78. O–H groups stabilize the metal ion, improve hydrogen bonding and increase the stability of NPs during growth77. N–H groups form hydrogen bonds with oxygen atoms, change the interfacial energy and form defect complexes78,79. Excessive functionalization can slow down growth, so balance is crucial for optimizing the synthesis of NPs79. The FT-IR spectrum reveals O–H stretching is crucial for ZnO-NP formation from S. ilicifolium extract, whereas Akbar et al.80 found O–H and N–H groups in marine alga ZnO-NPs. The synthesized NPs exhibit a distinctive peak at 2349.44 cm−1, indicating carbonate molecules and carboxyl groups, aligning with previous findings from Anand and Suresh81. The 1633.69 cm−1 peaks are likely due to the C = O stretching-carbonyl and C = O amide of the samples, as well as the N–H bending vibrations of amine or amide groups82. Furthermore, the peaks at 1277.17 cm−1 correspond to C–N stretching vibrations of aliphatic amines, while the asymmetric stretching vibration of the sulfate group is absent83. The study found peaks in the 400–900 cm-1 range, indicating Zn–O bonding, 666.21 cm−1 indicating alkynes with –C = C– stretching, and 1633.69 and 666.21 cm−1 likely due to ZnO stretching and deformation vibrations84. Ishwarya et al.85 found that metal oxides often absorb peaks with band patterns between 500 and 900 cm-1. Proteins and amino acid residues, with carbonyl and free NH2 groups, can bind to metals and form a coating to prevent aggregation and stabilization of NPs86. Proteins from seaweed effectively reduce metals, with their functional groups playing a crucial role in salt bioreduction and nanoparticle coating, alongside flavonoids OH group87.
XRD analysis
The X-ray diffraction pattern exhibited distinct peaks at 2θ values of 31.839°, 34.534°, 36.349°, 47.673°, and 56.696°, corresponding to the 001, 002, 101, 102, and 110 planes, respectively, which confirmed a highly crystalline structure (Fig. 4). The average crystal size of the NPs, calculated using the Debye–Scherrer equation, was estimated to be 34.44 nm. X-ray diffraction analysis revealed minor peaks, likely due to the crystallization of organic substances on the surface of the ZnO-NPs. Field emission scanning electron microscopy confirmed the absorption of ZnO-NPs by tomato seeds, indicating nanoparticle uptake within the seed endosperm. Our findings demonstrated that nano-priming, which involves the absorption of both water and ZnO-NPs, facilitates crossing of the seed coat and triggers reactive oxygen species (ROS) signaling, ultimately enhancing seed germination. Nano-priming has been claimed to facilitate seed coat penetration and increase germination rates88,89. ZnO-NPs significantly improve nutrient uptake and availability in crops, enhancing germination rates90. Studies show that priming chickpea, rice and corn seeds with ZnO-NPs increases water uptake and phosphorus and zinc uptake, leading to improved seed performance91,92. Thus, by promoting nutrient uptake, antioxidant qualities, and reactive oxygen species management, ZnO-NPs improve plant health and increase resistance to both abiotic and biotic stresses93.
Tomato seed ZnO nano-priming and percentage germination
The FE- SEM images of both control and treated seeds demonstrated the deposition of ZnO-NPs on their surfaces in varying sizes (Fig. 5). Priming the tomato seeds with various concentrations of nutrient solutions, including B-ZnO and ZnO-NPs, significantly increased germination compared to the control group treated with zinc acetate and hydropriming (P < 0.0001; Fig. 6 A). The germination rate was 91.5% in the treatment with 100 ppm B-ZnO compared to 74.02% in the control group. Additionally, seeds treated with 100 and 25 ppm ZnO-NPs presented 99.33% and 99.17% germination, respectively, higher than 74.02% in the control, eight days after treatment. Our findings showed that the germination rate of tomato seeds reaches to 91.5% with ZnO-NP treatment, compared to 74.02% in the control group. The ZnO-NP further enhances germination rates to 99.33% at 100 ppm and 99.17% at 25 ppm, both higher than the control group, such as found on germination of maize plants92. Thus, seed nano-priming, a cost-effective and simple strategy containing NPs, enhances plant growth, tolerance to stresses, and crop production capacity94,95.
Photosynthetic pigments contents in tomato leaves
The chlorophyll-a level in tomatoes treated with 10 ppm B-ZnO showed a significant increase compared to 5 ppm and the control group (P < 0.0001; Table 1). However, no significant difference was observed between the B-ZnO concentrations and the control for chlorophyll-b (P = 0.158; Table 1). The foliar application of ZnO-NPs at 100 ppm increases chlorophyll-a content, whereas chlorophyll-b increases at 10 ppm ZnO-NPs (P < 0.0001; Table 1). Similar to our findings, ZnO-NPs have been shown to increase plant zinc content and chlorophyll levels, thereby supporting plant growth by enhancing photosynthesis and CO₂ transport96,97.
Total carotenoid levels were significantly higher in tomato leaves treated with 50 ppm B-ZnO compared to the hydroprimed control and the 5 ppm concentration (P < 0.0001; Table 1). The ZnO-NPs nutrient solutions significantly increased total carotenoids at the 100 and 50 ppm concentrations in comparison with the control (P < 0.0001; Table 1). This research demonstrates that ZnO-NPs at concentrations of 50 and 100 ppm increase carotenoid content in plants, probably due to improvement of the glutamate-to-glycine ratio, promoting chlorophyll synthesis and tissue development98.
The secondary metabolites contents in tomato leaves and α-amylase activity in T. absoluta larvae
Increased TPC was observed in tomato treated with B-ZnO at the control, 100 and 50 ppm concentrations compared to the other concentrations (Table 2). Tomato leaves nano–primed with biogenic ZnO NPs at a concentration of 50 ppm significantly increased TPC compared to the concentration of 5 ppm and control (Table 2).The maximum value of TFC was measured at the 50 ppm concentration of the B-ZnO–treated mass compared to the 5 ppm (Table 2). In tomato leaves treated with biogenic ZnO–NPs at a concentration of 100 ppm, TFC levels were significantly increased compared to the control (Table 2). The highest value of TAC was significantly obtained at 50 and 5 ppm concentration of treated B-ZnO, while the lowest value was documented at control and 25 ppm, respectively (Table 2). The TAC values of tomato leaves nano–primed with biogenic ZnO–NPs increased at the concentrations of 50 and 100 ppm against the control (Table 2). Over the past decade, numerous studies have highlighted the significant impact of biologically derived NPs on nano-insecticidal toxicity, plant growth, and the biochemical properties of various plant species99,100. Plants increase the synthesis of defense compounds, including phenolic compounds, salicylic acid, and hydrogen peroxide to counteract abiotic and biological stresses, such as temperature fluctuations and diseases101. This research aims to enhance plant resilience and mitigate T. absoluta damage by focusing on B-ZnO and ZnO-NPs from brown seaweed extract.
Green-synthesized ZnO nanoparticles (ZnO-NPs) derived from brown algae have shown significant potential in enhancing the physiological efficiency and antioxidant activity of tomato plants. Specifically, tomato plants treated with 50 and 100 ppm ZnO-NPs exhibit a notable decrease in α-amylase activity in Tuta absoluta larvae (P < 0.0001; Table 3) and an increase in starch content (P < 0.0001; Fig. 6B), as well as higher levels of total phenolic compounds, flavonoids, and anthocyanins in tomato leaves (P < 0.0001; Table 2). These findings suggest that ZnO-NP treatments enhance the phytochemical profile of tomato plants, improving overall plant health and resilience against pests. The observed reduction in α-amylase activity in T. absoluta larvae could indicate a disruption in the larvae’s digestive enzymes, potentially hindering their ability to digest plant material effectively. The increase in secondary metabolites, including phenolics and flavonoids, is likely to play a crucial role in this process by acting as deterrents to herbivores and by interfering with the pests’ enzymatic digestion mechanisms.
Enzymes, as critical players in host–pathogen interactions, influence plant defense responses in numerous ways102. In the case of herbivory, enzymes like α-amylase and proteases in pests’ digestive systems are essential for breaking down starches and proteins in plant tissues103. By inhibiting the activity of these enzymes, ZnO-NPs may weaken the feeding efficiency of pests, thereby enhancing plant protection104. Additionally, secondary metabolites such as phenolic compounds, flavonoids, and tannins are often produced in response to both abiotic and biotic stress, including herbivore attack. These compounds not only serve as antioxidants to mitigate oxidative stress but also play antimicrobial and insecticidal roles, further contributing to plant defense 105. Zinc oxide nanoparticles have been shown to stimulate the accumulation of these secondary metabolites, strengthening the plant’s ability to fend off pathogens and pests.
Previous studies have demonstrated the impact of ZnO-NPs on secondary metabolite production in various plants. For instance, in potato plants, ZnO-NPs have been linked to an increase in total phenolic content in roots, enhancing the plant’s resistance to environmental stressors106. Similarly, García-López et al.107 reported that ZnO-NPs inhibited root growth while promoting the accumulation of non-enzymatic antioxidants, phenolics, flavonoids, and tannins, all of which contribute to a plant’s defense arsenal. Thus, NPs hold great potential in enhancing plant resistance, improving agricultural productivity, and reducing dependence on chemical pesticides, contributing to more sustainable and environmentally friendly farming practices6,7.
The protein and starch contents in tomato leaves and proteolytic activates, and TAG and protein contents in T. absoluta larvae
Proteases may play a role in neutralizing toxic proteins ingested by insects, underscoring their importance in nutrition and detoxification107. In our study, tomato plants treated with 10 ppm ZnO-NPs exhibited a significant increase in protein content (P < 0.0001; Fig. 6 C). Additionally, protease activity in the T. absoluta fed on plants treated with 5 and 10 ppm ZnO-NPs was higher than in those feeding on plants treated with 50 ppm (P < 0.0001; Table 3), likely reflecting increased energy demands. In addition, T. absoluta larvae feeding on plants treated with higher nanoparticle concentrations (50 and 100 ppm) had reduced protein and triglyceride levels (P < 0.0001; Table 3). This suggests that higher concentrations of ZnO-NPs may negatively impact the nutritional quality of the plants, leading to digestive disruptions and increased protein and lipid metabolization to meet energy needs. The action mechanism of ZnO-NPs likely involves the nanoparticles interacting with the insect’s digestive system, disrupting enzyme activities, and influencing metabolic pathways108. At higher concentrations, ZnO-NPs may induce oxidative stress in both the plant and the larvae, leading to altered nutritional content in the plant tissues, while also impairing the larvae’s ability to process and store proteins and lipids effectively109. This disruption results in decreased growth and developmental rates in the pests, as seen in studies showing a 50% reduction in mosquito larval growth rate due to nanoparticle exposure110.
Oxidative stress in T. absoluta larvae
In the current study, a significant decrease in alanine aminotransferase (ALT) and γ-Glutamyl transferase (γ-GT), as well as increase of aspartate aminotransferase (AST) activities occurred T. absoluta treated with ZnO-NPs plant extracts (P < 0.0001; Fig. 7A, B and C), probably due to increased energy demands associated with the Krebs cycle and detoxification processes. The aminotransferases have key functions in the maintenance of amino acid pool for protein synthesis, supply metabolites for energy metabolism and the catalysis between protein and carbohydrate metabolism, mainly in insects that have high contents of free amino acids. The reduction in ALT and γ-GT activity seems to be linked to impaired protein digestion rather than limitations in energy production or protein availability, as suggested by AST involvement in amino acid and ketoacid interconversion111. Phenolic compounds act as antioxidants by donating hydrogen and neutralizing free radicals, thereby mitigating oxidative stress from MDA and H₂O₂112. Hemolymph circulating amino acids are essential for osmoregulation, energy production, cuticle hardening, neural transmission, and oogenesis107. Their catabolism involves transamination, production of pyruvate and ammonium, and citric acid cycle intermediates. Alanine aminotransferase and AST are key enzymes in this pathway. ALT catalyzes the conversion of L-alanine to pyruvate and L-glutamate, both critical for proline metabolism and the Krebs cycle113. AST facilitates the conversion of aspartate and alpha-ketoglutarate into oxaloacetate, further contributing to the Krebs cycle113. γ-Glutamyl transferase (γ-GT) is involved in the γ-glutamyl cycle, which is vital for the synthesis and degradation of glutathione114.
In this study, T. absoluta larvae fed on plants treated with 50 ppm ZnO-NPs exhibited the highest levels of peroxidase (POX) and glucose-6-phosphate dehydrogenase (G6PD) activities, indicating enhanced metabolic responses (P < 0.0001; Table 4). In contrast, the highest ascorbate peroxidase (APX) activity was observed in larvae exposed to 100 ppm ZnO-NPs. Additionally, larvae treated with 100 ppm ZnO-NPs showed the highest malondialdehyde (MDA) concentration (P < 0.0001; Fig. 7E), a byproduct of fatty acid peroxidation, suggesting increased oxidative stress. High concentrations of ZnO-NPs also reduced triglyceride levels and an elevated thiol ratio (RSSR to RSH) (P < 0.0001; Fig. 7 F), indicating oxidative stress and a disruption in thiol-oxidase balance39. This finding aligns with previous studies by Wang et al.39 and Mardani-Talaee et al.54, which associated a higher oxidase-to-thiol reducer ratio with increased lipid peroxidation and MDA levels. Similar results were reported by Zorlu et al.115, who observed elevated MDA content in Galleria mellonella larvae fed TiO-NPs. The combination of ZnO-NPs with microalgae extracts has been tested, resulting in increased mortality rates in insects like Tenebrio molitor, indicating a synergistic effect that enhances toxicity116. Treatment with ZnONPs led to oxidative stress in the larvae, evidenced by a significant increase in hydrogen peroxide concentration110. This oxidative stress is a response to the toxic effects of the NPs . ZnO-NPs have been shown to induce chronic effects on insect larvae, such as Culex pipiens, leading to significant growth reduction and oxidative stress110.
The mechanism of action of ZnO-NPs likely involves the induction of oxidative stress in T. absoluta larvae, triggering changes in metabolic and enzymatic activities104. The ZnO-NPs may increase the production of reactive oxygen species (ROS), leading to alterations in enzyme activities such as ALT, AST, and γ-GT, which play essential roles in detoxification and amino acid metabolism90,117. Additionally, high concentrations of ZnO-NPs may disrupt cellular homeostasis, resulting in an imbalance of thiol-oxidase systems and elevated lipid peroxidation, as evidenced by the increased MDA levels. This oxidative stress likely impacts the larvae’s energy metabolism, impairing their ability to efficiently process nutrients.
Aldolase, a key enzyme in glycolysis, showed increased activity in T. absoluta larvae treated with 50 and 100 ppm ZnO-NPs (P < 0.0001; Table 4), suggesting enhanced glycolytic activity and elevated pyruvate production61. The increased pyruvate levels were further converted to lactate by lactate dehydrogenase, with larvae in the 100 ppm treatment exhibiting higher lactate concentrations (P < 0.0001; Fig. 7D). This increase in aldolase activity and lactate levels may also indicate tissue damage due to elevated secondary metabolites in the treated tomato leaves54.
Oxidative stress occurs when ROS levels exceed cellular thresholds38. Enzymes such as peroxidase (POX) and ascorbate peroxidase (APX) in chloroplasts, cytosol, mitochondria, and peroxisomes catalyze the reduction of hydrogen peroxide to water and oxygen117. APX is crucial for converting hydrogen peroxide into water, contributing to reducing lipid peroxidation. Similarly, the increased activity of APX and glucose-6-phosphate dehydrogenase (G6PD) in larvae consuming plants treated with 100 ppm ZnO-NPs suggests enhanced energy derivation through the glycolytic pathway. Catalase (CAT) reduces the accumulation of superoxide anions under oxidative stress. The increase in CAT activity observed in larvae feeding on plants treated with 50 and 100 ppm ZnO-NPs (P < 0.0001; Table 4) may be due to elevated secondary metabolites that contribute to tissue degradation, particularly in the digestive tract118. Belal and Gad119 reported an increase in CAT activity in Bombyx mori caterpillars treated with ZnO-NPs. This increase in CAT activity likely reflects an adaptive response to elevated hydrogen peroxide levels, with CAT facilitating its reduction to water and oxygen under oxidative stress conditions120.
Cluster analysis
Figure 8 depicts a cluster study of biochemical traits of the tomato leaves, across numerous biogenic ZnO-nano-priming and B-ZnO treatments. The results of cluster analysis of different biogenic ZnO-NPs and B-ZnO treatments based on biochemical traits show the presence of two groups: A and B; Group A consists of two subgroups: A1 (susceptible) and A2 (extremely susceptible). Subgroup A1 includes 10, 25, and 100 ppm B-ZnO treatments, whereas subgroup A2 includes the 5 ppm B-ZnO, control and 5 ppm ZnO-NPs treatments. Group B consists of two resistant subgroups: B1 (extremely resistant) and B2 (resistant). Subgroup B1 includes the treatments with 50 ppm ZnO-NPs and 50 ppm B-ZnO, while subgroup B2 includes the treatments with 100, 25 and 10 ppm ZnO-NPs. The induced resistance in treatment group B could be due to a higher activity of defense enzymes in the plant leaves121. For plants to be resistant to biotic stress, defense enzymes primarily control the deposition of cell wall components, especially lignin122. ZnO-NPs interact with plant cells to enhance antioxidant enzyme activity and stimulate secondary metabolite production123. These NPs alleviate stress conditions, improving physiological responses in plants. High concentrations of ZnO-NPs maintain elevated levels of antioxidant enzymes, crucial for detoxifying reactive oxygen species123,124. They also enhance secondary metabolite production, including flavonoids and phenolics, which play vital roles in plant defense mechanisms. It enhances their interaction with plant roots, facilitating the release of metabolites123,125. However, concerns about potential toxicity and accumulation in ecosystems warrant careful consideration in agricultural practices.
A cluster analysis of the biochemical characteristics of T. absoluta larvae across a variety of B-ZnO and biogenic ZnO-NPs treatments is shown in Fig. 9. Two categories, A and B, are shown by the findings of cluster analysis of several B-ZnO and ZnO-NPs based on biochemical features; Group A is made up of two subgroups, A1 (susceptible) and A2 (very susceptible). The 5 ppm B-ZnO treatment is part of subgroup A2, while the control and other treatments are part of subgroup A1. The B1 (very resistant) and B2 (resistant) are the two resistant subgroups that make up group B. The 50 ppm ZnO-NPs and 100 ppm ZnO-NPs treatments are included in subgroup B1, while the 100 ppm B-ZnO treatment is included in subgroup B2. Thus S. ilicifolium has synthesized ZnO-NPs (50 and 100 ppm) with strong antioxidant properties, surpassing standard antioxidants, potentially enhancing insects’ resilience against oxidative stress.
Based on the data obtained here, although hypothetical, it is plausible to suggest that ZnO-NPs derived from algae influence the physiology of tomato plants, enhancing their productivity by increasing chlorophyll levels, which in turn boosts protein and flavonoid content. This, in turn, confers greater resistance against T. absoluta, as observed by the partial inhibition of digestive enzyme activity, leading to oxidative stress in this pest, as evidenced by the increased activity of antioxidant enzymes.
Conclusion
Overall, our study shows that higher concentrations of ZnO-NPs, particularly at 50 and 100 ppm, not only enhanced germination rates, photosynthetic profiles, and related biochemical changes in tomato plants but also exhibited toxic effects on the insect pest T. absoluta. ZnO-NPs also increased antioxidant enzyme activities and significantly impacted malondialdehyde and lactate levels in the pest. The results indicate that foliar application of ZnO-NPs is more effective than conventional bulk zinc fertilizers. Employing ZnO-NPs as nano-fertilizers presents a promising, innovative strategy to improve plant performance, enhance agricultural productivity, and offer a sustainable alternative to chemical fertilizers.
Data availability
The data that support the findings of this study are available from the authors upon reasonable request. For details, please contact corresponding author Mozhgan Mardani-Talaee.
References
Rahman, A. et al. Larvicidal and antifeedant effects of copper nano-pesticides against Spodoptera frugiperda (JE Smith) and its immunological response. Insects 13, 1030. https://doi.org/10.3390/insects13111030 (2022).
Dilipan, E., Sivaperumal, P., Kamala, K., Ramachandran, M. & Vivekanandhan, P. Green synthesis of silver nanoparticles using seagrass Cymodocea serrulata (R. Br.) Asch. & Magnus, characterization, and evaluation of anticancer, antioxidant, and antiglycemic index. Biotechnol. Appl. Biochem. 70, 1346–1356. https://doi.org/10.1002/bab.2444 (2023).
Gowtham, H. G. et al. Toxicological effects of nanoparticles in plants: Mechanisms involved at morphological, physiological, biochemical and molecular levels. Plant Physiol. Biochem. 210, 108604. https://doi.org/10.1016/j.plaphy.2024.108604 (2024).
Pittarate, S. et al. Insecticidal effect of zinc oxide nanoparticles against Spodoptera frugiperda under laboratory conditions. Insects 12, 1017 (2021).
Chanu, T. T. & Upadhyaya, H. Zinc oxide nanoparticle-induced responses on plants: a physiological perspective. In Nanomaterials in Plants, Algae and Microorganisms 43–64 (Academic Press, 2019).
Singh, A. K. et al. Green synthesis, characterization and antimicrobial activity of zinc oxide quantum dots using Eclipta alba. Mater. Chem. Phys. 203, 40–48. https://doi.org/10.1016/j.matchemphys.2017.09.049 (2018).
Murali, M. et al. Zinc oxide nanoparticles prepared through microbial mediated synthesis for therapeutic applications: A possible alternative for plants. Front. Microbiol. 14, 1227951. https://doi.org/10.3389/fmicb.2023.1227951 (2023).
Murali, M. et al. Plant-mediated zinc oxide nanoparticles: advances in the new millennium towards understanding their therapeutic role in biomedical applications. Pharm. 13, 1662. https://doi.org/10.3390/pharmaceutics13101662 (2021).
Ma, W., Zhu, G., Zhang, Y. & Guo, J. Green synthesis of ZnO NPs with long-lasting and ultra-high antimicrobial activity. Surf. Interfaces. 50, 104506. https://doi.org/10.1016/j.surfin.2024.104506 (2024).
Hussain, R. T., Hossain, M. S. & Shariffuddin, J. H. Green synthesis and photocatalytic insights: A review of zinc oxide nanoparticles in wastewater treatment. Mater. Today Sustain. 26, 100764. https://doi.org/10.1016/j.mtsust.2024.100764 (2024).
Thema, F. T., Manikandan, E., Dhlamini, M. S. & Maaza, M. J. M. L. Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater. Lett. 161, 124–127. https://doi.org/10.1016/j.matlet.2015.08.052 (2015).
El-Beltagi, H. S. et al. Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar. Drugs 20, 342. https://doi.org/10.3390/md20060342 (2022).
Pokhrel, L. R. & Dubey, B. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci. Total. Environ. 45, 321–332. https://doi.org/10.1016/j.scitotenv.2013.02.059 (2013).
Abdel Latef, A. A. H., Abu Alhmad, M. F. & Abdelfattah, K. E. The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in Lupine (Lupinus termis) Plants. J. Plant Growth Regul. 36, 60–70. https://doi.org/10.1007/s00344-016-9618-x (2017).
Kashyap, P. L., Xiang, X. & Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 77, 36–51. https://doi.org/10.1016/j.ijbiomac.2015.02.039 (2015).
Ponnuchamy, K. & Jacob, J. A. Metal nanoparticles from marine seaweeds–a review. Nanotechnol. Rev. 5, 589–600. https://doi.org/10.1515/ntrev-2016-0010 (2016).
Abotaleb, S. I. et al. Biosynthesis of zinc oxide nanoparticles using seaweed: Exploring their therapeutic potentials. Appl. Sci. 14, 7069. https://doi.org/10.3390/app14167069 (2024).
Alprol, A. E., Mansour, A. T., El-Beltagi, H. S. & Ashour, M. Algal extracts for green synthesis of zinc oxide nanoparticles: promising approach for algae bioremediation. Materials 16, 2819. https://doi.org/10.3390/ma16072819 (2023).
Bai, Y. et al. Seaweed biomass as a sustainable resource for synthesis of ZnO nanoparticles using Sargassum wightii ethanol extract and their environmental and biomedical applications through Gaussian mixture model. Environ. Res. 249, 117464. https://doi.org/10.1016/j.envres.2023.117464 (2024).
Durga, M., Deepikaa, R., Vaishnavi, M. & Raksha, B. Study on phytochemical composition, biosynthesis and characterization of zinc oxide nanoparticle using Sargassum ilicifolium. Saudi J. Med. Pharm. Sci. 10, 489–501. https://doi.org/10.36348/sjmps.2024.v10i07.010 (2024).
Barciela, P. et al. Macroalgae as biofactories of metal nanoparticles; biosynthesis and food applications. Adv. Colloid Interface Sci. 311, 102829. https://doi.org/10.1016/j.cis.2022.102829 (2022).
El Khalloufi, F. & Oudra, B. Seaweeds as a new source of bioactive compounds and potent biotechnological applications. In Seaweed Biotechnology 229–287 (Apple Academic Press, 2023).
Itroutwar, P. D. et al. Seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of rice (Oryza sativa L.). J. Plant Growth Regul. 39, 717–728. https://doi.org/10.1007/s00344-019-10012-3 (2020).
Mughunth, R. J., Velmurugan, S., Mohanalakshmi, M. & Vanitha, K. A. review of seaweed extract’s potential as a biostimulant to enhance growth and mitigate stress in horticulture crops. Sci. Hortic. 334, 113312 (2024).
Mansour, A. T. et al. Green synthesis of zinc oxide nanoparticles using red seaweed for the elimination of organic toxic dye from an aqueous solution. Materials 15, 5169. https://doi.org/10.3390/ma15155169 (2022).
Guil-Guerrero, J. L. & Rebolloso-Fuentes, M. M. Nutrient composition and antioxidant activity of eight tomato (Lycopersicon esculentum) varieties. J. Food Compos. Anal. 22, 123–129. https://doi.org/10.1016/j.jfca.2008.10.012 (2009).
Stanton, C., Sanders, D., Krämer, U. & Podar, D. Zinc in plants: Integrating homeostasis and bio fortification. Mol. Plant. 15, 65–85. https://doi.org/10.1016/j.molp.2021.12.008 (2022).
Alloway BJ. Zinc in Soils and Crop Nutrition. 2nd Edition, IZA and IFA, Brussels, Belgium and Paris, France. 16. (2008). 139. 135p. https://www.fertilizer.org/wp-content/uploads/2023/01/2008_IZA_IFA_ZincInSoils.pdf.
Broadley, M. R., White, P. J., Hammond, J. P., Zelko, I. & Lux, A. Zinc in plants. New phytol. 173, 677–702 (2007).
Manvelian, J., Weisany, W., Tahir, N. A. R., Jabbari, H. & Diyanat, M. Physiological and biochemical response of safflower (Carthamus tinctorius L.) cultivars to zinc application under drought stress. Ind. Crops Prod. 172, 114069. https://doi.org/10.1016/j.indcrop.2021.114069 (2021).
Verbon, E. H. et al. Iron and immunity. Annu. Rev. Phytopathol. 55, 355–375. https://doi.org/10.1146/annurev-phyto-080516-035537 (2017).
Biondi, A. & Desneux, N. Special issue on Tuta absoluta: recent advances in management methods against the background of an ongoing worldwide invasion. J. Pest. Sci. 92, 1313–1315. https://doi.org/10.1007/s10340-019-01132-6 (2019).
Vivekanandhan, P., Swathy, K., Alahmadi, T. A. & Ansari, M. J. Biocontrol effects of chemical molecules derived from Beauveria bassiana against larvae of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Front. Microbiol. 15, 1336334. https://doi.org/10.3389/fmicb.2024.1336334 (2024).
Vivekanandhan, P., Swathy, K., Sarayut, P. & Patcharin, K. Biology, classification, and entomopathogen-based management and their mode of action on Tuta absoluta (Meyrick) in Asia. Front. Microbiol. 15, 1429690. https://doi.org/10.3389/fmicb.2024.1429690 (2024).
FAO. FAOSTAT, Pesticides Use. In: FAO. Rome. http://www.fao.org/faostat/en/#data/RP. (Cited July 2023). (2023).
Rai, M. & Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 94, 287–293 (2012).
Michiels, K. V., Damme, E. J. & Smagghe, G. Plant-insect interactions: what can we learn from plant lectins?. Arch. Insect Biochem. Physiol. 73, 193–212. https://doi.org/10.1002/arch.20351 (2010).
Vivekanandhan, P. et al. Toxicity of Metarhizium flavoviride conidia virulence against Spodoptera litura (Lepidoptera: Noctuidae) and its impact on physiological and biochemical activities. Sci. Rep. 12, 16775. https://doi.org/10.1038/s41598-022-20426-x (2022).
Wang, Y., Oberley, L. W. & Murhammer, D. W. Evidence of oxidative stress following the viral infection of two Lepidopteran insect cell lines. Free Radic. Biol. Med. 31, 1448–1455. https://doi.org/10.1016/S0891-5849(01)00728-6 (2001).
Devrim, S., Sinem, H. E. F., Birsen, A. & Can, A. M. Determination of relationship between lipid peroxidation, antioxidant defence, trace elements, and hemorheology in COPD. Trace Elem. Electroly. 2019, 1–6. https://doi.org/10.5414/TEX01567 (2018).
Tripathi, D. K., Singh, V. P., Prasad, S. M., Chauhan, D. K. & Dubey, N. K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 96, 189–198. https://doi.org/10.1016/j.plaphy.2015.07.026 (2015).
Murali, M. et al. Fate, bioaccumulation and toxicity of engineered nanomaterials in plants: Current challenges and future prospects. Sci. Total Environ. 811, 152249. https://doi.org/10.1016/j.scitotenv.2021.152249 (2022).
Lakshmeesha, T. R. et al. Biofabrication of zinc oxide nanoparticles from Melia azedarach and its potential in controlling soybean seed-borne phytopathogenic fungi. Saudi J. Biol. Sci. 27, 1923–1930. https://doi.org/10.1016/j.sjbs.2020.06.013 (2020).
García-Gómez, C., Obrador, A., González, D., Babín, M. & Fernández, M. D. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 589, 11–24. https://doi.org/10.1016/j.scitotenv.2017.02.153 (2017).
Włodarczyk, K., Smolińska, B. & Majak, I. The antioxidant potential of tomato plants (Solanum lycopersicum L.) under nano-ZnO treatment. Int. J. Mol. Sci. 24, 11833 (2023).
Hafezieh M, Ajdari A, Ajdehakosh Por D, Hosseini SH. Using Oman Sea Sargassum illicifolium meal for feeding white leg shrimp Litopenaeus vannamei. http://hdl.handle.net/1834/11713. (2014).
Lichtenthaler, H. K. [34] Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148, 350–382. https://doi.org/10.1016/0076-6879(87)48036-1 (1987).
Wagner, G. J. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 64, 88–93. https://doi.org/10.1104/pp.64.1.88 (1976).
Slinkard, K. & Singleton, V. L. Total phenol analyses: automation and comparison with manual methods. Am. J. Enol. Vitic. 28, 49–55. https://doi.org/10.5344/ajev.1977.28.1.49 (1997).
Chang, C. C., Yang, M. H., Wen, H. M. & Chern, J. C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 10, 178–182. https://doi.org/10.38212/2224-6614.2748 (2002).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Hellebust, J. A. & Craigie, J. S. Handbook of Physiological and Biochemical Methods 512 (Cambridge University Press, 1978).
Mardani-Talaee, M., Zibaee, A., Nouri-Ganbalani, G., Rahimi, V. & Tajmiri, P. Effects of potato cultivars on some physiological processes of Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). J. Econ. Entomol. 108, 2373–2382. https://doi.org/10.1093/jee/tov213 (2015).
Mardani-Talaee, M., Zibaee, A., Nouri-Ganblani, G. & Razmjou, J. Chemical and organic fertilizers affect physiological performance and antioxidant activities in Myzus persicae (Hemiptera: Aphididae). ISJ Invert. Surviv. J. 13, 122–133 (2016).
Mardani-Talaee, M., Zibaee, A., Abedi, Z. & Golizadeh, A. Digestion and protein metabolism of Trogoderma granarium (Coleoptera: Dermestidae) fed on different barley varieties. J. Stored Prod. Res. 73, 37–41. https://doi.org/10.1016/j.jspr.2017.06.002 (2017).
Bernfeld, P. Amylases, α and β. Methods Enzymol. 1, 149–158. https://doi.org/10.1016/0076-6879(55)01021-5 (1955).
Elpidina, E. N. et al. Compartmentalization of proteinases and amylases in Nauphoeta cinerea midgut. Arch. Insect Biochem. Physiol. 48, 206–216. https://doi.org/10.1002/arch.10000 (2001).
Fossati, P. & Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 28, 2077–2080 (1982).
Thomas, L. Clinical Laboratory Diagnostic 1st edn, 89–94 (Verlasgesellschaft Frankfurt, 1998).
Szasz, G. Reaction-rate method for gamma glutamyl transferase activity in serum. Clin. Chem. 22, 2051–2055 (1976).
Pinto, P. V. A., Kaplan, A. & Dreal, P. A. Aldolase: I. Colorimetric determination. Clin. Chem. 15, 349–360. https://doi.org/10.1093/CLINCHEM/15.5.339 (1969).
Addy, S. K. & Goodman, R. N. Polyphenol oxidase and peroxidase in apple leaves inoculated with a virulent or an avirulent strain for Ervinia amylovora. Indian Phytopathol. 25, 575–579 (1972).
Chance, B. & Maehly, A. C. [136] Assay of catalases and peroxidases. Methods Enzymol. 2, 764–775. https://doi.org/10.1016/S0076-6879(55)02300-8 (1955).
Balinsky, D. & Bernstein, R. E. The purification and properties of glucose- 6- phosphate dehydrogenase from human erythrocytes. Biochim. Biophys. Acta 67, 313–315. https://doi.org/10.1016/0006-3002(63)91828-6 (1963).
Bar-Or, D. et al. An analog of the human albumin N-terminus (Asp-Ala-His-Lys) prevents formation of copper-induced reactive oxygen species. Biochem. Biophys. Res. Commun. 284, 856–862. https://doi.org/10.1006/bbrc.2001.5042 (2001).
Khramtsov, V. V. et al. Quantitative determination and reversible modification of thiols using Imidazolidine biradical disulfide labl. J. Biochem. Biophys. Methods 35, 115–128. https://doi.org/10.1016/s0165-022x(97)00035-3 (1997).
Shimojo, N. et al. Test-strip method for measuring lactate in whole blood. Clin. Chem. 35, 1992–1994. https://doi.org/10.1093/clinchem/35.9.1992 (1989).
Guo, X., Wang, Y., Qin, Y., Shen, P. & Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 162, 618–628. https://doi.org/10.1016/j.ijbiomac.2020.06.180 (2020).
Keller, A. A. et al. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 44, 1962–1967. https://doi.org/10.1021/es902987d (2010).
Vivekanandhan, P., Deepa, S., Kweka, E. J. & Shivakumar, M. S. Toxicity of Fusarium oxysporum-VKFO-01 derived silver nanoparticles as potential inseciticide against three mosquito vector species (Diptera: Culicidae). J. Clust. Sci. 29, 1139–1149. https://doi.org/10.1007/s10876-018-1423-1 (2018).
Vivekanandhan, P., Panikar, S., Sethuraman, V., Usha-Raja-Nanthini, A. & Shivakumar, M. S. Toxic and synergetic effect of plant essential oils along with nano-emulsion for control of three mosquito species. Pestic. Res. J. 5, 100045. https://doi.org/10.1016/j.napere.2023.100045 (2023).
Ezealisiji, K. M., Siwe-Noundou, X., Maduelosi, B., Nwachukwu, N. & Krause, R. W. M. Green synthesis of zinc oxide nanoparticles using Solanum torvum (L) leaf extract and evaluation of the toxicological profile of the ZnO nanoparticles–hydrogel composite in Wistar albino rats. Int. Nano Lett. 9, 99–107. https://doi.org/10.1007/s40089-018-0263-1 (2019).
Banumathi, B., Malaikozhundan, B. & Vaseeharan, B. In vitro acaricidal activity of ethnoveterinary plants and green synthesis of zinc oxide nanoparticles against Rhipicephalus microplus (Boophilus). Vet. Parasitol. 216, 93–100. https://doi.org/10.1016/j.vetpar.2015.12.003 (2016).
Murugan, K. et al. Sargassum wightii-synthesized ZnO nanoparticles reduce the fitness and reproduction of the malaria vector Anopheles stephensi and cotton bollworm Helicoverpa armigera. Physiol. Mol. Plant Pathol. 101, 202–213. https://doi.org/10.1016/j.pmpp.2017.02.004 (2018).
Benelli, G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol. Res. 115, 23–34. https://doi.org/10.1007/s00436-015-4800-9 (2016).
Benelli, G. Green synthesized nanoparticles in the fight against mosquitoborne diseases and cancer e a brief review. Enzym. Microb. Technol. 95, 58–68. https://doi.org/10.1016/j.enzmictec.2016.08.022 (2016).
Bandyopadhyay, B., Reishus, K. N. & Duncan, M. A. Infrared spectroscopy of solvation in small Zn+ (H2O)n complexes. J. Phys. Chem. A 117, 7794–7803. https://doi.org/10.1021/jp4046676 (2013).
Boonchun, A., Lambrecht, W. R., Jiraroj, T. & Limpijumnong, S. Nitrogen pair−hydrogen complexes in ZnO and p-type doping. MRS Proc. https://doi.org/10.1557/opl.2012.246 (2012).
Esrafili, M. D., Behzadi, H. & Hadipour, N. L. Theoretical study of N-H··· O hydrogen bonding properties and cooperativity effects in linear acetamide clusters. Theor. Chem. Acc. 121, 135–146. https://doi.org/10.1007/s00214-008-0456-1 (2008).
Akbar, N., Aslam, Z., Siddiqui, R., Shah, M. R. & Khan, N. A. Zinc oxide nanoparticles conjugated with clinically-approved medicines as potential antibacterial molecules. AMB Exp. 11, 1–16. https://doi.org/10.1186/s13568-021-01261-1 (2021).
Anand, M. & Suresh, S. Marine seaweed Sargassum wightii extract as a low-cost sensitizer for ZnO photoanode based dye-sensitized solar cell. Adv. Nat. Sci. Nanosci. Nanotechnol. 6, 035008. https://doi.org/10.1088/2043-6262/6/3/035008 (2015).
Ebadi, M. et al. A bio-inspired strategy for the synthesis of zinc oxide nanoparticles (ZnO NPs) using the cell extract of cyanobacterium Nostoc sp. EA03: from biological function to toxicity evaluation. RSC Adv. 9, 23508–23525. https://doi.org/10.1039/c9ra03962g (2019).
Pandimurugan, R. & Thambidurai, S. Novel seaweed capped ZnO nanoparticles for effective dye photodegradation and antibacterial activity. Adv. Powder Technol. 27, 1062–1072. https://doi.org/10.1016/j.apt.2016.03.014 (2016).
Ramesh, M., Anbuvannan, M. & Viruthagiri, G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 136, 864–870. https://doi.org/10.1016/j.saa.2014.09.105 (2015).
Ishwarya, R. et al. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J. Photochem. Photobiol. B 178, 249–258. https://doi.org/10.1016/j.jphotobiol.2017.11.006 (2018).
El-Belely, E. F. et al. Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Arthrospira platensis (class: Cyanophyceae) and evaluation of their biomedical activities. Nanomaterials 11, 95. https://doi.org/10.3390/nano11010095 (2021).
Rabecca, R. et al. Facile synthesis of zinc oxide nanoparticle using algal extract and their antibacterial potential. Biomass Convers. Bior. https://doi.org/10.3390/polym15102335 (2022).
Khodakovskaya, M. V. et al. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc. Natl. Acad. Sci. U. S. A. 108, 1028–1033. https://doi.org/10.1073/pnas.1008856108 (2011).
Prerna, D. I. et al. Seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of rice (Oryza sativa L.). J. Plant Growth Regul. 39, 717–728. https://doi.org/10.1007/s00344-019-10012-3 (2020).
Das, I. & Borah, D. Microbial biosurfactant-mediated green synthesis of zinc oxide nanoparticles (ZnO NPs) and exploring their role in enhancing chickpea and rice seed germination. Discov. Nano 19, 1–21. https://doi.org/10.1186/s11671-024-04134-1 (2024).
Ahmad, W., Nepal, J., Xin, X. & He, Z. Agronomic Zn biofortification through nano ZnO application enhanced growth, photosystem efficiency, Zn and P nutrition in maize. Arch. Agron. Soil Sci. 69, 3328–3344. https://doi.org/10.1080/03650340.2023.2231350 (2023).
Donia, D. T. & Carbone, M. Seed priming with zinc oxide nanoparticles to enhance crop tolerance to environmental stresses. Int. J. Mol. Sci. 24, 17612. https://doi.org/10.3390/ijms242417612 (2023).
Subbaiah, L. V. et al. Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.). J. Agric. Food Chem. 64, 3778–3788. https://doi.org/10.1021/acs.jafc.6b00838 (2016).
Torre-Roche, R. D. L. et al. Seed biofortifcation by engineered nanomaterials: A pathway to alleviate malnutrition?. J. Agric. Food Chem. 68, 12189–12202. https://doi.org/10.1021/acs.jafc.0c04881 (2020).
Mickky, B. M. Seed Priming as a strategy to improve wheat productivity under abiotic stress: global meta-analysis. J. Plant Growth Regul. 41, 1397–1410. https://doi.org/10.1007/s00344-021-10403-5 (2022).
Mukherjee, Y. et al. Differential toxicity of bare and hybrid ZnO nanoparticles in green pea (Pisum sativum L.): a life cycle study. Front. Plant Sci. 12, 1242. https://doi.org/10.3389/fpls.2015.01242 (2016).
Wang, X. P., Li, Q. Q., Pei, Z. M. & Wang, S. C. Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol. Plant 62, 801–808. https://doi.org/10.1007/s10535-018-0813-4 (2018).
Salama, D. M., Osman, S. A., Abd El-Aziz, M. E., Abd Elwahed, M. S. & Shaaban, E. A. Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris). Biocatal. Agric. Biotechnol. 18, 101083. https://doi.org/10.1016/j.bcab.2019.101083 (2019).
Shahhoseini, R., Azizi, M., Asili, J., Moshtaghi, N. & Samiei, L. Effects of zinc oxide nanoelicitors on yield, secondary metabolites, zinc and iron absorption of Feverfew (Tanacetum parthenium L. Schultz Bip.). Acta Physiol. Plant 42, 1–18. https://doi.org/10.1007/s11738-020-03043-x (2020).
El-Saied, R. M. & Elsayed, M. S. Effect of different concentrations from zinc oxide nanoparticles prepared in date pits extract on pea (Pisum sativum L.) plant. Egypt. J. Chem. 65, 575–582 (2022).
Kulbat, K. The role of phenolic compounds in plant resistance. Food Sci. Biotechnol. 80, 97–108. https://doi.org/10.34658/bfs.2016.80.2.97-108 (2016).
Lorrai, R. & Ferrari, S. Host cell wall damage during pathogen infection: Mechanisms of perception and role in plant-pathogen interactions. Plants 10, 399. https://doi.org/10.3390/plants10020399 (2021).
Ortego, F. Physiological adaptations of the insect gut to herbivory. In Arthropod-Plant Interactions: Novel Insights and Approaches for IPM 75–88 (Springer, 2012).
Muhammad, A. et al. Dietary exposure of copper and zinc oxides nanoparticles affect the fitness, enzyme activity, and microbial community of the model insect, silkworm Bombyx mori. Sci. Total Environ. 813, 152608. https://doi.org/10.1016/j.scitotenv.2021.152608 (2022).
Tuladhar, P., Sasidharan, S. & Saudagar, P. Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. In Biocontrol Agents and Secondary Metabolites 419–441 (Woodhead Publishing, 2021).
Raigond, B. et al. Effect of zinc nanoparticles on antioxidative system of potato plants. J. Environ. Biol. 38, 435–439. https://doi.org/10.22438/jeb/38/3/MS-209 (2017).
García-López, J. I. et al. Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum annuum L. during germination. Agronomy 8, 215. https://doi.org/10.3390/agronomy8100215 (2018).
Shahzad, K. & Manzoor, F. Nanoformulations and their mode of action in insects: a review of biological interactions. Drug Chem. Toxicol. 44, 1–11. https://doi.org/10.1080/01480545.2018.1525393 (2021).
Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants?. Plant Physiol. Biochem. 144, 135–143. https://doi.org/10.1016/j.plaphy.2019.09.039 (2019).
Ibrahim, A. M. A. et al. Chronic effects induced by zinc oxide nanoparticles against larvae of the northern house mosquito Culex pipiens (Diptera: Culicidae). Int. J. Trop. Insect Sci. 43, 1937–1945. https://doi.org/10.1007/s42690-023-01092-6 (2023).
Elbanhawy, A. A., Elsherbiny, E. A., Abd El-Mageed, A. E. & Abdel-Fattah, G. M. Potential of fungal metabolites as a biocontrol agent against cotton aphid, Aphis gossypii Glover and the possible mechanisms of action. Pestic. Biochem. Physiol. 159, 34–40. https://doi.org/10.1016/j.pestbp.2019.05.013 (2019).
Nation, J. L. Insect Physiology and Biochemistry 2nd edn, 235 (CRC Press, 2008).
Wang, C. S., Chang, T. T., Yao, W. J., Wang, S. T. & Chou, P. Impact of increasing alanine aminotransferase levels within normal range on incident diabetes. J. Formos. Med. Assoc. 111, 201–208. https://doi.org/10.1016/j.jfma.2011.04.004 (2012).
Tate, S. S. & Meister, A. Gamma-glutamyl transpeptidase from kidney. Methods Enzymol. 113, 400–419. https://doi.org/10.1016/s0076-6879(85)13053-3 (1985).
Zorlu, T., Nurullahoğlu, Z. U. & Altuntaş, H. Infuence of dietary titanium dioxide nanoparticles on the biology and antioxidant system of model insect, Galleria mellonella (L.) (Lepidoptera: Pyralidae). J. Entomol. Res. Soc. 20, 89–103 (2018).
Rankic, I. et al. Nano/microparticles in conjunction with microalgae extract as novel insecticides against Mealworm beetles. Tenebrio Molitor. Sci. Rep. 11, 17125. https://doi.org/10.1038/s41598-021-96426-0 (2021).
Ibrahim, A. M. & Ali, A. M. Silver and zinc oxide nanoparticles induce developmental and physiological changes in the larval and pupal stages of Spodoptera littoralis (Lepidoptera: Noctuidae). J. Asia Pac. Entomol. 21, 1373–1378. https://doi.org/10.1016/j.aspen.2018.10.018 (2018).
Pardini, R. S., Pritsos, C. A., Bowen, S. M., Ahmad, S. & Blomquist, G. J. Adaptations to plant pro-oxidants in a phytophagous insect model: enzymatic protection from oxidative stress. In Oxygen Radicals in Biology and Medicine (eds Simic, M. G. et al.) 725–728 (Plenum Press, 1988).
Belal, R. & Gad, A. Zinc oxide nanoparticles induce oxidative stress, genotoxicity, and apoptosis in the hemocytes of Bombyx mori larvae. Sci. Rep. 13, 3520. https://doi.org/10.1038/s41598-023-30444-y (2023).
Meng, X. et al. Effects of Ag nanoparticles on growth and fat body proteins in silkworms (Bombyx mori). Biol. Trace Elem. Res. 180, 327–337. https://doi.org/10.1007/s12011-017-1001-7 (2017).
Gowtham, H. G. et al. Plant growth promoting rhizobacteria-Bacillus amyloliquefaciens improves plant growth and induces resistance in chilli against anthracnose disease. Biol. Control 126, 209–217. https://doi.org/10.1016/j.biocontrol.2018.05.022 (2018).
Naziya, B., Murali, M. & Amruthesh, K. N. Plant growth-promoting fungi (PGPF) instigate plant growth and induce disease resistance in Capsicum annuum L. upon infection with Colletotrichum capsici (Syd.) Butler & Bisby. Biomolecules 10, 41. https://doi.org/10.3390/biom10010041 (2019).
Salih, A. M. et al. Biosynthesis of zinc oxide nanoparticles using Phoenix dactylifera and their effect on biomass and phytochemical compounds in Juniperus procera. Sci. Rep. 11, 19136. https://doi.org/10.1038/s41598-021-98607-3 (2021).
Elsilk, S. E. et al. Green-synthesized zinc oxide nanoparticles by Enterobacter sp.: unveiling characterization, antimicrobial potency, and alleviation of copper stress in Vicia faba (L.) plants. BMC Plant Biol. 24, 474. https://doi.org/10.1186/s12870-024-05150-0 (2024).
Anusuya, S. & Rajan, K. Role of zinc and zinc oxide nanofertilizer in enhancing crop production. In Metal and Metal-Oxide Based Nanomaterials: Synthesis, Agricultural, Biomedical and Environmental Interventions 111–131 (Springer Nature, 2024).
Acknowledgements
This research was supported by the University of Mohaghegh Ardabili, Ardabil, Iran. We also extend our gratitude to the Agricultural Research Education and Extension Organization (AREEO), the Iranian Fisheries Science Research Institute (IFSRI), and the Offshore Research Center in Chabahar, Iran, for their collaboration in collecting the brown algae.
Author information
Authors and Affiliations
Contributions
Mozhgan Mardani-Talaee: Conceptualization, Investigation, Formal analysis, Writing—original draft. Jabraeil Razmjou: Conceptualization, Project administration, Supervision, Writing—review and editing, Funding acquisition. Ashkan Ajdari: Conceptualization, review and editing. José Eduardo Serrão: Validation and Writing review and editing. Perumal Vivekanandhan: Writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mardani-Talaee, M., Razmjou, J., Ajdari, A. et al. Green synthesis of zinc oxide nanoparticles from Sargassum ilicifolium to enhance tomato resistance against Tuta absoluta. Sci Rep 15, 13596 (2025). https://doi.org/10.1038/s41598-025-97535-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97535-w
Keywords
This article is cited by
-
Nanotechnology Applications for Enhancing Potato Productivity, Stress Resilience, and Post-Harvest Quality
American Journal of Potato Research (2026)
-
Multi-functional zinc oxide nanoparticles (ZnONps inspired from Myristica Fragrans seed extract against cervical Cancer, pyogenic bacteria and inflammatory response. a promising potential of ZnONps in cancer nanotherapy, infection control and inflammation management
3 Biotech (2026)
-
Investigating the alleviatory ability of bio-synthesized zinc oxide nanoparticles from Sargassum ilicifolium (Turner) C. Agardh on the tomato plants exposed to whitefly infestation
Scientific Reports (2025)
-
Sustainable synthesis of ZnO nanoparticles from Brickellia cavanillesii extract with enhanced photocatalytic and antimicrobial properties
Research on Chemical Intermediates (2025)